Summary
Background
Bariatric surgery (BS) is totally different from diabetes surgery (DS) in the patient characters, goals of surgery, and management although similar in surgical procedure. Comparison of BS and DS with long-term data is lacking.
Materials and methods
A retrospective review of patients who received BS and patients who received DS at Min-Sheng General Hospital from 2007 to 2013 was designed. All inpatient and outpatient follow-up data were analyzed. Patients undergoing BS for the treatment of morbid obesity were compared with patients undergoing metabolic surgery for the treatment of type 2 diabetes mellitus (T2DM). Patients who received revision surgeries were excluded. The main outcome measures were: (1) operation risk; (2) weight loss; and (3) diabetes remission.
Results
Between 2007 and 2013, 2073 patients who received BS and 741 patients who received DS were recruited from both centers. DS patients were older (41.1 ± 10.9 years vs. 33.1 ± 9.3 years, p < 0.05) and were more likely to be male (40.2% vs. 28.2%, p < 0.05) and to have diabetes (100% vs. 6.0%, p < 0.05), however, they had similar body mass index (BMI) (37.9 ± 8.0 vs. 38.5 ± 9.7, p = 0.78) compared to the BS patients. Surgical procedures are significantly different between the two groups (73.3% of the DS surgeries were gastric bypass procedure, whereas this procedure made up only 47.1% of BS surgeries). Although the major complication rates were similar (2.0% vs. 2.4%), the DS program had a significant higher mortality rate than the BS program (0.54% vs. 0.1%; p < 0.05). At the 5-year follow-up time point, 58.0% of the BS patients had achieved successful results (weight loss > 30%) and 80% of the DS patients had complete remission of their diabetes [hemoglobin A1c (HbA1c) < 6.0%]. Both the DS and the BS group had good results in up to 85% of the patients at the 5-year follow-up time point.
Conclusion
The clinical profiles were very different between the BS and the DS programs. Both programs achieved the desired outcomes equally well, however, the DS program had a higher risk than the BS program.
Keywords
Bariatric surgery;Diabetes surgery;Type 2 diabetes mellitus
1. Introduction
Obesity and type 2 diabetes mellitus (T2DM) are becoming epidemic diseases worldwide.1 ; 2 These two diseases are closely related and are both very difficult to treat.3 ; 4 Bariatric surgery (BS), aimed at weight reduction, has been proven to be a viable option for the treatment of severe obesity in comparison to conservative methods, resulting in long-lasting weight loss, improved quality-of-life, and the resolution of obesity-related comorbidities.5 Among all of the obesity-related comorbidities, BS has been proven especially successful in treating T2DM6; 7 ; 8 in morbidly obese patients [body mass index (BMI) > 35 kg/m2] as well as preventing the development of T2DM.9 Recently, gastrointestinal metabolic surgery has been proposed as a new treatment modality for T2DM in patients with BMI < 35 l g/m2.10 Several randomized trials have proven that metabolic surgery resulted in better glycemic control compared with medical treatment in T2DM patients with BMI < 35 kg/m2.11; 12; 13; 14; 15; 16 ; 17
We started our metabolic surgery program in 2007. An independent diabetes surgery (DS) center was set up in addition to the original BS center, because we realized that the patient characters, goals of surgery, and management are totally different. The two programs were different in their stated goals and were managed by different case coordinators, but offered similar procedures from the same surgeons. In this study, we sought to compare the characteristics of the patient populations, perioperative outcomes, and long-term effects of these two programs.
