Abstract
Systemic Lupus Eritematosus (SLE) is a systemic inflammatory disease often treated with the agent cyclophosphamide (CY), known by provoking important adverse reactions to the organism. Ader and Cohen have demonstrated an alternative way of administrating this agent based on pavlovian conditioning, in order to reduce the aggression caused by CY. Considering the influence of the temporal organization on learning and memory processes, the purpose of this study was to understand the temporal aspects involved in the conditioned immunomodulation. In a search for circadian modulation, we selected NZB/W (F1) female mice, a strain that spontaneously develop SLE. Divided into two major groups, the animals were submitted, in different phases of day, to a classical conditioning immunomodulation protocol, consisting in weekly parings of saccharin solution and CY injections. The success of the paradigm was evaluated by comparing lifespan among the groups. Simultaneously, it was monitored the water intake behavior, in order to correlate the stability of two rhythmic parameters, amplitude and spectral power density of the 24-h rhythm, with the progression of SLE. Our results indicate that mice could benefit from the conditioning task performed either in the light phase or in the dark phase of the LD cycle, as expressed by an increased lifespan. Concerning the rhythmic parameters, there was evidence of association between the rhythmic stability and the evolution of SLE, demonstrated by the maintenance of healthy levels of amplitude and spectral potency of the 24-h rhythm in animals exposed to the conditioning paradigm.
Keywords
Classical conditioning ; Circadian drinking behavior ; Immunomodulation ; Rhythmic stability ; NZB/W ; Learning
1. Introduction
Since Metalnikov and Chorine [1] , pioneers who described the relationship between the central nervous system (CNS) and the immune processes in the 1920s, neuroimmunomodulation has been extensively explored by different research groups aiming to unveil how the immune system interacts with the CNS, from cellular [2] to behavioral [3] levels. Particularly, It has been found that cellular and humoral immunity may be influenced by Pavlovian conditioning. Pairing a novel drinking solution (conditioned stimulus-CS) with an immunomodulatory drug (unconditioned stimulus-US) results in a modulation of the immune function. Findings in conditioned immunosuppression [4] raised the question of whether the development of an immune disorder could be delayed by the classical conditioning paradigm. To answer this question, Ader and Cohen [5] investigated the progression of proteinuria and the lifespan of spontaneous lupus prone mice under a classical conditioning paradigm in which a saccharin-flavored drink is paired with the injection of an effective immune-suppressing drug cyclophosphamide. The results were consistent with their hypothesis, confirming the biological impact of conditioned immunomodulation revealed by the modulation of the lifespan.
Although immune system conditioning has been extensively studied, the temporal aspect of this form of associative learning has been widely overlooked. A growing body of evidence indicates daily modulation in learning and states that both morphological and physiological integrity of circadian timekeeping system are critical for learning and memory processes [6] , [7] , [8] and [9] . Davies et al. [10] demonstrated a 24-h rhythm in passive avoidance behavior. Rats trained and tested with a 4-h interval throughout a 24-h cycle exhibited more pronounced memory retention during the light phase of the LD cycle than during the dark phase. Moreover, the “time-stamp” phenomenon, which comprises the strong relationship between the performance in a learning task and the circadian phase of prior training, has been reported both for reward-conditioned place preference and for avoidance-based place leaning in hamsters [11] and [12] even for animals carrying the Tau mutation and displaying a short 20 h-period of rest-activity rhythm [13] .
In addition to the interaction between learning and the circadian timekeeping system, immune function has also been found to be sensitive to daily temporal modulation. Daily physiological variations in circulating B and T cells [14] , cell migration [15] and response of T cells to antigen [16] are examples of essential immune functions under the control of the circadian system. Macrophages and monocytes have been extensively studied due to their robust intrinsic clock machinery and high amplitude circadian output, leading to excellent time givers to peripheral tissues [17] . Moreover, recruitment of macrophages and monocytes during infection is based on the expression of adhesion molecules whose activity of under the control of the circadian timekeeping system [18] . However, possible effects of immune functions on circadian system have not received the same attention. In an effort to study such interaction, Marpegan et al. [19] found that Escherichia coli lipopolysaccharide (LPS) significantly altered the pattern of activity of mice by promoting a delay in the rest-activity cycle and, specifically, inducing a different cell expression in the core site of the circadian timekeeping system, the suprachiasmatic nuclei.
