Summary
Introduction
The aim of this study was to evaluate the short- and long-term outcomes in patients who underwent open infrarenal aortic aneurysm repair.
Methods
Consecutive patients who underwent open repair of infrarenal aortic aneurysms at our institution from July 1st 1990 to June 30th 2012 were reviewed from a prospective collected departmental database. Short-term outcomes included 30-day mortality and peri-operative complications. Independent risk factors to predict 30-day mortality were identified. Long-term survival and secondary interventions were also reported.
Results
Three hundred and eighty-three patients (317 males, median age 72 years with a range of 15–90 years) underwent open infrarenal aortic aneurysm repair during the period, of whom 266 (69.5%) were elective, 18 (4.7%) were urgent for symptomatic but nonruptured cases, and 99 (25.8%) were emergency procedures for ruptured aneurysms. Mean aneurysm size was 6.5 cm (ranging from 2.5 cm to15 cm). All patients were followed up for at least 24 months with a mean follow up period 163 months. Overall 30-day mortality was 11.0% (36.4% for ruptured cases, 11.1% for symptomatic cases, and 1.5% for elective cases; p < 0.001). Preexisting renal disease and ruptured aneurysms were independent risk factors for 30-day mortality (p = 0.001 and p = 0.006 respectively). Systemic complications included 50 cardiac events, 52 respiratory events, six renal events, three cerebral vascular accidents, and one deep vein thrombosis/pulmonary embolism. Local complications included two anastomotic/graft hemorrhage, 10 distal thrombosis/embolisms, five bowel ischemias, one spinal cord ischemia, and 17 wound complications. The ruptured group presented survival rates of 53.5%, 50.5%, 47.5%, 42.3%, 38.0%, 21.9%, and 12.5% at 1 year, 2 years, 3 years, 4 years, 5 years, 10 years, and 15 years, respectively; while nonruptured survival rates were 91.5%, 88.0%, 83.7%, 78.3%, 73.0%, 43.0%, and 25.3%, respectively (log rank p < 0.001). For those who died 30 days after the operation, only six patients (1.8%) died from aneurysm related mortality. A total of three (0.9%) patients underwent late re-interventions, one for late aorto-enteric fistulae and two for anastomotic pseudoaneurysms.
Conclusion
In the current era of endovascular repair, open infrarenal aneurysm repair is effective and durable, and has very low secondary interventions rates.
Keywords
abdominal aortic aneurysm;abdominal aortic aneurysm repair;long term survival;reintervention
1. Introduction
Ever since Nicolai Volodos in 19861 and Juan Carlos Parodi in 19902 demonstrated the feasibility of aortic aneurysm exclusion with endografts, endovascular stent graft repair (EVAR) became the standard of care for infrarenal abdominal aortic aneurysms (AAA) at the end of past century. There was a resurge of interest in open repair when long-term results from the EVAR1 trial,3 DREAM trial,4 OVER trial,5 and ACE6 trial were published recently. They all showed that the early mortality advantage of EVAR was lost at around 2 years of follow up, and re-intervention, long term complications, and cost were significantly higher in the EVAR group compared to the open repair group. This observation was reconfirmed using meta-analysis.7
We present the long-term outcomes of patients who underwent open infrarenal AAA repair in our department. This is the first paper reporting the long-term durability of open repairs in the Han Chinese population. Patients and clinicians can now be informed about the relative merits of open treatment versus endovascular treatment.
2. Methods
We reviewed all patients who received open repair of infrarenal aortic aneurysm at our institution, a tertiary referral center in Hong Kong for the period of July 1st 1990 to June 30th 2012. Data were subtracted from a prospective collected departmental database, supplemented by clinical notes and computer records. Only infrarenal aneurysms were included; while pararenal, suprarenal, and thoracoabdominal aneurysms were excluded.
Patients presented with ruptures, either suspected clinically or diagnosed using imaging. These patients were treated as surgical emergencies. Midline laparotomy and infrarenal clamps were the preferred approaches. Often a supraceliac clamp may be needed temporarily. Asymptomatic patients were offered repair when the aneurysm size reached 5.0 cm. Symptomatic patients included those with pain, infection, embolic phenomenon, and an aneurysm expansion rate >0.5 cm/6 mo. They were treated in an early elective basis. Incision was rooftop or midline with an infrarenal clamp. Tube or bifurcated grafts of woven Dacron or knitted Dacron were used.
Patients were nursed in intensive care units after their operations. After discharge, patients were regularly followed up in the outpatient clinic. Surveillance duplex or computer tomography (CT) scans were not routine.
During the same period of time, our center also performed endovascular stenting but these patients were not reported in the current study.