2. Materials and methods
2.1. Setting
We began performing BS in 1997 and began performing laparoscopic BS in 1998.18 We launched our BS center in 2004. This center hosted our BS program and a multidisciplinary team was involved. The program was managed by a coordinator (Tsou JJ), and the patients were followed by surgeons with the consultation of the multidisciplinary team if necessary. An independent DS center was established in 2007 when we realized that patients requiring T2DM treatment had totally different clinical profiles and goals of surgery compared with the bariatric patients. The clinical management of the diabetic surgical patients is also totally different from that of the bariatric patients. This center is led by an endocrinologist (Chong K) and managed by a coordinator (Chen SC). The diabetic patients were managed by a multidisciplinary team, and each patient was followed by an endocrinologist. The two programs were different in their stated goals and were managed by different case coordinators, but offered similar procedures from the same group of surgeons (Lee WJ, Ser KH, Chen RC). Between 2007 and 2013, the 2073 patients who received BS and the 741 who received the DS were recruited from both centers. Patients who received revision surgeries were excluded. The demographics, operative time, length of stay, weight loss, and the effects on comorbidities and mortality were evaluated.
The study was conducted in Min-Sheng General Hospital of the National Taiwan University and was approved by the human research review board at Min-Sheng General Hospital. All of the clinical data were prospectively collected and stored in a personal computer data base.
2.2. Surgery
The surgical team performed various types of surgical procedures and had broad experience with bariatric/metabolic surgeries. The performed surgical procedures included three types of gastric bypass procedures: laparoscopic Roux-en-Y gastric bypass (LRYGB), laparoscopic mini gastric bypass (LMGB), and single anastomosis duodenojejunal bypass with sleeve gastrectomy (SADJB-SG), which have been published previously.19 ; 20 In brief, using a standard five-port laparoscopic technique, LRYGB was performed by the antecolic and antegastric route with 100 cm of biliopancreatic limb and 100–200 cm of alimentary limb. The gastric pouch was approximately 20 mL and the gastrojejunostomy was created by a stapler technique with an anastomosis 1.2 cm diameter wide. LMGB was performed first by creating a long sleeved gastric tube approximately 2.0 cm wide along the lesser curvature from the antrum to the angle of His. Then, a Billroth II type loop gastroenterostomy was created with the intestine at 150–250 cm distal to the ligament of Trietz. SADJB-SG was performed first by creating a sleeve gastrectomy over a 36Fr bougie and leaving a 4 cm long antrum. Then, the duodenum was transected 4 cm distal to the pyloric ring and a loop duodenojejunostomy was created with the intestine at 150–250 cm distal to the ligament of Treitz. Other procedures included the laparoscopic adjustable gastric banding (LAGB) and the laparoscopic sleeve gastrectomy (LSG).21 ; 22 The type of operation performed is usually codecided by the patient themselves and the surgeon after several comprehensive seminars with the multidisciplinary team.
2.3. Patients and outcome measures
Patient follow up was scheduled at the 1st, 3rd, 6th, and 12th month for the 1st year and then annually. Body weight loss and a laboratory evaluation of nutritional parameters were recorded during every visit. The success of BS was defined as very successful (weight loss > 30%) and successful (weight loss > 20% but < 30%). The success of DS was defined as very successful [complete remission of T2DM, hemoglobin A1c (HbA1c) < 6%] and successful (partial remission of T2DM, HbA1c < 6.5%).23 Patients were also asked to grade their satisfaction with the surgery at the annual visits. The classification was divided into five grades ranging from excellent to poor.
All patients received a quality-of-life questionnaire evaluation at their preoperative assessments and postoperative follow up. Quality-of-life was measured by the Gastrointestinal Quality-of-Life Index (GIQLI), a 36-item questionnaire.24 In the analysis, the results of the questionnaire were divided into four domains: symptoms (19 items), physical status (7 items), psychological emotions (6 items), and social functioning (4 items). Each item was scored on a range from 0 to 4 (from the worst to the best option). The maximum score was 144. This questionnaire had been verified in previous studies19; 22; 25; 26; 27 ; 28 and the normal range was from 118 to 125.28
Perioperative complications were defined using the Clavien-Dindo classification for grading the severity of surgical complications.29 Grade I (minor deterioration from the normal postoperative course) and Grade II (complications can be treated by drugs, blood transfusion, physiotherapy, or nutritional support) are classified as minor complications. Grade III (complications require interventional or operative treatment), and Grade IV (complications are life-threatening complications with the need for intensive care unit management) are classified as major complications. Grade V is defined as mortality of the patient.
