The closed-loop pathways of signaling molecules
Authors: Yang LIU*
Affiliations:
The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong.
- To whom correspondence should be addressed: mmyliu@polyu.edu.hk
Background. The pathways of signaling molecules are important to understanding how signaling molecules regulate physiological function and also in predicting the pathological development which is important to therapeutic strategy, however the thorough knowledge of these pathways is still lack.
Methods. In this paper, we used the big data concept to analyze the pathways of signaling molecules and categorize these molecules into five groups according to their origin and effect on the five organs of heart-spleen-lung-kidney-liver.
Results. Heart group includes insulin-line growth factor (IGF), Ang and Mg; Angiotensin (Ang), and Magnesium (Mg); spleen group includes atrial natriuretic peptide (ANP), aldosterone, retinoic acid and ghrelin; lung group includes fibroblast growth factor-7 (FGF7) and vascular endothelial growth factor (VEGF), ascorbic acid, and hypoxia inducible factor (HIF); kidney group includes calcitonin, parathyroid hormone-related protein (PTHrP), Wnt, nitric oxide (NO); and liver group includes erythropoietin (EPO), superoxide dismutase (SOD), renin, aldo-keto reductase (AKR), and glutathione (GSH).
Discussion. We found that each group of molecules have assisting effect on the other organ in the order of heart-spleen-lung-kidney-liver-heart, and have regulating effect on the other organ in the order of heart-lung-liver-spleen-kidney-heart. Moreover, the pathways of molecules of each group also follow these two arrangements, in which the pathways of molecules form a closed-loop that may lead to new therapeutic strategies.
Key words: Signaling molecules, pathways, closed-loop, therapeutic strategy.
1. Introduction
The pathways of signaling molecules are important to understanding how signaling molecules regulate physiological function and also in predicting the pathological development which is important to therapeutic strategy. However, despite the documented importance of signaling molecules to human life, we still lack thorough knowledge of pathways of signaling molecules. It is well known that heart, spleen, lung, kidney and liver generate/secret important signaling molecules which have significant effect on human physiological processes through signaling pathways. Where liver generates insulin-line growth factor (IGF), heart produces atrial natriuretic peptide (ANP), spleen generates fibroblast growth factor-7 (FGF7) and vascular endothelial growth factor (VEGF), lung secrets calcitonin and parathyroid hormone-related protein (PTHrP), and kidney produces erythropoietin (EPO). These are key signaling molecules, and there are other molecules that regulate or inhibit these key signaling molecules. Here we used Big Data concept to analyze the pathways of these signaling molecules, and categorize these signaling molecules into five groups according to their origin and effect on a particular organ. We named these group as heart group, spleen group, lung group, kidney group and liver group. Heart group includes IGF, Angiotensin (Ang), and Magnesium (Mg); spleen group includes ANP, aldosterone, retinoic acid and ghrelin; lung group includes FGF7, VEGF, ascorbic acid, and hypoxia inducible factor (HIF); kidney group includes calcitonin, PTHrP, Wnt, nitric oxide (NO); and liver group includes EPO, superoxide dismutase (SOD), renin, aldo-keto reductase (AKR), and glutathione (GSH). As shown in Figure 1, within each group, the signaling molecules regulate each other and form a small closed-loop of pathways. Among those five groups, each group have assisting effect on other organ in the order of heart-spleen-lung-kidney-liver-heart, and have regulating effect on the other organ in the order of heart-lung-liver-spleen-kidney-heart. Moreover, the molecules of each group regulate those in other group also in the orders of heart-spleen-lung-kidney-liver-heart and heart-lung-liver-spleen-kidney-heart. Therefore, we may constitute the closed-loop pathways of these signaling molecules.
2. Method
We apply the following selection method to identify the signaling molecules.
Step 1:identify the key molecules
It is known that heart generates ANP, spleen produces FGF7 and VEGF, lung secrets calcitonin and PTHrP, kidney generates EPO, and liver generates IGF respectively. Since IGF has significant effect on heart, ANP has significant effect on spleen, FGF7 and VEGF have significant effect on lung, calcitonin and PTHrP have significant effect on kidney, and EPO has significant effect on liver, we set IGF as key molecule of “heart group”, ANP as key molecule of “spleen group”, FGF7 and VEGF as key molecules of “lung group”, calcitonin and PTHrP as key molecules of “kidney group”, and EPO as key molecule of “liver group”.
Step 2: explore the relationships among the key molecules
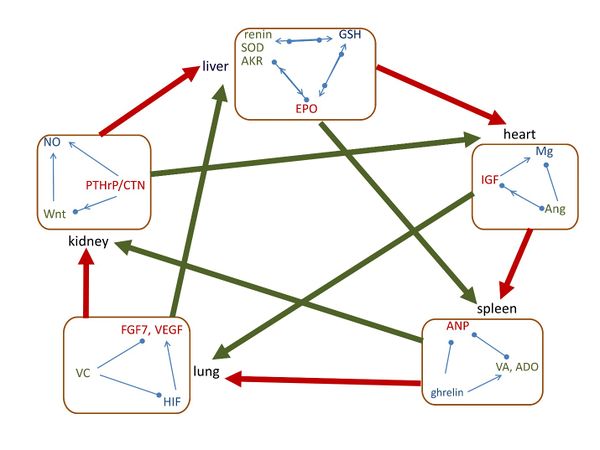
In Figure 1, along the outer circle of heart-spleen-lung-kidney-liver-heart, the key molecule of each group is generated by the previous organ.
Step 3: identify the signaling molecules in each group
These key signaling molecules usually have proliferating or warming effect. There should exist other signaling molecules that have shrinkage or cooling effect and also have significant effect on the same organ, i.e., within the same group, the molecules should have contrary effect or regulate each other, thus the functions of these signaling molecules should be
Φ1: proliferation or warming, (for key signaling molecule),
Φ2: shrinkage or cooling,
Φ3: activation or regulation.
Suppose the signaling molecules form the following set,
X = {x: signaling molecules}.
Then, we find the signaling molecules for each group as following set:
Heart group= x: Φ1, Φ2, Φ3,
Spleen group = x: Φ1, Φ2, Φ3,
Lung group = x: Φ1, Φ2, Φ3,
Kidney group = x: Φ1, Φ2, Φ3,
Liver group = x: Φ1, Φ2, Φ3.