Taking into account the already described influence of the circadian timekeeping system on learning processes and the clear interface between immune function and the circadian timekeeping system, it would be important to address the significance of the circadian organization in the progression of immune diseases. For instance, Systemic lupus erythematosus (SLE) is an autoimmune disease that describes a whole range of systemic affections, including skin, joints, central nervous system and kidney. A recent British cohort study stated that although incidence presented an annual 1.8% decrease, prevalence changed from 64.99/100.000 to 97.04/100.00, from 1999 to 2012 [20] . In addition, despite substantial advances in the therapeutic approaches of this autoimmune disease, the mean age of death of patients with systemic lupus erythematosus is 44 y [21] , thus representing a life threatening condition that impacts people during their high productive working-age.
Besides the well established kidney and vascular diseases associated with SLE, it is considered poorly understood its relationship with the circadian timekeeping system. Melatonin daily pattern, a well stablished and gold marker of the circadian phase, seems to be affected by SLE, as patients show significantly lower daily melatonin levels in comparison to healthy women during short photoperiod [22] . It is a significant finding since melatonin is considered one of the most prominent endogenous synchronizer needed to maintain the stability of phase relationship and reinforce the different circadian rhythms in the whole organism [23] . Moreover, the prevalence of sleep disorders in SLE as well as the contributing factors to their occurrence remain poorly understood, despite its prevalence rates ranging between noteworthy 55% and 85% according to distinct study approaches [24] .
NZB/W (F1) mice offers a well-established mouse model of SLE since mice develop, spontaneously, the disease that resembles human SLE, including antinuclear antibodies, hemolytic anemia, proteinuria and a fatal progressive glomerulonephritis [25] . This model has also been submitted to classical conditioning immunosuppression protocols [5] and to different regimes of administration of immunosuppression drugs, including Cyclophosphamide (CY), a chemotherapeutic agent that exhibits a notably diurnal rhythm in toxicity [26] . All these features of the animal model and of the therapeutic agent open a wide range of opportunity to the study of a possible diurnal modulation in conditioned immunosuppression in a murine model of the Human Lupus Disease.
Therefore, our objective is to evaluate the interaction between the circadian organization and the survival of the NZB/W(F1) mice, as well as to search for a distinct effect of the conditioned immunosuppression according to the time of the day the protocol is administered, regarding both the progression of renal commitment and lifespan.
2. Materials and methods
2.1. Animals
Female New Zealand mice (NZBx NZW F1) were 6 months old at the beginning of the experiment. They were housed individually in standard polypropylene cages and were kept in an air-conditioned, soundproof holding room, at an ambient temperature of 22±2 °C and under a 12:12 h light-dark cycle (LD 12:12, lights on at 07:00 h). Food and water were available ad libitum. All experiments were performed in accordance with the guidelines of the Brazilian National Board for handling and care of laboratory Animals. Our study protocol was approved by local ethics committee (Instituto de Ciências Biomédicas – Universidade de São Paulo, Brazil).
2.2. Experimental procedures
2.2.1. Animals selection and proteinuria
Animals were tested for proteinuria using Multistix™ reagent strips (Bayer Diagnostics) at eight months of age, based on the findings of clear dichotomy in the development of renal disease, as proposed by Daikh and Wofsy [27] . Animals displaying proteinuria levels of 100 mg/dl or below (mild renal disease) were excluded from the experiment. Proteinuria was measured on freshly expressed urine samples of the remaining animals (N =42) fortnightly, throughout the experiment.
2.2.2. Drinking behavior apparatus
During the entire experiment, drinking behavior was recorded with “The Mouse Watcher” TMW10 equipment (Consultoria Eletronica, Brazil). Each touch on the sipper tube surface activated an electronic microswitch and was recorded as one pulse of drinking. Data were collected continuously in 5-min bins and transmitted to a computer for further analysis.