2.4. Statistical analysis
All statistical analyses were performed using SPSS version 12.01 (SPSS Inc., Chicago, IL, USA), with a baseline comparison made using Chi-square tests and two-sample t tests. Continuous variables were expressed as the mean (and standard deviation). The differences in pertinent characteristics were established using the t test for independent samples. A two-sided p value = 0.05 was considered statistically significant.
3. Results
3.1. Participants
Overall 2814 patients (2073 in the BS program and 741 in the DS program) were evaluated. As shown in Table 1, the DS patients were older (41.1 ± 10.9 years vs. 33.1 ± 9.3 years, p < 0.001) and were more likely to be male (40.2% vs. 26.2%, p < 0.001) and to have diabetes (100% vs. 6.0%, p < 0.001); however, they had BMI values similar to those of the BS patients (37.9 ± 8.0 vs. 38.5 ± 7.9, p = 0.78). The DS patients also had a significantly higher prevalence of hypertension.
| Bariatric (n = 2073) | Diabetes (n = 741) | P | |
|---|---|---|---|
| Age (y) | 33.1 ± 9.3 | 41.1 ± 10.9 | <0.001 |
| Sex (female) | 1529 (73.8) | 443 (59.8) | <0.001 |
| Body weight (kg) | 105.2 ± 26.3 | 103.9 ± 25.4 | 0.245 |
| Body height (cm) | 165.4 ± 31.9 | 165.2 ± 8.4 | 0.879 |
| BMI (kg/m2) | 38.5 ± 7.9 | 37.9 ± 8.0 | 0.780 |
| Waist circumference (cm) | 116.2 ± 44.2 | 115.7 ± 18.1 | 0.805 |
| Uric acid (mg/dL) | 6.9 ± 1.8 | 6.6 ± 1.9 | <0.001 |
| Hypertension | 1063 (51.3) | 450 (60.7) | < 0.001 |
| Dyslipidemia | 968 (46.7) | 357 (48.2) | 0.531 |
| AST (U/L) | 28.9 ± 31.5 | 37.2 ± 30.4 | <0.001 |
| Albumin (mg/dL) | 4.5 ± 1.9 | 4.4 ± 0.3 | 0.224 |
| WBC (103/uL) | 9.5 ± 10.3 | 8.4 ± 2.3 | <0.001 |
| Creatinine (mg/dL) | 0.7 ± 0.2 | 0.8 ± 0.3 | <0.001 |
| Patients with T2DM | 125 (6.0) | 741 (100) | <0.001 |
| Duration of T2DM (y) | 0.1 ± 0.3 | 3.9 ± 4.6 | <0.001 |
| HbA1c (%) | 5.8 ± 0.7 | 8.4 ± 2.9 | <0.001 |
| Glucose (mg/dL) | 94.0 ± 17.8 | 162.1 ± 68.9 | <0.001 |
| Insulin usage (case) | 5 (4.0) | 126 (17.0) | <0.001 |
Data are presented as mean ± standard deviation or n (%).
AST = aspartate aminotransferase; BMI = body mass index; HbA1c = hemoglobin A1c; T2DM = type 2 diabetes; WBC = white blood cell.