Within each group, the molecules form a closed-loop regulating relationship.
Step 4: determining the intrinsic relationships among molecules of these five groups
By comparison and iteration, we determine the interrelationships each group of molecules with organs, and the pathways of molecules among these five groups.
Step 5: refine the selection and iterate from Step 2
Thus, we could establish a “large” closed-loop pathways among the molecules of these five groups.
3. Results
3.1 The signaling molecules in each group and their regulating relationship
The molecules in each group are tabulated in Table 1.
3.1.1 Heart group consists of IGF, Ang and Mg
IGF is secreted by liver and is important to heart function. IGF-1 signaling regulates metabolism, contractility, senescence, autophagy, hypertrophy, and apoptosis in the heart; it activates canonical and noncanonical signaling pathways in the heart; deficiency in IGF-1 may drive cardiovascular disease, and local IGF-1 therapy can prevent heart injuries in experimental models (Troncoso et al. 2014). IGF-1 has warming effect (Sanchez-Alavez et al. 2011).
Ang is secreted by liver; Ang II regulates cardiac and blood vessel contractility, and is involved in cardiac growth, remodeling, and apoptosis (De Mello & Danser 2000). Ang II induces hypothermia (Wilson & Fregly 1985),which is contrary to IGF.
Mg is important to normal heart rhythm and is a cofactor in more than 300 enzyme systems that regulate diverse biochemical reactions in the body, including blood pressure regulation and blood glucose control (NIH-library-Mg).
IGF, Ang and Mg regulate each other and form the small closed-loop pathways, as shown in the heart group of Figure 1. It was found that Ang II infusion in rat decreases levels of circulating and skeletal muscle IGF-1 (Song et al. 2005), Ang II stimulates cardiac IGF-1 (Brink et al. 1999) and transcription of IGF-1 receptor in vascular smooth muscle cells (Ma et al. 2006), and IGF-1 reduces Ang II (Kajstura et al. 2001). Moreover, the exposure of the platelets to Ang II significantly decreases intracellular Mg concentrations (Touyz & Schiffrin 1993), and Mg levels are strongly associated with IGF-1 (Maggio et al. 2011).
3.1.2 Spleen group consists of ANP, aldosterone, retinoic acid, and ghrelin
ANP is secreted by heart muscle cells that intervenes in the short- and long-term control of blood pressure and of water and electrolyte balance (Debold 1985). The spleen is an important site of ANP-induced fluid extravasation into the systemic lymphatic system. ANP enhances the extravasation of isoncotic fluid from the splenic vasculature both by raising intrasplenic microvascular pressure and by increasing filtration area (Sultanian et al. 2001). Moreover, maintaining body temperature is an important function of ANP (Palmer & Clegg 2015).
Aldosterone retains needed salt in human body and helps control blood pressure, the balance of electrolytes in blood, and the distribution of fluids in the body. An intracardiac production of aldosterone was recently found in rat (Delcayre et al. 2000). Aldosterone increases spleen size and weight (McGraw et al. 2013). Retinoic acid (vitamin A) controls the homeostasis of splenic cells (Klebanoff et al. 2013) and is generated by the epicardium (Chen et al. 2002). Moreover, either aldosterone or retinoic acid inhibits heat generation in body (Marzolla et al. 2012; Murholm et al. 2013), which are contrary to ANP.
Ghrelin is a gastric peptide that regulates the distribution and consumption of energy. Ghrelin inhibits proliferation of splenic T cells (Xia et al. 2004) and effectively regulates food intake and energy homeostasis (Ueno et al. 2005).
The spleen group in Figure 1 shows the contrary effect and supportive relationship between ANP and aldosterone/retinoic acid. ANP has exactly opposite function of aldosterone, ANP decreases circulating aldosterone and vice versa (Klabunde 2012). Retinoic acid signaling markedly stimulates natriuretic peptide receptor-A gene expression (Kumar et al. 2014). Moreover, it was found that ANP level decreases significantly after treatment with ghrelin in a rat model (Torsello et al. 2003); ghrelin elevates aldosterone (Milosevic et al. 2010); and applying ghrelin supplementation to normal lungs increased retinoic acid receptor α/γ expression (Pereira-Terra et al. 2015).
3.1.3 Lung group consists of FGF7, VEGF, ascorbic acid and HIF
FGF7 is secreted by spleen (Suzuki et al. 1993) and is a potent mitogen that enhances cell proliferation in various organs, including the lung, skin, intestine, breast, and liver. It controls the lung morphogenesis, respiratory epithelial cell differentiation and proliferation (Tichelaar et al. 2000).
VEGF is critical for the development and maintenance of the lung and also plays a role in several acute and chronic lung diseases (Voelkel et al. 2006). It was found that splenic T cells produce VEGF (Owen et al. 2003). VEGF is important for brown adipose tissue development and maintenance in which the function of brown adipose tissue is to generate body heat (Bagchi et al. 2013).
Ascorbic acid (Vitamin C) was identified in the early 1900s in the search for a deficient substance responsible for scurvy which was directly linked to pneumonia. Vitamin C is a physiological antioxidant protecting host cells against oxidative stress caused by infections, and plays a role on preventing and treating pneumonia (Hemila & Louhiala 2007) and other lung disease (Fisher et al. 2011). It was found that vitamin C content in spleen is much higher than that in other organs (Tsao et al. 1987) and there exists an extracellular pool of ascorbic acid in lung maintained even during scurvy (Willis & Kratzing 1976).
HIF which is a highly conserved transcription factor that is present in almost all cell types, is tightly regulated by oxygen availability, and regulates the expression of hundreds of genes. HIF system plays a critical role in pulmonary development (Shimoda & Semenza 2011).
The lung group in Figure 1 shows the contrary and regulating interrelationship of HIF, FGF7, VEGF and vitamin C. HIF-1 activates VEGF transcription in hypoxic cells (Forsythe et al. 1996). HIF promotes the expression of FGF7 mRNA levels (Tsuji et al. 2014). Vitamin C prevents endothelial VEGF and VEGFR-2 overexpression (Rodriguez et al. 2005) and inhibits NO-induced HIF-1α stabilization and accumulation (Muellner et al. 2010).