2.2.3. The conditioning paradigm
Animals were assigned to two major groups: The morning group “M” (subjected to the procedure two hours after lights on) and the night group “N” (subjected to the procedure two hours after light off).
Each major group was divided into 4 groups, according to Ader and Cohen Protocol [5] . Table 1 clarifies the group division and the protocol for each group.
| Groups M | Groups N | Pairings (n ) | Protocol |
|---|---|---|---|
| C100M | C100N | 6 | 6X Sac1 +CY2 |
| C50M | C50N | 6 | 3X Sac1 +CY2 and 3X Sac1 +Sal3 (random) |
| NC50M | NC50N | 3+3 | 3X Sac1 +CY2 and 3X Sac1 +Sal3 (not paired) |
| CTLM | CTLN | 6 | 6X Sac1 +Sal3 |
The behaviorally conditioned immunosupression paradigm consists of 6 pairings, on a weekly basis, of a saccharin solution and an intraperitoneal injection of cyclophosphamide or saline solution under a specific physical context (black chamber and menthol odor).
C100 groups (M and N), or standard treatment groups, were subjected on a weekly basis for 6 weeks, to an intraperitoneal injection of cyclophosphamide (30 mg/kg) (CY) immediately after a saccharin solution (SAC). By the end of the experiment, animals from C100 groups have received a total amount of 180 mg/kg each. In turn, conditioned groups (C50M and C50N) received SAC solution on 3 times in 6 weeks, in random order, followed by an intraperitoneal injection of cyclophosphamide (30 mg/kg). On the other 3 occasions that the animals were not subjected to the drug, they received an intraperitoneal injection of saline after SAC. Consistently, by the end of the experiment, animals from C50 groups have received a total amount of 90 mg/kg each, or 50% the amount of each animal from C100 groups.
Animals from NC50 groups (non-conditioned groups NC50M and NC50N) were also submitted to CY injections following SAC presentations once every 2 weeks, but on a noncontingent basis (the SAC and the drug injections were not paired). Thus, by the end of the experiment, despite the fact that each animal from NC50 groups have received the same amount of drug that C50 groups received, there was no regular temporal relationship between SAC and CY (pairings). Control groups (CTLM and CTLN) were not subjected to cyclophosphamide, at all. Actually, they only received SAC solution followed by saline injections, on a weekly and noncontingent basis.
2.3. Statistical analyses
Following the conditioning paradigm, drinking behavior data were extracted 5 times at week 1, 3, 5, 7 and 9, each containing a time series of 10 days. Drinking behavior was analyzed using the integrated package for chronobiology ‘‘El Temps” v.251 (A. Díez-Noguera, Universitat de Barcelona, 2011). Time series were submitted to the Cosinor method of cosine curve adjustment [28] , for the detection of circadian rhythmicity. Moreover, in order to search for periodic patterns and to determine spectral power density, Sokolove–Bushell Periodograms were used. For intergroup comparison (at weeks 1, 3, 5, 7 and 9), Kruskal–Wallis ANOVA was performed. The significance level was set at (P <0.05). Log-rank test was used to compare survival rates between groups for lifespan analysis.
3. Results
3.1. Lifespan
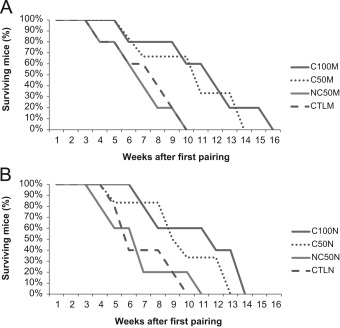
Chemotherapeutic regimens extended the lifespan of animals subjected to 6 administrations of cyclophosphamide, for both morning (C100M) and night (C100N) groups, when compared to their respective controls (log-rank test, P= 0.03 and P= 0.05, respectively). Precisely, mice from groups C100M and C100N began to die at week 9, whereas mice from control groups (CTLM and CTLN) began to die at week 5.