3.2. Surgical complications
Surgical procedures are significantly different between the two groups (73.3% of the DS patients received gastric bypass procedures whereas only 47.1% of the BS patients received bypass surgery). The surgical time was significantly longer for the DS patients (145.8 ± 40.6 minutes vs. 131.7 ± 39.1 minutes, p < 0.05). The DS patients also had a greater intraoperative blood loss and a longer mean hospital stay compared with the BS patients ( Table 2). The overall complication rate was higher in the DS program than the BS program (10.5% vs. 7.4%), but the major complication rate was similar between the two groups (2.4% vs. 2.0%, p = 0.07). Among 50 (2.4%) of the BS patients who had major complications; only two (0.1%) died, while the other 48 recovered without severe sequelae. One patient died of sleep apnea in the perioperative period after an unsuccessful resuscitation. The other patient died of multiorgan failure after a complication of internal bleeding, omentum infarction, and sepsis. Among the 15 (2.0%) DS patients with major complications, four (0.54%) died, and three (0.40%) had severe neurologic sequelae due to vascular complications. One case died of liver failure after prolonged treatment of a minor leakage after gastric bypass. Two cases died of multiorgan failure after delayed diagnosis of leakage after gastric bypass. One case died of multiorgan failure after delayed diagnosis of internal bleeding. Overall, the DS group had a significantly higher surgical mortality and complication with severe sequelae (0.94% vs. 0.1%; p < 0.01).
| Bariatric (n = 2073) | Diabetes (n = 741) | p | |
|---|---|---|---|
| Procedure | <0.001 | ||
| LRYGB | 472 (22.8) | 257 (34.7) | |
| LMGB | 489 (23.6) | 217 (29.3) | |
| LSG | 765 (36.9) | 137 (18.5) | |
| SADJB-SG | 14 (0.7) | 69 (9.3) | |
| LAGB | 128 (6.2) | 20 (2.7%) | |
| Mean operative time (min) | 131.7 ± 39.1 | 145.8 ± 40.6 | <0.001 |
| Intraoperative blood loss (mL) | 41.9 ± 44.8 | 54.0 ± 114.7 | <0.001 |
| Postoperative flatus passage (d) | 1.7 ± 0.7 | 1.8 ± 0.6 | 0.21 |
| Postoperative hospital stay (d) | 3.3 ± 1.7 | 4.2 ± 1.7 | <0.001 |
| Early postoperative complication | |||
| Minor | 105 (5.0) | 63 (8.5) | 0.001 |
| Major | 50 (2.4) | 15 (2.0) | 0.547 |
| Leakage | 31 (1.5) | 7 (1.0) | |
| Bowel obstruction | 3 (0.1) | 1 (0.1) | |
| Major bleeding | 8 (0.4) | 3 (0.4) | |
| Hepatic failure | 0 | 1 (0.1) | |
| Renal failure | 0 | 1 (0.1) | |
| Stricture of anastomosis | 1 (0.05) | 1 (0.1) | |
| Respiratory failure | 1 (0.05) | 0 | |
| Heart failure | 1 (0.05) | 0 | |
| Mortality | 2 (0.1) | 4 (0.54) | 0.017 |
Data are presented as mean ± standard deviation or n (%).
LAGB: laparoscopic adjustable gastric banding; LMGB: laparoscopic mini gastric bypass; LRYGB: laparoscopic Roux-en-Y gastric bypass; LSG: laparoscopic sleeve gastrectomy; SADJB-SG: single anastomosis duodenojejunal bypass-sleeve gastrectomy.
3.3. Success of surgery
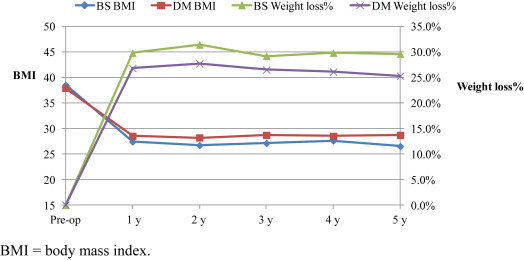
Most of the patients were regularly followed at outpatient clinics; some patients were referred to local hospitals or clinics for further follow-up due to the long travel distance. At follow-up, the BS patients had achieved superior weight loss and a lower mean BMI compared with the DS patients at the 5-year follow-up time point (Figure 1). At the 5-year follow-up time point, a significant higher portion of the BS patients had very successful weight loss compared with the DS patients (58% vs. 33.9%, p < 0.05).