Patients' baseline characteristics and follow-up periods were reported. Short-term outcomes included peri-operative morbidities and mortalities. Variables including age, sex, comorbidities, preoperative hemoglobin level, preoperative base excess, whether the aneurysm had ruptured or not, and American Society of Anesthesiologists (ASA) grades were used to predict the 30-day mortality using a binary logistic regression model. Long-term survival and secondary interventions were reported. Chi-square test was used to differentiate significant differences between categorical variables, while Student t test was used for continuous variables. Statistical analysis was calculated by SPSS version 22 (SPSS Inc., Chicago, IL, USA). A p value <0.05 was defined as significant.
Definitions of comorbidities were as follows. Cardiac history included stable angina, unstable angina, myocardial infarction, congestive heart failure, and arrhythmia. Pulmonary history included all patients with dyspnea or chest roenterogramic changes. Renal impairment was defined as serum creatinine level >120 μmol/L. Positive smoking history included all patients who had quit <10 years.
Thirty-day mortality was defined as death ≤30 days after index open repair. Late aneurysm-related mortality was defined as death >30 days after index operation and as a direct result of aneurysm rupture. Late re-intervention was defined as further intervention >30 days after the index operation. Graft related events were defined as those occurring as a direct consequence of prosthetic aortic replacement and consisted of graft thrombosis, pseudoaneurysm formation, graft infection, and graft-enteric fistula. Other aneurysms remote from the index operation, e.g., thoracic or iliac aneurysm, requiring further intervention were not counted and were reported separately.
3. Results
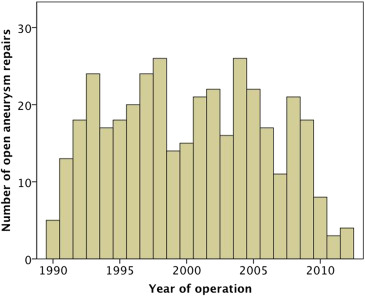
Three hundred and eighty-three patients underwent open infrarenal aortic aneurysm repair during the study period (Fig. 1). Three hundred and seventeen (82.8%) were males while 66 (17.2%) were female. The median age was 72 years ranging from 15 years to 90 years, of whom 266 (69.5%) were elective, 18 (4.7%) were urgent for symptomatic but nonruptured cases, and 99 (25.8%) were emergency procedures for ruptured aneurysms. Mean aneurysm size was 6.5 cm ranging from 2.5 cm to 15.0 cm. Other baseline characteristics are shown in Table 1. All patients were followed up for at least 24 months, with a mean follow up period 163 months.
|
|
|
Fig. 1. Number of open infrarenal abdominal aortic aneurysm repairs over the years. |
| Variable | No. | % |
|---|---|---|
| Age | Median age 72 y (range, 15–90 y) | |
| ≥70 y | 230 | 60.1 |
| <70 y | 153 | 39.9 |
| Gender | ||
| Male | 317 | 82.8 |
| Female | 66 | 17.2 |
| Presentation | ||
| Ruptured | 99 | 25.8 |
| Symptomatic | 18 | 4.7 |
| Elective | 266 | 69.5 |
| Coexisting condition | ||
| Smoking | 226 | 59.0 |
| Hypertension | 213 | 55.6 |
| Diabetes mellitus | 39 | 10.2 |
| Cardiac disease | 129 | 33.7 |
| Cerebral vascular accident | 44 | 11.5 |
| Pulmonary disease | 49 | 12.8 |
| Renal impairment | 89 | 23.2 |
| Family history of aneurysm | 3 | 0.8 |
| Aneurysm size | Median size 6.5 cm (range, 2.5–15.0 cm) | |
| <5 cm | 21 | 5.5 |
| 5–7 cm | 251 | 65.5 |
| >7 cm | 111 | 29.0 |
3.1. Short-term results
Overall, 30-day mortality was 11.0% (36.4% for ruptured cases, 11.1% for symptomatic cases, and 1.5% for elective cases; p < 0.001). Univariate logistic regression analysis showed age, smoking history, hypertension, pre-existing renal disease, preoperative hemoglobin level, preoperative base excess, ruptured aneurysm, ASA grades 3–5, and aneurysm size were predictors of 30-day mortality ( Table 2) With subsequent multivariate logistic regression, only pre-existing renal disease and ruptured aneurysms were found to be independent significant risk factors for 30-day mortality (p = 0.001 and p = 0.006, respectively; Table 3).