On the other hand, lifespan of animals from both groups NC50M and NC50N, who received half of the drug in a non-contingent, unpaired basis, was not statistically different from control animals (log-rank test, P= 0.7 and P= 0.9, respectively). At week 9, only 20% of the total number of animals from NC50 (M and N) and CTL (M and N) groups were alive.
Additionally, besides being subjected to the same amount of drug, when compared with NC50M and NC50N groups, animals from the conditioned groups C50M and C50N survived significantly longer than their respective controls (log-rank test, P= 0.04 and P=0.05, respectively). Survival rate was higher for those submitted to the conditioning protocol and 66.66% of animals of C50 groups were still alive by week 9. Fig. 1 summarizes the results from log-rank test and respective survival curves for all groups.
|
|
|
Fig. 1. Survival Profiles of NZB/W (F1) mice. Log-rank test used to compare survival rates between groups for lifespan analysis. A. Comparison of groups submitted to procedures during the light phase of LD cycle. B. Comparison of groups submitted to procedures during the dark phase of LD cycle. Extended lifespan of animals subjected to 6 administrations of cyclophosphamide, for both morning (C100M) and night (C100N) groups, when compared to their respective controls (log-rank test, P =0.03 and P =0.05, respectively). No difference found for both groups NC50M and NC50N, when compared to control animals (log-rank test, P =0.7 and P =0.9, respectively). Animals from the conditioned groups C50M and C50N survived significantly longer than their respective controls (log-rank test, P =0.04 and P =0.05, respectively). |
Therefore, no differences regarding time of day – ZT02 and ZT14, were found for animals that received the total amount or 50% of CY under the conditioned protocol.
3.2. Drinking behavior
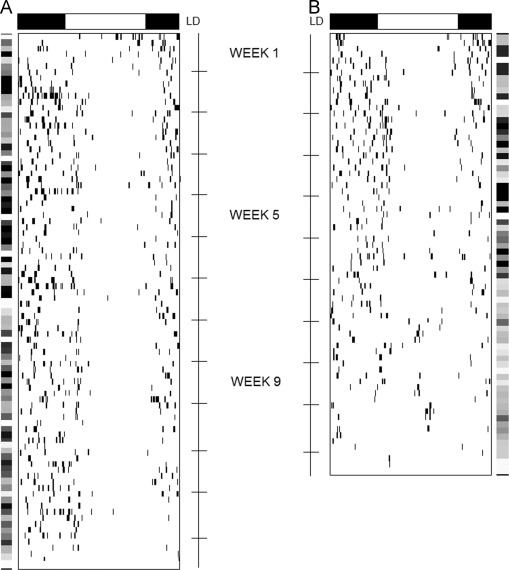
All mice presented daily rhythm of drinking behavior at the beginning of the conditioning protocol with a dominant 24-h periodicity (Sokolove–Bushell periodogram) and a significant 24-h rhythm identified by Cosinor analysis. Our hypothesis was confirmed, animals not submitted to the total amount of drug or not conditioned (NC50 and CTL groups) would demonstrate earlier decreases in rhythmic parameters of the drinking behavior rhythm along the lifespan, when compared to animals from the C50 or C100 groups. Fig. 2 shows an example of distinct water consumption pattern from week 1, 3, 5, 7, 9 and over time, including spectral power density as graphic matrix of the 1st harmonic analysis (24 h).