|
|
|
Figure 1. Weight loss (%) after bariatric and diabetes surgery. BMI = body mass index. |
Complete remission of T2DM was achieved in 80% of the DS patients at the 5-year follow-up time point (Table 3). Another 9.2% of the DS patients achieved partial remission. The mean HbA1c level decreased from a preoperative value of 8.4 ± 2.9% to 6.2 ± 1.3 at the 5-year follow-up time point.
| Variable | BS (n = 450) | DS (n = 168) | p |
|---|---|---|---|
| BMI (kg/m2) | 26.5 ± 4.8 | 28.7 ± 6.3 | 0.055 |
| Excess body weight loss (%) | 77.1 ± 25.1 | 63.3 ± 19.0 | 0.050 |
| Total weight loss (%) | 29.6 ± 12.7 | 25.3 ± 10.3 | 0.030 |
| Great success (>30%) | 261 (58.0) | 57 (33.9) | 0.017 |
| Success (20–30%) | 123 (27.3) | 69 (41.1) | |
| Partial success (<20%) | 66 (14.7) | 42 (25) | |
| Remission of T2DM | |||
| Great success (HbA1c <6.0%) | – | 156 (80) | |
| Success (HbA1c 6.0–6.5%) | – | 18 (9.2) | |
| Partial success (HbA1c >6.5%) | – | 21 (10.8) |
Data are presented as mean ± standard deviation or n (%).
BMI = body mass index; HbA1c = hemoglobin A1c; T2DM = type 2 diabetes.
3.4. Quality-of-life
The preoperative GIQLI scores were similar in the two groups (Table 4). Five years after surgery, the mean GIQLI score was significantly higher than the preoperative score in both groups. Significantly higher subtotals were found in the general quality-of-life assessment, including in the domains of physical, social, and emotional function (Table 4). There was a significant decrease in the symptoms score after surgery in both groups due to the postoperative presence of gastrointestinal symptoms in both groups.
| GIQLI | Bariatric (preoperative)n = 1401 | Bariatric (postoperative 5 years)n = 470 | Diabetes (preoperative)n = 564 | Diabetes (postoperative 5 years)n = 86 |
|---|---|---|---|---|
| Overall | 108.4 ± 15.7 | 110.7 ± 16.5 * | 109.3 ± 18.5 | 116.0 ± 14.4 * |
| Symptoms | 64.5 ± 6.6 | 57.6 ± 9.2 * | 64.3 ± 8.5 | 59.6 ± 9.0 * |
| Physical | 16.6 ± 5.3 | 20.4 ± 5.0* | 17.2 ± 5.5 | 22.5 ± 3.5 * |
| Emotional | 12.1 ± 4.0 | 15.9 ± 2.6* | 12.8 ± 3.9 | 16.1 ± 3.1 * |
| Social | 15.2 ± 3.7 | 16.8 ± 2.8* | 15.0 ± 4.3 | 17.9 ± 2.2 |
- p < 0.05 compared with the preoperative data.
3.5. Patient satisfaction
At the 5-year follow-up time point, the patients satisfaction was very high in both groups (Table 5). Up to 88% of the patients had excellent or very good satisfaction with their surgery, however, more patients in the BS group felt excellent than in the DS group (53.2% vs. 29.4%, p < 0.05).
| Bariatric (n = 470) | Diabetes (n = 86) | p | |
|---|---|---|---|
| Excellent | 247 (53.2) | 26 (29.4) | 0.206 |
| Very good | 172 (36.2) | 50 (58.8) | |
| Good | 39 (8.5) | 6 (5.9) | |
| Fair | 12 (2.1) | 4 (5.9) | |
| Worse | 0 | 0 |
Data are presented as n (%).