| Variable | Odd ratio | 95% Confidence interval | p | |
|---|---|---|---|---|
| Male | 1.046 | 0.443 | 2.469 | 0.918 |
| Age | 1.055 | 1.012 | 1.100 | 0.013 |
| Smoking | 0.269 | 0.135 | 0.537 | <0.001 |
| Hypertension | 0.401 | 0.206 | 0.782 | 0.007 |
| DM | 0.411 | 0.095 | 1.770 | 0.233 |
| Cardiac disease | 1.390 | 0.721 | 2.680 | 0.325 |
| CVA | 0.792 | 0.269 | 2.337 | 0.673 |
| Pulmonary disease | 2.052 | 0.915 | 4.602 | 0.081 |
| Renal disease | 2.541 | 1.302 | 4.959 | 0.006 |
| Preoperative hemoglobin level | 0.669 | 0.578 | 0.773 | <0.001 |
| Preoperative base excess | 0.883 | 0.832 | 0.936 | <0.001 |
| AAA size | 1.314 | 1.097 | 1.573 | 0.003 |
| Ruptured | 26.476 | 10.695 | 65.546 | <0.001 |
| ASA 3–5 | 5.807 | 1.747 | 19.303 | 0.004 |
AAA = abdominal aortic aneurysm; ASA = American Society of Anaesthesiologist; CVA = cerebrovascular accident; DM = diabetes mellitus.
a. By binary logistic regression. Age, preoperative hemoglobin level, preoperative base excess, and AAA size are continuous variables, while others are categorical variables.
| Variable | Odd ratio | 95% Confidence interval | p | |
|---|---|---|---|---|
| Age | 1.037 | 0.960 | 1.119 | 0.358 |
| Smoking | 1.005 | 0.322 | 3.138 | 0.994 |
| Hypertension | 0.385 | 0.106 | 1.389 | 0.145 |
| Renal disease | 8.434 | 2.349 | 30.284 | 0.001 |
| Preoperative hemoglobin level | 0.867 | 0.668 | 1.124 | 0.280 |
| Preoperative base excess | 0.952 | 0.889 | 1.019 | 0.156 |
| AAA size | 1.193 | 0.909 | 1.566 | 0.203 |
| Ruptured | 7.803 | 1.778 | 34.233 | 0.006 |
| ASA 3–5 | 2.266 | 0.223 | 23.032 | 0.489 |
AAA = abdominal aortic aneurysm; ASA = American Society of Anesthesiologists score.
a. By binary logistic regression. Age, preoperative hemoglobin level, preoperative base excess, and AAA size are continuous variables, while others are categorical variables.
Compared with nonruptured aneurysms, ruptured aneurysms led to significant longer aortic clamp time, prolonged operative duration, larger amount of blood loss, and increased postoperative ventilator assisted time (Table 4). Systemic complications included 50 cardiac events, 52 respiratory events, six renal events, three cerebral vascular accidents, and one deep vein thrombosis/pulmonary embolism. Local complications included two anastomotic/graft hemorrhage, 10 distal thrombosis/embolism, five bowel ischemias, one spinal cord ischemia, and 17 wound complications. Compared with nonruptured aneurysms, ruptured aneurysms led to significantly more adverse events in cardiac, respiratory, renal, and bowel ischemia complication subgroups (Table 4).
| Overall (n = 383) | Ruptured (n = 99) | Nonruptured (n = 284) | p | |
|---|---|---|---|---|
| Mean aortic clamp time (min) | 62.4 | 72.8 | 59.5 | <0.001a |
| Mean operative time (min) | 202.5 | 180.3 | 209.7 | 0.001a |
| Blood loss (mL) | 1748 | 3858 | 1055 | <0.001a |
| Mean days of assisted ventilation (d) | 2.2 | 7.6 | 0.5 | <0.001a |
| 30 d mortality | 42 (11.0) | 36 (36.4) | 6 (2.1) | <0.001b |
| Systemic complications | ||||
| Cardiac | 50 (13.0) | 21 (21.2) | 29 (10.2) | 0.009b |
| Respiratory | 52 (13.6) | 32 (32.3) | 20 (7.0) | <0.001b |
| Renal | 6 (1.6) | 5 (5.1) | 1 (0.4) | 0.005b |
| CVA | 3 (0.8) | 1 (1.0) | 2 (0.7) | 1.000b |
| DVT/PE | 1 (0.3) | 1 (1.0) | 0 (0) | 0.258b |
| Local complications | ||||
| Graft complication | 2 (0.5) | 0 (0) | 2 (0.7) | 1.000b |
| Distal thrombosis/embolism | 10 (2.6) | 5 (5.1) | 5 (1.8) | 0.134b |
| Bowel ischemia | 5 (1.3) | 4 (4.0) | 1 (0.4) | 0.017b |
| Spinal ischemia | 1 (0.3) | 1 (1.0) | 0 (0) | 0.258b |
| Wound complication | 17 (4.4) | 7 (7.1) | 10 (3.5) | 0.158b |
Data are presented as n or n (%).