|
|
|
Fig. 2. Representative data of conditioned group mice and control group. Profile of water consumption of a mouse from C50M group, submitted to the conditioning protocol during the light phase of LD cycle raw data throughout the experiment (A). Profile of water consumption of a mouse from CTLM group, submitted to saline during the light phase of LD cycle. Raw data throughout the experiment (B). Detail for external bars indicating the graphic matrix of the 24-h 1st harmonic power spectral serial analysis. Gray levels ranging 0.00 (extreme light) to 10.0 (extreme dark) arbitrary units. The darkest, the more significant circadian rhythmicity. |
Intergroup comparison of spectral power density in the circadian range showed no difference among all groups at week 1 (Kruskal–Wallis test, H =7.7, df=7, P =0.35). At week 3 spectral power density remained unchanged among all groups (Kruskal–Wallis test, H =6.2, df=7, P =0.51). At week 5 the comparison revealed a significant reduction in spectral power density for the 24-h component only for CTL (M and N) and NC50 (M and N) groups (Kruskal–Wallis test, H =16.9, df=7, P <0.05). In addition to the reduced spectral power density, we found a statistical significant decline in the amplitude of the circadian rhythm of drinking for CTL (CTLM and CTLN) and NC50 (NC50M and NC50N) groups at week 5 (Kruskal–Wallis, H =11.8, df=7, P <0.05) using the best-fit sinusoidal curve by the Cosinor method. At week 7, both spectral power density and amplitude displayed the same level of expression for each group, adding support to the reductions observed at week 5 for CTL and NC50 groups.
None of the animals from CTL and NC50 groups expressed statistically significant 24-h rhythms with the Cosinor analysis during week 9, which made the comparison impossible. Nevertheless, for the groups of animals that received all six administrations of cyclophosphamide (C100M and C100N) or received half of the total amount in the conditioning protocol (C50M and C50N), neither spectral power density nor amplitude levels were different among them (Kruskal–Wallis test, H =1.69, df=3, P =0.63).
4. Discussion
To our knowledge, this is the first effort to search for a diurnal variation in conditioned immunosuppression, linking together apparent distinct fields of research, such as the need for a proper output from the circadian timekeeping system, the diurnal variation of immunomodulation and toxicity, the conditioned immunosuppression, and the interaction between the progression of an autoimmune disease and the circadian timekeeping system.
The chemotherapeutic agent cyclophosphamide exhibits a notably diurnal rhythm in toxicity. Scheving et al. [26] demonstrated that in mice with leukemia, administration of this agent during the light phase of the light-dark (LD) cycle resulted in a success rate of 44%, compared to 94% of success when administered during the dark phase.
Since the circadian timekeeping system interacts with both the effectiveness of the cyclophosphamide and primary learning faculties11 , [29] , we find it necessary to study the effect of cyclophosphamide on immunosupression using a conditioning paradigm that incorporates the concept of diurnal variation.
Despite the lack of exact comparative literature on diurnal variations in conditioned immunomodulation, we expected performance of nocturnal rodents (such as mice) to be better only during their active phase (i.e. the dark phase during LD cycle). The behavioral literature provides vast evidence for diurnal and circadian modulation in learned behaviors [7] , [8] and [30] . For instance, Chaudhury and Colwell [9] found intriguing results in a fear conditioning task and suggested that this apparent phase dependency may be a special feature of aversive (or fear) conditioning. Moreover, they concluded that the light phase was a fearful time for nocturnal animals and this phase dependency could lead to improved performance in aversive conditioning. However, other studies have shown better performance in nocturnal animals during the dark phase of the LD cycle in different protocols learning [31] and [32] . These findings demonstrated possible species–specific differences and variation in training protocol and behavioral paradigms, possibly explaining our results and the conflicting reports and lack of consensus in the literature. Therefore, contrary to our expectations, the results indicated that mice could benefit from the conditioning task performed either in the light phase or in the dark phase of the LD cycle, being the success represented by an increased lifespan.
Concerning the interaction between the progression of an autoimmune disease and the circadian timekeeping system, the disruption of the circadian system may occur in tumor tissue, tumor-bearing animals, and terminal cancer patients. Such rhythmic disruption includes decrease in amplitude, phase shifts, period changes, and erratic peaks and troughs in endocrine, metabolic, immunological, and rest-activity cycles [33] . Experimental challenging of the circadian timekeeping system leading to Chronodisruption due to chronic phase shifts of the light-dark (LD) cycle [34] or short 20-h LD cycle [35] have also been proved to adversely affects immune function. Additionally, changes in daily rhythms of heart rate, heart rate variability and blood pressure [36] , as well as rhythmic expression of corticosterone, leptin and clock genes [37] were detected during the course of autoimmune disease progression in an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE).