4. Discussion
This study confirmed that BS can be performed as a metabolic surgery for the treatment of T2DM, and that BS achieved very good glycemic control up to 5 years with very high patient satisfaction. The result is consistent with the recently reported results of laparoscopic BS.30 ; 31 However, although the major complication rate was similar between the two groups, patients who received DS tended to develop more severe complications that resulted in higher mortality rates. This finding corroborated with a recent report from Inabnet et al31 that BS carried a higher risk in patients with metabolic syndrome compared with patients without metabolic syndrome. The reason for the more severe complication in the DS patients might be related to the compromised cardiovascular system and depressed immunity in patients with poorly controlled diabetes.32 ; 33
In this study, the patient characteristics are significantly different. Patients requesting diabetes treatment were significantly older and had more comorbidities than patients requesting BS. These differences are reasonable and in accordance with the previous report.34 Although the DS patients were sicker than the BS patients, more patients in the DS group received the more complicated gastric bypass procedures. This is because excluding the duodenum in the bypass procedure had been proven to be more effective in glycemic control than a procedure without duodenum exclusion, such as the LSG or the LAGB.5; 8; 12; 13 ; 17 Therefore, we only advised patients with high BMI, a short duration of T2DM, and good B-cell preservation to undergo an LSG procedure.35 By contrast, in patients without T2DM, LSG is becoming the leading type of BS. Recent studies have supported that LSG is a durable BS comparable to gastric bypass procedures.36 ; 37 The other advantages of LSG include the avoidance of gastric cancer risk in the excluded stomach and a reduction of the incidence of micronutrient deficiency. These factors result in the discordance of surgical procedures between the BS and DS groups.
In DS, a novel procedure, SADJB-SG, has been developed and is specifically designed for metabolic surgery.20 This operation avoids the risk of remnant gastric cancer arising from excluded stomach. By preserving the pylorus, this operation may decrease the incidence of dumping syndrome and a hypoglycemic episode. A single loop anastomosis also simplifies the operation and decreases the surgical risks in this group of vulnerable patients. Currently, approximately 30% of our diabetes surgeries use the SADJB-SG procedure. We are continually investigating the long-term efficacy and functional advantages of this novel procedure.
In this study, patients with diabetes do lose less weight than patients without diabetes. Starting with a similar BMI, the BS group achieved a higher weight loss and a lower BMI compared with the DS group 5 years after surgery. However, although weight loss is inferior in the DS group, the remission of diabetes remained high in the DS group, which highlights the role of weight loss in diabetes treatment and the difficulty of losing weight by medical treatment in this group of patients. The selection of the proper patients to receive DS is very important for the success of the DS. In our previous study, BMI, duration of diabetes, and C-peptide levels are three independent predictors for the success of DS.38 Together with age, an ABCD score had been proposed for patient selection for DS.39 In our diabetic program, we not only excluded patients with long-term disease and poor B-cell preservation, we also limited patient age to <65 years.
Although DS can be considered a treatment option for patients with poorly controlled diabetes despite the high remission rate and patients satisfaction rate, safety is still the first priority in DS. This group of patients is relatively vulnerable, and may be subject to possible vascular complications due to a long history of diabetes, especially in patients with poorly controlled disease. In this study, we performed a routine cardiac stress test for all of our patients. Some patients were found to have coronary artery diseases and received stent treatment before proceeding with the diabetes surgeries. We also intensively monitored our patients within the first 24 hours with a low threshold for laboratory and computer tomography study. Patients with poorly controlled diabetes or other metabolic disorders, such as extreme hyperlipidemia, were admitted 2 days before surgery and received a short-term intensive treatment to optimize their medical condition prior to surgery. In our experience, these policies reduce the risks and improve the safety of DS.
In conclusion, this study is the first to report the outcomes of DS compared with BS after 5 years of follow up. The results support the application of bariatric/metabolic surgery in diabetes treatment and highlight the importance of different strategies for managing these two groups of patients.