3.1.4 Kidney group consists of Calcitonin, PTHrP, Wnt and NO
Both calcitonin and PTHrP are secreted from lung (Gomez-Roman et al. 2002; Hastings et al. 1994). Calcitonin augments the renal reabsorptive capacity for calcium (Hsu et al. 2010), PTHrP is potent renal regulating factor and mitogenic for various renal cell types, and plays a role in renal development (Esbrit & Egido 2000). Both calcitonin and PTHrP have “warming” effect (Fargeas et al. 1985; Kir et al. 2014).
Wnt signaling regulates cell-to-cell interactions during development and adult tissue homeostasis, and is associated with kidney development and kidney diseases (Pulkkinen et al. 2008). Several Wnt genes are expressed in lung (Dean et al. 2005). Wnt signaling blocks thermogenesis (Kang et al. 2005) which is contrary to calcitonin and PTHrP.
NO is the second messenger and a major regulator in the cardiovascular, immune, and nervous systems. In the kidney NO has numerous important functions including the regulation of renal hemodynamics, maintenance of medullary perfusion, mediation of pressure-natriuresis, blunting of tubuloglomerular feedback, inhibition of tubular sodium reabsorption, and modulation of renal sympathetic neural activity (Mount & Power 2006).
The spleen group in Figure 1 shows the interrelationships among calcitonin, PTHrP, Wnt and NO. Calcitonin stimulates expression of sclerostin (Gooi et al. 2010) which is an inhibitor of Wnt, and administration of PTHrP can increase Wnt signaling (Lopez-Herradon et al. 2013). Moreover, calcitonin increases plasma NO levels (Tas et al. 2002), PTHrP activates NO release (Kalinowski et al. 2001), and Wnt-5a increases NO production (Munoz et al. 2014).
3.1.5 Liver group consists of EPO, renin, SOD, AKR, and GSH
EPO is produced principally in the liver during fetal gestation and mainly in the kidney for adult (Zanjani et al. 1981). EPO is a glycoprotein that promotes the proliferation and differentiation of erythrocyte precursors. It was reported that high-dose EPO increases liver regeneration by affecting the biochemical, morphological, and histopathological parameters after liver resection (Gul et al. 2013). EPO increases brown fat gene expression (Wang et al. 2013).
Renin is produced in kidney and exerts significant effect on liver. The renin-substrate concentration in patients with liver disease is much lower than that of normal subjects (Ayers 1967). Blockade of the renin-angiotensin system improves liver regeneration (Koh et al. 2013), i.e., renin is not favor liver proliferation.
SOD is an important antioxidant defense in nearly all living cells exposed to oxygen and is intensively expressed in kidney (Suh et al. 1997). SOD mimetic improves the function, growth and survival of small size liver grafts after transplantation in rats (Cui et al. 2012) and Cu/Zn SOD is broadly distributed in liver (Okado-Matsumoto & Fridovich 2001). It was found that cold-exposure activates brown adipose tissue thermogenesis but decreases SOD expression (Petrovic et al. 2010), indicating SOD has “cooling” effect.
AKR is expressed in kidney (MacLeod et al. 2010). The AKR superfamily comprises of several enzymes that catalyze redox transformations involved in intermediary metabolism, detoxification and biosynthesis (Barski et al. 2008). AKR-7A protects liver cells and tissues from acetaminophen-induced oxidative stress and hepatotoxicity (Ahmed et al. 2011). Cold-exposure suppresses AKR level significantly (Shore et al. 2013), indicating AKR has “cooling” effect.
GSH is a substance produced naturally by the liver and effectively scavenges free radicals and other reactive oxygen species (Wu et al. 2004). GSH plays a key role in the liver in detoxification reactions.
The liver group in Figure 1 shows the interrelationships among EPO, renin, SOD, AKR and GSH. When treated with EPO, the GSH and SOD levels in kidney of rats are significantly decreased (Hussein et al. 2011). The extracellular SOD is a major repressor of hypoxia-induced EPO gene expression (Zelko & Folz 2005), but treatment with EPO increases vascular expression of SOD1 (d'Uscio et al. 2010). SOD and GSH work together to prevent or repair the damage caused by reactive oxygen species. SODs convert superoxide radical into hydrogen peroxide and molecular oxygen, while the GSH peroxidases (GPx) convert hydrogen peroxide into water, and GSH helps the productivity of GPx (Weydert & Cullen 2010). GSH and AKR are also interrelated, since GSH levels are reduced in AKR-deficient strains (Chang & Petrash 2008). Furthermore, renin-angiotensin system stimulates EPO secretion (Vlahakos et al. 1995), and inhibition of GSH increases plasma renin activity (Murakami et al. 1989).
3.2 The assisting relationship between molecule groups and organs
Moreover, as shown in Figure 1, we found that molecules of heart group assist spleen, spleen group assists lung, lung group assists kidney, kidney group assists liver, and liver group assists heart. Along the outer circle it follows the assisting sequence of heart-spleen-lung-kidney-liver-heart. We have also found that the regulating sequence of heart-lung-liver-spleen-kidney-heart, i.e., the diagonal direction. However, the inversed direction may have opposite effect.
3.2.1 The assisting relationship of heart-spleen-lung-kidney-liver-heart
The signaling molecules of heart group have assisting effect on spleen. For example, Mg deficiency causes morphological and immunological alterations in the spleen (Malpuech-Brugere et al. 1998); it was found that localized IGF-1 secretion enhances erythropoiesis in the spleen of murine embryos (Tan et al. 2015), lower IGF-I status is associated with higher spleen longitudinal diameter(Savastano et al. 2011), and IGF-I is necessary for survival or transition of myeloid progenitors into the spleen(Welniak et al. 2004); Ang II has a role in the establishment of an efficient T cell response in the spleen (Silva-Filho et al. 2013).
The signaling molecules of spleen group have assisting effect on lung. For example, ANP attenuates Lipopolysaccharides-induced lung vascular leak (Birukova et al. 2010); Retinoic acid regulates lung morphogenesis (Malpel et al. 2000); aldosterone may be used as a strategy to increase lung edema clearance (Olivera et al. 2000); and ghrelin is produced early by the fetal lung and promotes lung growth (Santos et al. 2006).