CVA = cerebrovascular accident; DVT = deep vein thrombosis; PE = pulmonary embolism.
a. Student t test.
b. Chi-square test.
3.2. Long-term results
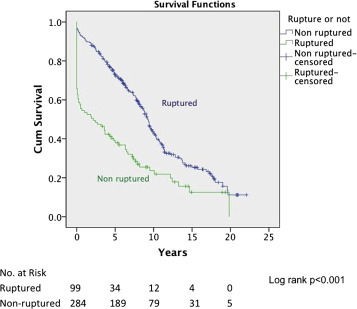
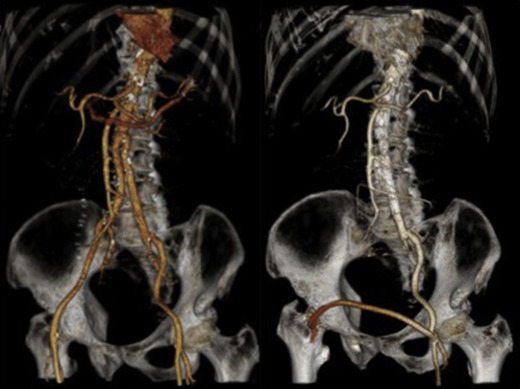
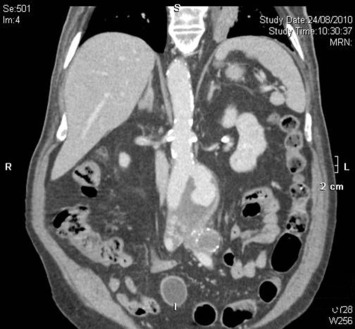
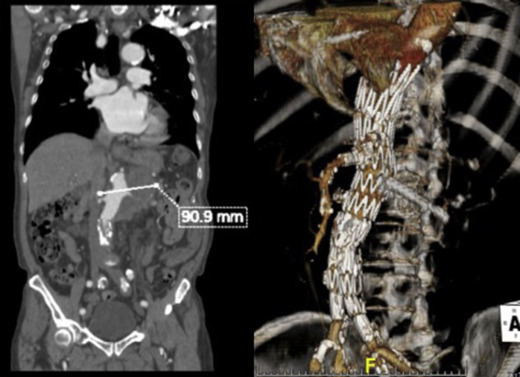
The ruptured group presented survival rates of 53.5%, 50.5%, 47.5%, 42.3%, 38.0%, 21.9%, and 12.5% at 1 year, 2 years, 3 years, 4 years, 5 years, 10 years, and 15 years, respectively; while the nonruptured group had survival rates of 91.5%, 88.0%, 83.7%, 78.3%, 73.0%, 43.0%, and 25.3% (log rank p < 0.001; Fig. 2). For those who died 30 days after their operation, only six patients died from aneurysm related causes, giving a late aneurysm-related mortality of 1.8% (Table 5). There were a total of three (0.9%) patients who underwent late re-interventions due to graft related events. The first patient underwent open repair in 1994 at the age of 66 years. The 4.4 cm infrarenal aortic aneurysm and 5.7 cm left iliac aneurysm were replaced with aorto-bi-iliac knitted Dacron graft. He presented with recurrent gastrointestinal bleeding of unknown origin 6 years later. Upper endoscopy and colonoscopy failed to localize the bleeding source. Laparotomy and enteroscopy showed suspicious bleeding from the proximal jejunum and possible graft-to-jejunum fistula. Bleeding was settled with aorto-uni-iliac endovascular stenting and cross femoral bypass. The patient is still alive to date (Fig. 3). The second patient underwent open repair with aorto-bi-femoral woven Dacron graft in 1993 at the age of 72 years. Seven years later, he was incidentally found to have a pulsatile mass at the epigastrium with a CT scan showing an infrarenal 8 cm proximal anastomosis pseudoaneurysm. Treatment was standard endovascular repair (Fig. 4). The last patient had open repair surgery with aorto-bi-iliac woven Dacron graft in 1993 at the age of 76 years. He complained of epigastric pain 20 years later with a CT scan showing a 9 cm proximal perivisceral pseudoaneurysm, which was settled with endovascular stenting with fenestration to all four visceral vessels (Fig. 5; Table 6). In addition, 17 (5.0%) patients developed thoracic or iliac aneurysms in later life. They were treated with open or endovascular repair (Table 7).