References
- 1 S. Wild, G. Roglic, A. Green, R. Sicree, H. King; Global prevalence of diabetes for the year 2000 and projections for 2030; Diabetes Care, 27 (2004), pp. 1047–1052
- 2 K.M. Flegal, M.D. Carroll, B.K. Kit, C.L. Ogden; Prevalence of obesity and trends in the distribution of body mass index among US adults. 1999–2010; JAMA, 307 (2012), pp. 491–497
- 3 P. Zimmer, K.G. Alberti, J. Shaw; Global and societal implications of the diabetes epidemic; JAMA, 414 (2013), pp. 782–787
- 4 Y. Xu, L. Wang, J. He, et al.; Prevalence and control of diabetes in Chinese adults; JAMA, 310 (2013), pp. 948–958
- 5 H. Buchwald, Y. Avidor, E. Braunwald, et al.; Bariatric surgery: a systematic review and meta-analysis; JAMA, 292 (2004), pp. 1724–1737
- 6 W.J. Pories, M.S. Swanson, K.G. MacDonald, et al.; Who would have thought it? An operation provides to be the most effective therapy for adult onset diabetes mellitus; Ann Surg, 222 (1995), pp. 339–352
- 7 L. Sjostrom, K. Narbro, D. Sjostrom, et al.; Effect of bariatric surgery on mortality in Swedish obese subjects; N Engl J Med, 357 (2007), pp. 741–752
- 8 S.A. Brethauer, A. Amino, H. Ramero-Talamas, et al.; Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus; Ann Surg, 258 (2013), pp. 628–637
- 9 L.M. Carrison, M. Peltonen, S. Ahlin, et al.; Bariatric surgery and prevention of type 2 diabetes in Sweden obese subjects; N Engl J Med, 367 (2012), pp. 2683–2693
- 10 J.B. Dixon, P. Zimmet, K.G. Alberti, F. Ribino; Bariatric surgery: an IDF statement for obese type 2 diabetes; Diabet Med, 28 (2011), pp. 628–642
- 11 J.B. Dixon, P.E. O'Brien, J. Playfair, et al.; Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial; JAMA, 299 (2008), pp. 316–323
- 12 W.J. Lee, K. Chong, K.H. Ser, et al.; Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial; Arch Surg, 146 (2011), pp. 143–148
- 13 P.R. Schauer, S.R. Kashyap, K. Wolski, et al.; Bariatric surgery versus intensive medical therapy in obese patients with diabetes; N Engl J Med, 366 (2012), pp. 1567–1576
- 14 S. Ikramuddin, J. Korner, W.J. Lee, et al.; Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: The diabetes surgery study randomized clinical trial; JAMA, 309 (2013), pp. 2240–2249
- 15 Z. Liang, Q. Wu, B. Chen, P. Yu, H. Zhao, X. Ouyang; Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial; Diabetes Res Clin Pract, 101 (2013), pp. 50–56
- 16 J. Wentworth, J. Playfair, C. Lavrie, et al.; Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomized controlled trial; Lancet Diabetes Endocrinol, 2 (2014), pp. 545–552
- 17 P.R. Schauer, D.L. Bhatt, J.P. Kirwan, et al.; Bariatric surgery versus intensive medical therapy for diabetes; N Engl J Med, 371 (2014), pp. 681–682
- 18 W.J. Lee, I.R. Lai, M.T. Huang, C.C. Wu, P.L. Wei; Laparoscopic versus open vertical banded gastroplasty for the treatment of morbid obesity; Surg Laparosc Endosc Percutan Tech, 11 (2001), pp. 9–13
- 19 W.J. Lee, P.J. Yu, W. Wang, T.C. Chen, P.L. Wei, M.T. Huang; Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial; Ann Surg, 242 (2005), pp. 20–28
- 20 W.J. Lee, K.T. Lee, K. Kasama, et al.; Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): short-term result and comparison with gastric bypass; Obes Surg, 24 (2014), pp. 109–113
- 21 W.J. Lee, W. Wang, P.L. Wei, M.T. Huang; Gastrointestinal quality of life following laparoscopic adjustable gastric banding in Asia; Obes Surg, 16 (2006), pp. 586–591