The signaling molecules of lung group have assisting effect on kidney. For example, HIF is involved in the regulation of a multitude of biological processes that are relevant to kidney function under physiological and pathological conditions, including glucose and energy metabolism, angiogenesis, erythropoiesis and iron homeostasis, cell migration, and cell-cell and cell-matrix interactions (Haase 2006); FGF-7 is part of the signaling pathway controlling collecting system size and nephron number in the kidney during development (Qiao et al. 1999); and VEGF is required for growth and proliferation of glomerular and peritubular endothelial cells (Schrijvers et al. 2004).
The signaling molecules of kidney group have assisting effect on liver. For example, PTHrP activates human hepatic stellate cells(Liang et al. 2013) ; calcitonin intensificate the processes of conjugation of bile acids with amino acids taurine and glycine in hepatocytes and canalicular secretion that result in improvement of solubilization properties of the bile, ability of the bile to hold cholesterol in solute state and prevent the formation of calculi in biliary tracts (Gorenko et al. 2012); short time lower dose of NO administration is good for liver, but long time high dose of NO administration is harmful for liver (Hon et al. 2002); Wnt signaling contributes to liver physiology and pathology by regulating various basic cellular events, including differentiation, proliferation, survival, oxidative stress, and morphogenesis (Thompson & Monga 2007).
The signaling molecules of liver group have assisting effect on heart. For example, low GSH has high risk of cardiovascular disease (Shimizu et al. 2004); treated with EPO, a significant improvement in cardiac function and symptoms can be found (van der Meer et al. 2004); extracellular SOD protects the heart against oxidative stress and hypertrophy after myocardial infarction (van Deel et al. 2008); and AKR is activated in the ischemic heart (Kaiserova et al. 2006).
1.1.2 The regulating relationship of heart-lung-liver-spleen-kidney-heart
The signaling molecules of heart group has regulating effect on lung. For example, IGF-1 induces lung fibroblast activation, and blockade of IGF pathway in murine model of lung fibrosis improved outcome and decreased fibrosis (Hung et al. 2013); Ang II could mediate the response to lung injury (Marshall 2003); and Mg deficiency contributes to pulmonary complications (Landon & Young 1993).
The signaling molecules of lung group has regulating effect on liver. It was found that FGF7 promotes liver regeneration (Takase et al. 2013); VEGF promotes proliferation of hepatocytes through reconstruction of liver sinusoids by proliferation of sinusoidal endothelial cells (Taniguchi et al. 2001); vitamin C deficiency promotes fatty liver disease development (Ipsen et al. 2014) and vitamin C plus E combination treatment is a safe and effective treatment option in patients with fatty liver disease (Ersoz et al. 2005); and HIF plays a role across a range of hepatic pathophysiology (Nath & Szabo 2012).
The signaling molecules of liver group has regulating effect on spleen, for example, EPO is necessary for the development of erythroid colonies in spleen (Schooley 1966).
The signaling molecules of spleen group has regulating effect on kidney, for example, ANP can act directly or indirectly (via inhibition of aldosterone biosynthesis) on the kidney to alter sodium transport and may regulate fluid distribution within the extracellular space (Blaine 1990); Aldosterone is crucial for sodium conservation in the kidney (Arai & Chrousos 2016); retinoic acid is required for kidney development (Mallipattu & He 2015); the kidney degrades ghrelin, and increased total ghrelin levels in chronic kidney disease are primarily due to the decreased degradation of ghrelin in the kidney (Yoshimoto et al. 2002).
The signaling molecules of kidney group also have regulating effect on heart organ. For example, NO regulates cardiac function through both vascular-dependent and -independent effects (Massion et al. 2003); congestive heart failure is associated with increased circulating calcitonin gene-related peptide (CGRP), but CGRP attenuates the development of myocardial infarction (Franco-Cereceda & Liska 2000); PTHrP has a protective effect for heart (Ross & Schlüter 2005); Wnt controls heart development but is also modulated during adult heart remodeling (Bergmann 2010).
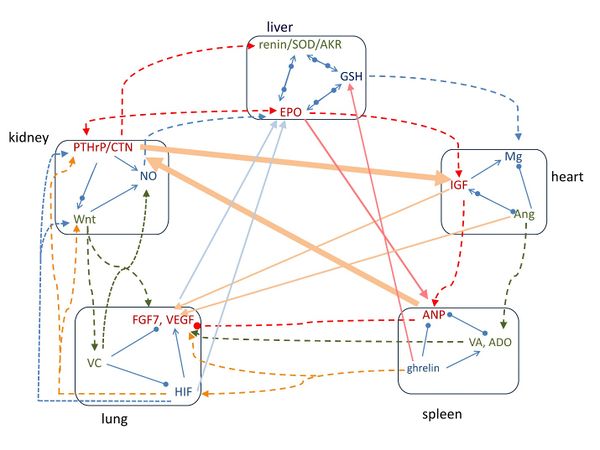
3.3 The interrelationships among groups of molecules
Figure 2 shows the pathways of the signaling molecules of the five groups. Along the outer circle heart-spleen-lung-kidney-liver-heart, the signaling molecules activate each other and form a clockwise closed-loop. As between liver group and heart group, EPO administration increases plasma IGF-I levels (Sohmiya & Sohmiya 2010),
GSH enhances intracellular Mg both in vivo and in vitro (Barbagallo et al. 1999). Between heart group and spleen group, it was found that IGF-II increases the protein level of ANP (Chu et al. 2008), and Ang causes the release of aldosterone from the adrenal glands. Between spleen group and lung group, ghrelin administration significantly increased HIF-1α and VEGF mRNAs (Konturek et al. 2006), Aldosterone increases VEGF-A production in human neutrophils (Walczak et al. 2011), and retinoic acid increases HIF-1α (Fernandez-Martinez et al. 2012) and markedly stimulates FGF7 expression (Mackenzie & Gao 2001); however, ANP blocks VEGF production and signaling in vitro (Lara-Castillo et al. 2009). Between lung group and kidney group, HIF modulates Wnt/β-catenin signaling (Mazumdar et al. 2010), induces PTHrP (Pelosi et al. 2013), and is regulated by NO (Agani et al. 2002); Wnt activates FGF7 during epidermal stratification (Zhu et al. 2014), and regulates vitamin C biosynthesis (Nejak-Bowen et al. 2009); and vitamin C increases nitric oxide synthase activity (d'Uscio et al. 2003). Between kidney group and liver group, calcitonin gene-related peptide (CGRP) and PTHrP stimulate renin secretion (Atchison et al. 2012; Kurtz et al. 1988), PTHrP and EPO are positively correlated with each other(Feng et al. 2013), PTHrP upregulates AKR 1C3(Downs et al. 2011), and NO stimulates EPO(Cokic et al. 2014).