|
|
|
Fig. 2. Survival curve of ruptured versus nonruptured (log rank p < 0.001). |
| Causes | No. (n = 341) | |
|---|---|---|
| Cardiovascular related | 41 | |
| Noncardiovascular related | 108 | |
| Ruptured aneurysm/complications | 6 | 1 type A dissection with hemopericardium 1 ruptured arch aneurysm 3 ruptured thoracic aneurysm 1 suspected perigraft infection |
| Unknown | 71 | |
| Still alive | 115 |
|
|
|
Fig. 3. Late intervention, Patient 1. |
|
|
|
Fig. 4. Late intervention Patient 2. |
|
|
|
Fig. 5. Late intervention, Patient 3. |
| Causes | No. (n = 341) | Treatments |
|---|---|---|
| Aorto-enteric fistula | 1 | EVAR |
| Proximal anastomotic pseudoaneurysm | 2 | EVAR |
| Types of aneurysm | No. (n = 341) | Treatments |
|---|---|---|
| Thoracoabdominal aneurysm | 10 | TEVAR |
| 1 | Open | |
| Iliac aneurysm | 5 | EVAR |
| 1 | Open |
(T)EVAR = (Thoracic) endovascular aortic repair.
4. Discussion
Our indications for surgical management of infrarenal abdominal aortic aneurysm were similar to well established standards.8; 9; 10; 11; 12 ; 13 One-fourth of open repairs were for ruptured cases with 36.4% 30-day mortality. The mortality rate for open elective infrarenal aortic aneurysm repair was 1.5%. Symptomatic aneurysms only account for a small proportion.
World reported perioperative mortality and morbidity varied greatly. A meta-analysis of the reported literature showed 30-day or in-hospital mortality from ruptured aneurysm ranging from 27% to 69%.14 As for elective cases, 30-day or in-hospital mortality ranged from 1.4% to 6.5%. Overall 30-day mortality of the open arm of EVAR1,3 DREAM,4 OVER,5 and ACE6 trials was 3.69% (range, 0.6–4.6%). While certain high volume centers reported excellent mortality rates of 0% to 1.2%,15; 16 ; 17 multi-institutional nationwide data continued to show higher incidences of mortality ranging from 4.2% to 8.4%.16 ; 17 There were strong relationships between operative outcomes and the case volume of the surgeon or hospital. Birkmeyer et al18 ; 19 reported an elective AAA repair mortality rate of 7.8% in low volume centers (<17 cases/y), as opposed to 4.9% in high volume centers (>79 cases/y).
Despite advancements in operative, anesthetic, and intensive care, perioperative mortality of ruptured aneurysms was still extremely high. Several meta-analyses are available in literature. Hoornweg et al20 reported an overall mortality of 48.5%. Bown et al21 reported the rate as 48%. Kantonen et al22 reported the rate as 68%. This high mortality rate is most likely related to factors such as delays in recognition and intervention, hemodynamic instability, profound blood loss, and suboptimal perioperative care in emergency setting.21 ; 22 The relatively low rates in our institution were probably related to short travel times to the hospital, availability of dedicated vascular surgeons, and outstanding anesthetic and intensive care.
Several factors have been identified as predictive factors for operative mortality. These included advanced age, women, elevated body mass index (BMI), ruptured aneurysm, large aneurysm size, smoking, renal impairment, chronic obstructive pulmonary disease, coronary artery disease, prolonged aortic clamp time, greater blood loss, and positive fluid balance.23; 24; 25; 26; 27; 28; 29; 30; 31; 32; 33 ; 34 Furthermore, a few risk scoring systems have been developed to identify high risk patients undergoing open AAA repair. The Glasgow Aneurysm score (GAS),35 Vascular Physiology only-Physiological and Operative Severity Score for enUmeration of Mortality [V(p)-POSSUM],36 Vascular Biochemical and Hematological Outcome Model (VBHOM),37 Lees Revised Cardiac Risk Index (RCRI),38 and Preoperative Risk Score of the Estimation of Physiological Ability and Surgical Stress Score (PRS of E-PASS)39 were the best known predictors. Bryce et al40 and Tang et al41 showed that V(p)-POSSUM and E-PASS better predict outcome than the other two. However, due to the size of our patient cohort, our study only showed that pre-existing renal disease and ruptured aneurysms were associated with increased mortality in elective surgery and in emergency open repair. The most commonly reported risk factors of early mortality, such as advanced age and female gender, were insignificant in this study.