In this study we observed that animals from the groups that received the entire amount of immunosupression drug, as well as those subjected to 50% of cyclophosphamide under the conditioning protocol, expressed stability in daily rhythm of drinking behavior throughout the experiment, in contrast with the decrease in both amplitude from the adjusted 24-h cosine curve and 24-h rhythm spectral power density shown by animals from control and non-conditioned groups.
Finally, to keep the proper functioning of the circadian system throughout life seems to be imperative for the ordinary physiological expression of multiple functions, from lifespan to memory acquisition and consolidation. Disruption of rhythmicity reduces lifespan and suprachiasmatic implants from young animals restores higher amplitude rhythms in old rodents [38] . Moreover, animals exposed to chronic phase shifts, which has been previously to lead to circadian dysfunction, exhibit learning and memory dysfunction [39] .
In conclusion, our results indicate that mice could benefit from the conditioning task performed either in the light phase or in the dark phase of the LD cycle, as expressed by an increased lifespan. Concerning the rhythmic parameters, there was evidence of association between the stability of the signal from the circadian timekeeping system and the evolution of SLE, demonstrated by the maintenance of healthy levels of amplitude and spectral power density of the 24 h rhythm in animals exposed to the conditioning paradigm.
Some limitations should be stressed. In spite of our results in terms of the proteinuria, a marker of the success of the pavlovian conditioning paradigm, one limitation of the present research was the lack of histopathological observations of renal tissue and blood analysis, the standard measure of Lupus progression. It is noteworthy, however, that our approach was intended to be as non-invasive as possible, privileging the behavioral data instead of such markers of disease progression. In addition, we only tested animals in two different phases of day, making it impossible to generalize our results to the other phases of the day.
References
- [1] Metalnikov S, Chorine V. Rôle des reflexes conditionnels dans l’immunité. Annales de l’Institut Pasteur, 1926. XL(II).
- [2] M.A. Erickson, K. Dohi, W.A. Banks; Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier; Neuroimmunomodulation, 19 (2012), pp. 121–130
- [3] F.A. Costa-Pinto, et al.; Behavior: a relevant tool for brain-immune system interaction studies; Ann N Y Acad Sci, 1153 (2009), pp. 107–119
- [4] R. Ader, N. Cohen; Behaviorally conditioned immunosuppression; Psychosom Med, 37 (1975), pp. 333–340
- [5] R. Ader, N. Cohen; Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus; Science, 215 (1982), pp. 1534–1536
- [6] S.W. Cain, T. Chou, M.R. Ralph; Circadian modulation of performance on an aversion-based place learning task in hamsters; Behav Brain Res, 150 (2004), pp. 201–205
- [7] V.S. Valentinuzzi, L. Menna-Barreto, G.F. Xavier; Effect of circadian phase on performance of rats in the Morris water maze task; J Biol Rhythm, 19 (2004), pp. 312–324
- [8] F.K. Stephan, N.S. Kovacevic; Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions; Behav Biol, 22 (1978), pp. 456–462
- [9] D. Chaudhury, C.S. Colwell; Circadian modulation of learning and memory in fear-conditioned mice; Behav Brain Res, 133 (2002), pp. 95–108
- [10] J.A. Davies, V. Navaratnam, P.H. Redfern; A 24-hour rhythm in passive-avoidance behaviour in rats; Psychopharmacologia, 32 (1973), pp. 211–214
- [11] M.R. Ralph, et al.; The significance of circadian phase for performance on a reward-based learning task in hamsters; Behav Brain Res, 136 (2002), pp. 179–184
- [12] S.W. Cain, R.J. McDonald, M.R. Ralph; Time stamp in conditioned place avoidance can be set to different circadian phases; Neurobiol Learn Mem, 89 (2008), pp. 591–594