For the diagonal arrangement of heart-lung-liver-spleen-kidney-heart, the signaling molecules in each group also enhances those in following group. For example, between heart group and lung group, IGF-1 induces lung fibroblast activation (Hung et al. 2013) and induces VEGF (Slomiany & Rosenzweig 2004); and Ang II increases FGF7 mRNA levels (Stirling et al. 1990) and stimulates VEGF synthesis (Pupilli et al. 1999). Between lung group and liver group, VEGF is crucial for EPO-induced improvement of cardiac function (Westenbrink et al. 2010); vitamin C elevates red blood cell glutathione in healthy adults (Johnston et al. 1993); HIF induces EPO production (Wang et al. 1995) and induction of HIF-1 varies with the GSSG/GSH ratio (Tajima et al. 2009). Between liver group and spleen group, EPO stimulates ANP secretion (Porat et al. 1996), and ghrelin treatment increases GSH levels(Cao et al. 2013) and activity of SOD (Zwirska-Korczala et al. 2007). Between spleen group and kidney group, ANP increases calcitonin gene-related peptide (CGRP) within human circulation (Vesely et al. 1997). Between kidney group and heart group, calcitonin increases concentration of IGF (Farley et al. 2000), and IGF signaling antagonizes the Wnt pathway (Schlupf & Steinbeisser 2014).
4. Discussion
Based on the five organs we categorize the signaling molecules into five groups, heart group, spleen group, lung group, kidney group and liver group, respectively. The assisting and regulating relationships follow the outer circle and diagonal arrangement in Figure 1. As shown in Figure 1, along the outer circle arrangement of heart-spleen-lung-kidney-liver-heart, the key molecule of each group is generated/secreted in the previous organ, for example, IGF in heart group is generated in liver, and so on. Within each group, the molecules are contrary but interdependent and regulate each other. Along the outer circle arrangement of heart-spleen-lung-kidney-liver-heart, the molecules of each group have assisting effect on the corresponding organ and the following organ. For example, the molecules in heart group have assisting effect on heart and spleen, the molecules of spleen group have assisting effect on spleen and lung, the molecules in lung group have assisting effect on lung and kidney, the molecules in kidney group have assisting effect on kidney and liver, and the molecules of liver group have assisting effect on liver and heart. If in reversed order, then the key molecule of each group may have negative effect on the previous organ. For example, circulating ANP, the key molecule of spleen group, is greatly increased in congestive heart failure as a result of increased synthesis and release of this hormone (Brandt et al. 1993); VEGF, the key molecule of lung group, increases mobilization and recruitment of hematopoietic stem cells and circulating endothelial precursor cells to the spleen resulting in splenomegaly (Hattori et al. 2001); for the key molecules of kidney group, small cell carcinoma of lung is frequently associated with abnormally raised plasma calcitonin (Gropp et al. 1980), and lung cancer is associated with high level of PTHrP (Furlhata et al. 1996); for the key molecule of liver group, patients with polycystic kidney disease had higher levels of EPO (Chandra et al. 1985). However, IGF of heart group has positive effect on liver, for example the concentration of IGF-1 is low in patients with chronic liver disease mainly due to the decreased liver function (Møller & Becker 1992).
Along the diagonal arrangement heart-lung-liver-spleen-kidney-heart, the molecules of each group also have positive/regulating effect on the following organ. For example, the molecules of heart group have positive effect on lung, the molecules of lung group have positive effect on liver, the molecules of liver group have positive effect on spleen, the molecules of spleen group have positive effect on kidney, and the molecules of kidney group have positive effect on heart. If in reversed order, the key molecule of each may have negative effect on the previous organ. For example, for the key molecule of heart group, higher serum IGF-1 levels were positively associated with chronic kidney disease (Teppala et al. 2010); for the key molecule of kidney group, calcitonin gene-related peptide suppresses spleen T lymphocyte proliferation (Haberstock-Debic et al. 1999) and endotoxin induces PTHrP gene expression in splenic stromal cells (Funk et al. 1995); for the key molecule of liver group, the correlation between serum-EPO and lung function indices is negative, indicating a negative effect of EPO on lung function (Graudal et al. 1991). However, the key molecule of spleen group has positive effect on liver, as ANP protects liver from hypoxic injury (Carini et al. 2003); and the key molecules of lung group also have positive effect on heart, for example VEGF and its receptors play a role in many different aspects of cardiovascular development, including stem cell differentiation into cardiomyocytes, stem cell migration and survival, and heart development (Madonna & De Caterina 2009), and treatment with VEGF and FGF7 can help to optimize the development of epicardium (Smart et al. 2007).
The pathways of the signaling molecules also follow the closed-loop arrangement of heart-spleen-lung-kidney-liver-heart. As shown in Figure 2, the molecules in heart group activate those in spleen group, the molecules in spleen group regulate those of lung group, the molecules of lung group regulate those of kidney group, the molecules of kidney group activate those of liver group, and the molecules of liver group regulate those of heart group.
Moreover, particularly the key molecule of each group follows the pathway of heart-lung-liver-spleen-kidney-heart, the diagonal arrangement in Figure 2. The molecules of heart group activate those in lung group; the molecules of lung group activate the molecule in liver group; the key molecule of liver group activate the key molecule of spleen group, but ghrelin in spleen group activates GSH and SOD in liver group; the key molecule of spleen group activates the key molecule of kidney group; and the key molecule in kidney group activates the key molecule of heart group.
In conclusion, we categorized the signaling molecules into five groups according to their origin and effect on organs, and found the intrinsic relationships among these five group of molecules. These intrinsic relationships form the closed-loop of pathways of signaling molecules and follow the particular pathways. This enables us to investigate the effect of balance of signaling molecules on human health and understand the closed-loop of pathways of these molecules, that would lead to new therapeutic strategies and insight into the rules governing physiological processes.