- 22 K.H. Ser, W.J. Lee, Y.C. Lee, J.C. Chen, Y.H. Su, S.C. Chen; Experience in laparoscopic sleeve gastrectomy for morbidly obese Taiwanese: staple-line reinforcement is important for preventing leakage; Surg Endosc, 24 (2010), pp. 2253–2259
- 23 J.B. Buse, S. Laughlin, S. Caprio, et al.; How do we define cure of diabetes?; Diabetes Care, 32 (2009), pp. 2133–2135
- 24 E. Espasch, J.L. Williams, S. Wood-Dauphinee; Gastrointestinal quality of life index: development validation and application of new instrument; Br J Surg, 82 (1995), pp. 216–222
- 25 K. Slim, J. Bousquet, F. Kwiatkowski; Quality of life before and after laparoscopic fundoplication; Am J Surg, 180 (2001), pp. 41–45
- 26 G. Decker, F. Borie, D. Bouamrirene; Gastrointestinal quality of life before and after laparoscopic Heller myotomy with partial posterior fundoplication; Ann Surg, 236 (2002), pp. 750–758
- 27 R. Peterli, B. Wolnerhanssen, T. Peters, et al.; Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial; Ann Surg, 250 (2009), pp. 234–241
- 28 S.E. Overs, R.A. Freeman, N. Zarshenas, K.L. Walton, J.O. Jorgensen; Food tolerance and gastrointestinal quality of life following three bariatric procedures: adjustable gastric banding, Roux-en-Y gastric bypass, and sleeve gastrectomy; Obes Surg, 22 (2012), pp. 536–543
- 29 D. Dindo, N. Demartines, P.A. Clavien; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey; Ann Surg, 240 (2004), pp. 205–213
- 30 The longitudinal assessment of bariatric surgery (LABS) Consortium; Perioperative safety in the longitudinal assessment of bariatric surgery; NEJM, 361 (2009), pp. 445–454
- 31 W. Inabnet, D.A. Winegar, B. Sherif, M.G. Sarr; Early outcomes of bariatric surgery in patients with metabolic syndrome: an analysis of the bariatric outcomes longitudinal data base; J Am Coll Surg, 214 (2012), pp. 550–557
- 32 A.S. Dronge, M.F. Perkal, S. Kancir, J. Concato, M. Aslan, R.A. Rosenthal; Long-term glycemic control and postoperative infectious complications; Arch Surg, 141 (2006), pp. 375–380
- 33 C. Umpierrez, D. Smiley, S. Jacobs, et al.; Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 Surgery); Diabetes Care, 34 (2011), pp. 256–261
- 34 F. Rubino, A. Shukla, A. Pomp, M. Moreira, S.M. Ahn, G. Dakin; Bariatric, metabolic, and diabetes surgery: whats in a name; Ann Surg, 259 (2014), pp. 117–122
- 35 W.J. Lee, K.H. Ser, K. Chong, et al.; Laparoscopic sleeve gastrectomy for diabetes treatment in non-morbidly obese patients: efficacy and change of insulin secretion; Surgery, 147 (2010), pp. 664–669
- 36 R. Peterli, Y. Borbély, B. Kern, et al.; Early results of the Swiss multicentre bypass or sleeve study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass; Ann Surg, 258 (2013), pp. 690–695
- 37 S. Trastulli, J. Disiderio, S. Guarino, et al.; Laparoscopic sleeve gastrectomy compared with other bariatric surgical procedures: a systematic review of randomized trials; Surg Obes Relat Dis, 9 (2013), pp. 816–829
- 38 J. Dixon, L.M. Chung, K. Chong, et al.; Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes; Diabetes Care, 36 (2013), pp. 20–26
- 39 W.J. Lee, K. Hur, M. Lakadawala, et al.; Predicting the success of metabolic surgery: age, body mass index, C-peptide, and duration score; Surg Obes Relat Dis, 9 (2013), pp. 379–384
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?