The merits of open repair compared with endovascular repair were lower late aneurysm-related mortality and lower late re-intervention rates. Both EVAR13 and DREAM4 trials reported their long-term survival after a mean follow up of 6 years and 6.4 years respectively. Late aneurysm related mortality after open repair was 2.2% and 0.6% in EVAR1 and DREAM respectively. Late re-intervention rate was 1.7% per year in the EVAR1 trial, and 18.1% in the DREAM trial. Both studies demonstrated significant lower re-intervention rates after open repair compared with those who underwent endovascular repair (5.1%/y and 29.6%/y in the EVAR1 trial and DREAM trial respectively). The Mayo clinic reported re-intervention rates for late aortic graft-related events of 5.1–5.3% at a median follow up of 5.8–7 years, with an associated operative mortality rate of 17–19%.42 ; 43 Biancari et al44 from Finland reported a re-intervention rate of 10.6% at the median follow up of 8 years, with an associated operative mortality rate of 9%. Adam et al45 from Australia reported a re-intervention rate of 3.4% at the median follow up of 41 months in nonruptured aneurysms and a rate of 5% at the median follow up of 30 months in ruptured cases. Our data of 1.8% late aneurysm-related mortality and 0.9% late re-intervention rate demonstrated that open AAA repair has excellent long-term durability in our population, and furthermore, the results compare favorably with previous reports from North America, Europe, and Australia (Table 8).
| Late aneurysm related mortality (%) | Late reintervention rate (%) | Mean follow up period | |
|---|---|---|---|
| EVAR 1 | 2.2 | 1.7%/y | 6 y |
| DREAM | 0.6 | 18.1 | 6.4 y |
| Mayo clinic | 5.1–5.3 | 5.8–7 y | |
| Biancari et al44 (Finland) | 10.6 | 8 y | |
| Adam et al45 (Australia) | 3.4 (nonruptured) 5 (ruptured) | 41 mo 30 mo | |
| Our data | 1.8 | 0.9 | 13.6 y |
Graft occlusion and anastomotic pseudoaneurysm were the more frequent graft-related indications for re-intervention, while graft infection and graft-enteric fistula occurred infrequently.42; 43; 44; 45; 46 ; 47 All re-interventions in our study were for symptomatic complications, while it may be difficult to identify asymptomatic complications as imaging follow ups after open repair were not routine in our center. CT scans were included in the follow up protocol for patient randomized to open repair in EVAR1 trial,48 and it is anticipated that further reports from the trial will determine the true incidence of late graft-related events.
There are several limitations in this study, as this study had a relatively small case number and incomplete long-term data collection. Completeness of patient follow up was an inherited limitation in our retrospective study. Being a tertiary referral center, every patient with symptomatic late graft related events would be referred back to us for management. The late re-intervention rate should therefore be accurate. However, the causes of death of a vast number of patients were unknown despite every effort to determine the cause of death, adding uncertainty to the late aneurysm-related mortality. Another important limitation of this study was the lack of comparison with the endovascular group. Our center began endovascular treatment for AAA in 1999. We were eagerly waiting the long-term outcomes of endovascular AAA repair. In the current era of endovascular repair, open infrarenal aneurysm repair is safe and effective, and still has its place in cases with difficult necks and access. As our study spanned from 1990 to 2012, there is always an issue with different emerging techniques over time, but nonetheless it represents one of the largest databases of Chinese patients receiving open AAA management.
Acknowledgments
This study received no financial support.
References
- 1 N.L. Volodos, I.P. Karpovich, V.I. Troyan, et al.; Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction; Vasa Suppl, 33 (1991), pp. 93–95
- 2 J.C. Parodi, J.C. Palmaz, H.D. Barone; Transfemoral intraluminal graft implantation for abdominal aortic aneurysms; Ann Vasc Surg, 5 (1991), pp. 491–499
- 3 The United Kingdom EVAR Trial Investigators; Endovascular versus open repair of abdominal aortic aneurysm; N Engl J Me, 362 (2010), pp. 1863–1871