References
Agani FH, Puchowicz M, Chavez JC, Pichiule P, and LaManna J. 2002. Role of nitric oxide in the regulation of HIF-1 alpha expression during hypoxia. American Journal of Physiology-Cell Physiology 283:C178-C186. 10.1152/ajpcell.00381.2001
Ahmed MME, Wang T, Luo Y, Ye SL, Wu Q, Guo ZS, Roebuck BD, Sutter TR, and Yang JY. 2011. Aldo-Keto Reductase-7A Protects Liver Cells and Tissues From Acetaminophen-Induced Oxidative Stress and Hepatotoxicity. Hepatology 54:1322-1332. 10.1002/hep.24493
Arai K, and Chrousos GP. 2016. Aldosterone deficiency and resistance.
Atchison DK, Westrick E, Szandzik DL, Gordish KL, and Beierwaltes WH. 2012. Parathyroid hormone-related protein stimulates plasma renin activity via its anorexic effects on sodium chloride intake. American Journal of Physiology-Endocrinology and Metabolism 303:E457-E463.
Ayers CR. 1967. Plasma Renin Activity and Renin-Substrate Concentration in Patients with Liver Disease. Circulation Research 20:594-&.
Bagchi M, Kim LA, Boucher J, Walshe TE, Kahn CR, and D'Amore PA. 2013. Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. Faseb Journal 27:3257-3271. 10.1096/fj.12-221812
Barbagallo M, Dominguez LJ, Tagliamonte MR, Resnick LM, and Paolisso G. 1999. Effects of glutathione on red blood cell intracellular magnesium - Relation to glucose metabolism. Hypertension 34:76-82.
Barski OA, Tipparaju SM, and Bhatnagar A. 2008. The Aldo-Keto Reductase Superfamily and Its Role in Drug Metabolism and Detoxification. Drug Metabolism Reviews 40:553-624. 10.1080/03602530802431439
Bergmann MW. 2010. WNT signaling in adult cardiac hypertrophy and remodeling. Circulation research 107:1198-1208.
Birukova AA, Xing J, Fu P, Yakubov B, Dubrovskyi O, Fortune JA, Klibanov AM, and Birukov KG. 2010. Atrial natriuretic peptide attenuates LPS-induced lung vascular leak: role of PAK1. American Journal of Physiology-Lung Cellular and Molecular Physiology 299:L652-L663.
Blaine EH. 1990. Atrial natriuretic factor plays a significant role in body fluid homeostasis. Hypertension 15:2-8.
Brandt RR, Wright RS, Redfield MM, and Burnett JC. 1993. Atrial natriuretic peptide in heart failure. Journal of the American College of Cardiology 22:A86-A92.
Brink M, Chrast J, Price SR, Mitch WE, and Delafontaine P. 1999. Angiotensin II stimulates gene expression of cardiac insulin-like growth factor I and its receptor through effects on blood pressure and food intake. Hypertension 34:1053-1059.
Cao YK, Tang J, Yang T, Ma H, Yi DH, Gu CH, and Yu SQ. 2013. Cardioprotective Effect of Ghrelin in Cardiopulmonary Bypass Involves a Reduction in Inflammatory Response. PloS one 8. ARTN e55021
10.1371/journal.pone.0055021
Carini R, De Cesaris MG, Splendore R, Domenicotti C, Nitti MP, Pronzato MA, and Albano E. 2003. Mechanisms of hepatocyte protection against hypoxic injury by atrial natriuretic peptide. Hepatology 37:277-285.
Chandra M, Miller M, Mossey R, and McVicar M. 1985. Serum immunoreactive erythropoietin levels in patients with polycystic kidney disease as compared with other hemodialysis patients. Nephron 39:26-29.
Chang Q, and Petrash JM. 2008. Disruption of aldo-keto reductase genes leads to elevated markers of oxidative stress and inositol auxotrophy in Saccharomyces cerevisiae. Biochimica Et Biophysica Acta-Molecular Cell Research 1783:237-245. 10.1016/j.bbamer.2007.08.008
Chen THP, Chang TC, Kang JO, Choudhary B, Makita T, Tran CM, Burch JBE, Eid H, and Sucov HM. 2002. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Developmental Biology 250:198-207. 10.1006/dbio.2002.0796
Chu CH, Tzang BS, Chen LM, Kuo CH, Cheng YC, Chen LY, Tsai FJ, Tsai CH, Kuo WW, and Huang CY. 2008. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via G alpha q interaction and protein kinase C-alpha/CaMKII activation in H9c2 cardiomyoblast cells. Journal of Endocrinology 197:381-390. 10.1677/Joe-07-0619
Cokic BBB, Cokic VP, Suresh S, Wirt S, and Noguchi CT. 2014. Nitric oxide and hypoxia stimulate erythropoietin receptor via MAPK kinase in endothelial cells. Microvascular research 92:34-40.
Cui YY, Qian JM, Yao AH, Ma ZY, Qian XF, Zha XM, Zhao Y, Ding Q, Zhao J, Wang S, and Wu J. 2012. SOD Mimetic Improves the Function, Growth, and Survival of Small-Size Liver Grafts After Transplantation in Rats. Transplantation 94:687-694. 10.1097/TP.0b013e3182633478
d'Uscio LV, Milstien S, Richardson D, Smith L, and Katusic ZS. 2003. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circulation Research 92:88-95. 10.1161/01.Res.0000049166.33035.62
d'Uscio LV, Smith LA, and Katusic ZS. 2010. Erythropoietin Increases Expression and Function of Vascular Copper- and Zinc-Containing Superoxide Dismutase. Hypertension 55:998-1004. 10.1161/Hypertensionaha.110.150623
De Mello WC, and Danser AHJ. 2000. Angiotensin II and the heart - On the intracrine renin-angiotensin system. Hypertension 35:1183-1188.