- [13] S.W. Cain, et al.; Retention of a 24-h time memory in Syrian hamsters carrying the 20-h short circadian period mutation in casein kinase-1epsilon (ck1epsilontau/tau); Neurobiol Learn Mem, 114 (2014), pp. 171–177
- [14] C.M. Steel, J. Evans, M.A. Smith; Physiological variation in circulating B cell:T cell ratio in man; Nature, 247 (1974), pp. 387–389
- [15] E. Richens, et al.; Circadian rhythms in cell migration in vitro, and its effect on antigen-induced migration inhibition; Clin Exp Immunol, 19 (1975), pp. 343–346
- [16] E.E. Fortier, et al.; Circadian variation of the response of T cells to antigen; J Immunol, 187 (2011), pp. 6291–6300
- [17] N. Labrecque, N. Cermakian; Circadian clocks in the immune system; J Biol Rhythm, 58 (2015), pp. 50–56
- [18] S. Sato, et al.; A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of CCl2 expression ; J Immunol, 192 (2014), pp. 407–417
- [19] L. Marpegan, et al.; Circadian responses to endotoxin treatment in mice; J Neuroimmunol, 160 (2005), pp. 102–109
- [20] F. Rees, et al.; The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012; Ann Rheum Dis, 72 (2014), pp. 100–108
- [21] R. Cervera, et al.; Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1000 patients; Medicine, 82 (2003), pp. 299–308
- [22] R. Robeva, et al.; Decreased daily melatonin levels in women with systemic lupus erythematosus – a short report; Balk Med J, 30 (2013), pp. 273–276
- [23] P. Pévet, et al.; Melatonin in the multi‐oscillatory mammalian circadian world; Chrono- Int, 23 (2006), pp. 39–51
- [24] L. Palagini, et al.; Sleep disorders and systemic lupus erythematosus; Lupus, 23 (2014), pp. 115–123
- [25] B.H. Hahn; Lessons in lupus: the mighty mouse; Lupus, 10 (2001), pp. 589–593
- [26] L.E. Scheving, et al.; Circadian bioperiodic response of mice bearing advanced L1210 leukemia to combination therapy with adriamycin and cyclophosphamide; Cancer Res, 40 (1980), pp. 1511–1515
- [27] D.I. Daikh, D. Wofsy; Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide; J Immunol, 166 (2001), pp. 2913–2916
- [28] W. Nelson, et al.; Methods for cosinor-rhythmometry; Chronobiologia, 6 (1979), pp. 305–323
- [29] B.D. Devan, et al.; Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task; Neurobiol Learn Mem, 75 (2001), pp. 51–62
- [30] F.A. Holloway, R. Wansley; Multiphasic retention deficits at periodic intervals after passive-avoidance learning; Science, 180 (1973), pp. 208–210
- [31] R.R. Pagano, R.H. Lovely; Diurnal cycle and ACTH facilitation of shuttlebox avoidance; Physiol Behav, 8 (1972), pp. 721–723
- [32] W.B. Ghiselli, R.A. Patton; Diurnal variation in performance of free-operant avoidance behavior of rats; Psychol Rep, 38 (1976), pp. 83–90
- [33] S. Sephton, D. Spiegel; Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease?; Brain Behav Immun, 17 (2003), pp. 321–328
- [34] K.L. Adams, et al.; Environmental circadian disruption elevates the IL-6 response to lipopolysaccharide in blood; J Biol Rhythm, 28 (2013), pp. 272–277
- [35] D.J. Phillips, M.I. Savenkova, I.N. Karatsoreos; Environmental disruption of the circadian clock leads to altered sleep and immune responses in mouse; Brain Behav Immun (2012)
- [36] A.C. Buenafe, et al.; A telemetric study of physiologic changes in mice with induced autoimmune encephalomyelitis; Lab Anim, 37 (2008), pp. 361–368
- [37] A.C. Buenafe; Diurnal rhythms are altered in a mouse model of multiple sclerosis; J Neuroimmunol, 243 (2012), pp. 12–17
- [38] M.W. Hurd, M.R. Ralph; The significance of circadian organization for longevity in the golden hamster; J Biol Rhythm, 13 (1998), pp. 430–436
- [39] R.J. McDonald, et al.; Multiple effects of circadian dysfunction induced by photoperiod shifts: alterations in context memory and food metabolism in the same subjects; Physiol Behav, 118 (2013), pp. 14–24
Document information
Published on 20/10/16
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?