- 4 J.L. De Bruin, A.F. Baas, J. Buth, et al.; Long-term outcome of open or endovascular repair of abdominal aortic aneurysm; N Engl J Med, 362 (2010), pp. 1881–1889
- 5 F.A. Lederle, J.A. Frieschlag, T.C. Kyriakides, et al.; Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial; JAMA, 302 (2009), pp. 1535–1542
- 6 J.P. Becquemin; The ACE trial: a randomized comparison of open versus endovascular repair in good risk patients with abdominal aortic aneurysm; J Vasc Surg, 50 (2009), pp. 222–2224 discussion 224
- 7 G. Dangas, D. O'Connor, B. Firwana, et al.; Open versus endovascular stent graft repair of abdominal aortic aneurysms: a meta-analysis of randomized trials; JACC Cardiovasc Interv, 5 (2012), pp. 1071–1080
- 8 Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants; Lancet, 352 (1998), pp. 1649–1655
- 9 F.A. Lederle, S.E. Wilson, J.R. Johnson, et al.; Immediate repair compared with surveillance of small abdominal aortic aneurysms; N Engl J Med, 346 (2002), pp. 1437–1444
- 10 P. Cao, P. De Rango, F. Verzini, et al.; Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial; Eur J Vasc Endovasc Surg, 41 (2011), pp. 13–25
- 11 K. Ouriel; The PIVOTAL study: a randomized comparison of endovascular repair versus surveillance in patients with smaller abdominal aortic aneurysms; J Vasc Surg (2009) 49266–2669
- 12 J.L. Eliason, W.D. Clouse; Current management of infrarenal abdominal aortic aneurysms; Surg Clin North Am, 87 (2007), pp. 1017–1033
- 13 E.L. Chaikof, D.C. Brewszter, R.L. Dalman, et al.; The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines; J Vasc Surg, 50 (2009), pp. S2–49
- 14 A. Hallin, D. Bergqvist, L. Holmberg; Literature review of surgical management of abdominal aortic aneurysm; Eur J Vasc Endovasc Surg, 22 (2001), pp. 197–204
- 15 N.R. Hertzer, E.J. Mascha, M.T. Karafa, P.J. O'Hara, L.P. Krajewski, E.G. Beven; Open infrarenal abdominal aortic aneurysm repair: the Cleveland Clinic experience from 1989 to 1998; J Vasc Surg, 35 (2002), pp. 1145–1154
- 16 P.F. Lawrence, C. Gazak, L. Bhirangi, et al.; The epidemiology of surgically repaired aneurysms in the United States; J Vasc Surg, 30 (1999), pp. 632–640
- 17 J.B. Dimick, J.A. Cowan Jr., J.C. Stanley, P.K. Henke, P.J. Pronovost, G.R. Upchurch Jr.; Surgeon specialty and provider volumes are related to outcome of intact abdominal aortic aneurysm repair in the United States; J Vasc Surg, 38 (2003), pp. 739–744
- 18 J.D. Birkmeyer, A.E. Siewers, E.V.A. Finlayson, et al.; Hospital volume and surgical mortality in the United States; N Engl J Med, 346 (2002), pp. 1128–1137
- 19 J.D. Birkmeyer, T.A. Stukel, A.E. Siewers, et al.; Surgeon volume and operative mortality in the United States; N Engl J Med, 349 (2003), pp. 2117–2127
- 20 L.L. Hoornweg, M.N. Storm-versloot, D.T. Ubbink, M.J. Koelemay, D.A. Legemate, R. Balm; Meta analysis on mortality of ruptured abdominal aortic aneurysms; Eur J Vasc Endovasc Surg, 35 (2008), pp. 558–570
- 21 M.J. Bown, A.J. Sutton, P.R. Bell, R.D. Sayers; A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair; Br J Surg, 89 (2002), pp. 714–730
- 22 I. Kantonen, M. Lepäntalo, M. Brommels, M. Luther, J.P. Salenius, K. Ylönen; Mortality in ruptured abdominal aortic aneurysms. The Finnvasc Study Group; Eur J Vasc Endovasc Surg, 17 (1999), pp. 208–212
- 23 E.P. Bauer, C. Redaelli, L.K. von Segesser, M.I. Turina; Ruptured abdominal aortic aneurysms: predictors for early complications and death; Surgery, 114 (1993), pp. 31–315
- 24 S. Sasaki, K. Takigami, T. Kunihara, et al.; Abdominal aortic aneurysms in aged patients: analysis of risk factors in non-ruptured cases; J Cardiovasc Surg (Torino), 40 (1999), pp. 1–5
- 25 A.J. Berry, R.B. Smith, W.S. Weintraub, et al.; Age versus comorbidities as risk factors for complications after elective abdominal aortic reconstructive surgery; J Vasc Surg, 33 (2001), pp. 345–352
- 26 V.L. Roger, D.J. Ballard, J.W. Hallett Jr., P.J. Osmundson, P.A. Puetz, B.J. Gersh; Influence of coronary artery disease on morbidity and mortality after abdominal aortic aneurysmectomy: a population-based study, 1971–1987; J Am Coll Cardiol, 14 (1989), pp. 1245–1252