Dean CH, Miller LAD, Smith AN, Dufort D, Lang RA, and Niswander LA. 2005. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Developmental Biology 286:270-286. 10.1016/j.ydbio.2005.07.034
Debold AJ. 1985. Atrial Natriuretic Factor - a Hormone Produced by the Heart. Science 230:767-770. DOI 10.1126/science.2932797
Delcayre C, Silvestre JS, Garnier A, Oubenaissa A, Cailmail S, Tatara E, Swynghedauw B, and Robert V. 2000. Cardiac aldosterone production and ventricular remodeling. Kidney International 57:1346-1351. DOI 10.1046/j.1523-1755.2000.00973.x
Downs TM, Burton DW, Araiza FL, Hastings RH, and Deftos LJ. 2011. PTHrP stimulates prostate cancer cell growth and upregulates aldo–keto reductase 1C3. Cancer letters 306:52-59.
Ersoz G, Gunsar F, Karasu Z, Akay S, Batur Y, and Akarca US. 2005. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turk J Gastroenterol 16:124-128.
Esbrit P, and Egido J. 2000. The emerging role of parathyroid hormone-related protein as a renal regulating factor. Nephrology Dialysis Transplantation 15:1109-1111. DOI 10.1093/ndt/15.8.1109
Fargeas MJ, Fioramonti J, and Bueno L. 1985. Central Actions of Calcitonin on Body-Temperature and Intestinal Motility in Rats - Evidence for Different Mediations. Regulatory Peptides 11:95-103. Doi 10.1016/0167-0115(85)90070-9
Farley J, Dimai HP, Stilt-Coffing B, Farley P, Pham T, and Mohan S. 2000. Calcitonin increases the concentration of insulin-like growth factors in serum-free cultures of human osteoblast-line cells. Calcified Tissue International 67:247-254. DOI 10.1007/s002230001112
Feng C-c, Ding G-x, Song N-h, Li X, Wu Z, Jiang H-w, and Ding Q. 2013. Paraneoplastic hormones: parathyroid hormone-related protein (PTHrP) and erythropoietin (EPO) are related to vascular endothelial growth factor (VEGF) expression in clear cell renal cell carcinoma. Tumor Biology 34:3471-3476.
Fernandez-Martinez AB, Jimenez MIA, and Cazana FJL. 2012. Retinoic acid increases hypoxia-inducible factor-1 alpha through intracrine prostaglandin E-2 signaling in human renal proximal tubular cells HK-2. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids 1821:672-683. 10.1016/j.bbalip.2012.01.010
Fisher BJ, Seropian IM, Kraskauskas D, Thakkar JN, Voelkel NF, Fowler AA, and Natarajan R. 2011. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Critical Care Medicine 39:1454-1460. 10.1097/CCM.0b013e3182120cb8
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, and Semenza GL. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and Cellular Biology 16:4604-4613.
Franco-Cereceda A, and Liska J. 2000. Potential of calcitonin gene-related peptide in coronary heart disease. Pharmacology 60:1-8.
Funk JL, Lausier J, Moser AH, Shigenaga JK, Huling S, Nissenson RA, Strewler GJ, Grunfeld C, and Feingold KR. 1995. Endotoxin induces parathyroid hormone-related protein gene expression in splenic stromal and smooth muscle cells, not in splenic lymphocytes. Endocrinology 136:3412-3421.
Furlhata M, Sonobe H, Iwata J, Ido E, Ohtsukl Y, Asahi Y, Kubonishl I, and Miyoshi I. 1996. Lung squamous cell carcinoma producing both parathyroid hormone‐related peptide and granulocyte colony stimulating factor. Pathology international 46:376-379.
Ghambarali Z, Bidmeshkipouri A, Akrami H, Azadbakht M, and Rabzia A. 2014. Ethanolic extract of Ficus carica leave Suppresses Angiogenesis by Regulating VEGF-A and Integrin beta3 mRNA Expression in Human umbilical vein endothelial cells. Indian J Physiol Pharmacol 58:407-415.
Gomez-Roman JJ, Martinez JMC, Rozas SF, and Val-Bernal JF. 2002. Hormone expression and opioid receptors in fetal and adult lung. Archivos De Bronconeumologia 38:362-366.
Gooi JH, Pompolo S, Karsdal MA, Kulkarni NH, Kalajzic I, McAhren SHM, Han B, Onyia JE, Ho PWM, Gillespie MT, Walsh NC, Chia LY, Quinn JMW, Martin TJ, and Sims NA. 2010. Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone 46:1486-1497. 10.1016/j.bone.2010.02.018
Gorenko ZA, Karbovska LS, Vascheka IP, and Veselsky SP. 2012. The Influence of Calcitonin on the Liver Bile Formation Function in Rats. International Journal of Physiology and Pathophysiology 3.
Graudal N, Galløe A, and Nielsen O. 1991. Erythropoietin in chronic obstructive pulmonary disease. Respiration 58:141-144.
Gropp C, Havemann K, and Scheuer A. 1980. Ectopic hormones in lung cancer patients at diagnosis and during therapy. Cancer 46:347-354.
Gul M, Comert M, Cakmak GK, Kertis G, Ugurbas E, and Oner MO. 2013. Effect of erythropoietin on liver regeneration in an experimental model of partial hepatectomy. International Journal of Surgery 11:59-63. 10.1016/j.ijsu.2012.11.012
Haase VH. 2006. Hypoxia-inducible factors in the kidney. American Journal of Physiology-Renal Physiology 291:F271-F281.
Haberstock-Debic H, Weyns A, Marotti T, and De Potter W. 1999. Calcitonin gene-related peptide receptors in pig spleen and the involvement of the CGRP 1 receptor in the splenocyte function. Neuropeptides 33:47-53.
Hastings RH, Duong HS, Burton DW, and Deftos LJ. 1994. Alveolar Epithelial-Cells Express and Secrete Parathyroid Hormone-Related Protein. American Journal of Respiratory Cell and Molecular Biology 11:701-706.
Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, and Crystal RG. 2001. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. The Journal of experimental medicine 193:1005-1014.
Hemila H, and Louhiala P. 2007. Vitamin C may affect lung infections. Journal of the Royal Society of Medicine 100:495-498. DOI 10.1258/jrsm.100.11.495
Hon WM, Lee KH, and Khoo HE. 2002. Nitric oxide in liver diseases. Annals of the New York Academy of Sciences 962:275-295.