- 27 D.H. Stone, P.P. Goodney, J. Kalish, et al.; Severity of chronic obstructive pulmonary disease is associated with adverse outcomes in patients undergoing elective abdominal aortic aneurysm repair; J Vasc Surg, 57 (2013), pp. 1531–1536
- 28 R.C. Lo, R.P. Bensley, A.D. Hamdan, et al.; Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England; J Vasc Surg, 57 (2013), pp. 1261–1268 e1–5
- 29 N.A. Kennedy, L.M. Flynn, R.M. Berg, D.R. Lorelli, K. Rama, Y. Rizk; The evaluation of morbidity and mortality in abdominal aortic aneurysm repair patients as related to body mass index; Am J Surg, 199 (2010), pp. 369–371 discussion 371
- 30 S.W. Grant, G.L. Hickey, A.D. Grayson, D.C. Mitchell, C.N. McCollum; National risk prediction model for elective abdominal aortic aneurysm repair; Br J Surg, 100 (2013), pp. 645–653
- 31 N.R. Hertzer, E.J. Mascha; A personal experience with factors influencing survival after elective open repair of infrarenal aortic aneurysms; J Vasc Surg, 42 (2005), pp. 898–905
- 32 G.T. McArdle, G. Price, A. Lewis, et al.; Positive fluid balance is associated with complications after elective open infrarenal abdominal aortic aneurysm repair; Eur J Vasc Endovasc Surg, 34 (2007), pp. 522–527
- 33 A.W. Beck, P.P. Goodney, B.W. Nolan, et al.; Predicting 1-year mortality after elective abdominal aortic aneurysm repair; J Vasc Surg, 49 (2009), pp. 838–843 discussion 843–844
- 34 D.P. Nathan, C.J. Brinster, B.M. Jackson, et al.; Predictors of decreased short- and long-term survival following open abdominal aortic aneurysm repair; J Vasc Surg, 54 (2011), pp. 1237–1243
- 35 A.K. Samy, G. Murray, G. MacBain; Glasgow aneurysm score; Cardiovasc Surg, 2 (1994), pp. 41–44
- 36 D.R. Prytherch, G.L. Sutton, J.R. Boyle; Portsmouth POSSUM models for abdominal aortic aneurysm surgery; Br J Surg, 88 (2001), pp. 958–963
- 37 T. Tang, S.R. Walsh, D.R. Prytherch, et al.; VBHOM, a data economic model for predicting the outcome after open abdominal aortic aneurysm surgery; Br J Surg, 94 (2007), pp. 717–721
- 38 T.H. Lee, E.R. Marcantonio, C.M. Mangione, et al.; Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery; Circulation, 100 (1999), pp. 1043–1049
- 39 T. Tang, S.R. Walsh, T.R. Fanshawe, et al.; Estimation of physiologic ability and surgical stress (E-PASS) as a predictor of immediate outcome after elective abdominal aortic aneurysm surgery; Am J Surg, 194 (2007), pp. 176–182
- 40 G.J. Bryce, C.J. Payne, S.C. Gibson, D.B. Kingsmore, D.S. Byrne, C. Delles; Risk stratification scores in elective open abdominal aortic aneurysm repair: are they suitable for preoperative decision making?; Eur J Vasc Endovasc Surg, 44 (2012), pp. 55–61
- 41 T.Y. Tang, S.R. Walsh, T.R. Fanshawe, et al.; Comparison of risk-scoring methods in predicting the immediate outcome after elective open abdominal aortic aneurysm surgery; Eur J Vasc Endovasc Surg, 34 (2007), pp. 505–513
- 42 J.W. Hallett Jr., D.M. Marshall, T.M. Petterson, et al.; Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience; J Vasc Surg, 25 (1997), pp. 277–284 discussion 285–286
- 43 J.S. Cho, P. Gloviczki, E. Martelli, et al.; Long-term survival and late complications after repair of ruptured abdominal aortic aneurysms; J Vasc Surg, 27 (1998), pp. 813–819 discussion 819–820
- 44 F. Biancari, K. Ylönen, V. Anttila, et al.; Durability of open repair of infrarenal abdominal aortic aneurysm: a 15-year follow-up study; J Vasc Surg, 35 (2002), pp. 87–93
- 45 D.J. Adam, R.A. Fitridge, S. Raptis; Late reintervention for aortic graft-related events and new aortoiliac disease after open abdominal aortic aneurysm repair in an Australian population; J Vasc Surg, 43 (2006), pp. 701–705 discussion 705–706
- 46 E.S. Crawford, S.A. Saleh, J.W. Babb 3rd, D.H. Glaeser, P.S. Vaccaro, A. Silvers; Infrarenal abdominal aortic aneurysm: factors influencing survival after operation performed over a 25-year period; Ann Surg, 193 (1981), pp. 699–709
- 47 G. Plate, L.A. Hollier, P. O'Brien, P.C. Pairolero, K.J. Cherry, F.J. Kazmier; Recurrent aneurysms and late vascular complications following repair of abdominal aortic aneurysms; Arch Surg, 120 (1985), pp. 590–594
- 48 Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial; Lancet, 365 (2005), pp. 2179–2186
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?