Hsu YJ, Dimke H, Hoenderop JGJ, and Bindels RJM. 2010. Calcitonin-stimulated renal Ca2+ reabsorption occurs independently of TRPV5. Nephrology Dialysis Transplantation 25:1428-1435. 10.1093/ndt/gfp645
Hung CF, Rohani MG, Lee SS, Chen P, and Schnapp LM. 2013. Role of IGF-1 pathway in lung fibroblast activation. Respiratory Research 14. Artn 102
10.1186/1465-9921-14-102
Hussein AM, Shokeir AA, Sarhan ME, El-Menabawy FR, Abd-Elmoneim HA, El-Nashar EM, and Barakat NM. 2011. Effects of combined erythropoietin and epidermal growth factor on renal ischaemia/reperfusion injury: a randomized experimental controlled study. Bju International 107:323-328. 10.1111/j.1464-410X.2010.09328.x
Ipsen DH, Tveden-Nyborg P, and Lykkesfeldt J. 2014. Does Vitamin C Deficiency Promote Fatty Liver Disease Development? Nutrients 6:5473-5499. 10.3390/nu6125473
Johnston CS, Meyer CG, and Srilakshmi JC. 1993. Vitamin-C Elevates Red-Blood-Cell Glutathione in Healthy-Adults. American Journal of Clinical Nutrition 58:103-105.
Kaiserova K, Srivastava S, Hoetker JD, Awe SO, Tang X-L, Cai J, and Bhatnagar A. 2006. Redox activation of aldose reductase in the ischemic heart. Journal of Biological Chemistry 281:15110-15120.
Kajstura J, Fiordaliso F, Andreoli AM, Li BS, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, and Anversa P. 2001. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 50:1414-1424. DOI 10.2337/diabetes.50.6.1414
Kalinowski L, Dobrucki LW, and Malinski T. 2001. Nitric oxide as a second messenger in parathyroid hormone-related protein signaling. Journal of Endocrinology 170:433-440. DOI 10.1677/joe.0.1700433
Kang S, Bajnok L, Longo KA, Petersen RK, Hansen JB, Kristiansen K, and MacDougald OA. 2005. Effects of wnt signaling on brown adipocyte differentiation and metabolism mediated by PGC-1 alpha. Molecular and Cellular Biology 25:1272-1282. 10.1128/Mcb.25.4.1272-1282.2005
Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, and Spiegelman BM. 2014. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513:100-+. 10.1038/nature13528
Klabunde RE. 2012. Cardiovascular physiology concepts. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.
Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, Sukumar M, Muranski P, Scott CD, Hall JA, Ferreyra GA, Leonardi AJ, Borman ZA, Wang JS, Palmer DC, Wilhelm C, Cai RM, Sun JF, Napoli JL, Danner RL, Gattinoni L, Belkaid Y, and Restifo NP. 2013. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. Journal of Experimental Medicine 210:1961-1976. 10.1084/jem.20122508
Koh SL, Ager E, Malcontenti-Wilson C, Muralidharan V, and Christophi C. 2013. Blockade of the renin-angiotensin system improves the early stages of liver regeneration and liver function. Journal of Surgical Research 179:66-71. 10.1016/j.jss.2012.09.007
Konturek PC, Brzozowski T, Walter B, Burnat G, Hess T, Hahn EG, and Konturek SJ. 2006. Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. European Journal of Pharmacology 536:171-181. 10.1016/j.ejphar.2006.02.032
Kumar P, Periyasamy R, Das S, Neerukonda S, Mani I, and Pandey KN. 2014. All-Trans Retinoic Acid and Sodium Butyrate Enhance Natriuretic Peptide Receptor A Gene Transcription: Role of Histone Modification. Molecular Pharmacology 85:946-957. 10.1124/mol.114.092221
Kurtz A, Muff R, Born W, Lundberg J, Millberg B, Gnädinger M, Uehlinger D, Weidmann P, Hökfelt T, and Fischer J. 1988. Calcitonin gene-related peptide is a stimulator of renin secretion. Journal of Clinical Investigation 82:538.
Landon RA, and Young EA. 1993. Role of magnesium in regulation of lung function. J Am Diet Assoc 93:674-677.
Lara-Castillo N, Zandi S, Nakao S, Ito Y, Noda K, She H, Ahmed M, Frimmel S, Ablonczy Z, and Hafezi-Moghadam A. 2009. Atrial natriuretic peptide reduces vascular leakage and choroidal neovascularization. The American journal of pathology 175:2343-2350.
Liang F-F, Liu C-P, Li L-X, Xue M-M, Xie F, Guo Y, and Bai L. 2013. Activated effects of parathyroid hormone-related protein on human hepatic stellate cells. PloS one 8:e76517.
Lopez-Herradon A, Portal-Nunez S, Garcia-Martin A, Lozano D, Perez-Martinez FC, Cena V, and Esbrit P. 2013. Inhibition of the canonical Wnt pathway by high glucose can be reversed by parathyroid hormone-related protein in osteoblastic cells. Journal of Cellular Biochemistry 114:1908-1916. 10.1002/jcb.24535
Ma YW, Zhang LP, Peng T, Cheng JZ, Taneja S, Zhang JQ, Delafontaine P, and Du J. 2006. Angiotensin II stimulates transcription of insulin-like growth factor I receptor in vascular smooth muscle cells: Role of nuclear factor-kappa B. Endocrinology 147:1256-1263.
Mackenzie IC, and Gao ZR. 2001. Keratinocyte growth factor expression in human gingival fibroblasts and stimulation of in vitro gene expression by retinoic acid. Journal of Periodontology 72:445-453. DOI 10.1902/jop.2001.72.4.445
MacLeod AK, Kelly VP, Higgins LG, Kelleher MO, Price SA, Bigley AL, Betton GR, and Hayes JD. 2010. Expression and Localization of Rat Aldo-Keto Reductases and Induction of the 1B13 and 1D2 Isoforms by Phenolic Antioxidants. Drug Metabolism and Disposition 38:341-346. 10.1124/dmd.109.030544
Madonna R, and De Caterina R. 2009. VEGF receptor switching in heart development and disease. Cardiovascular research 84:4-6.
Maggio M, Ceda GP, Lauretani F, Cattabiani C, Avantaggiato E, Morganti S, Ablondi F, Bandinelli S, Dominguez LJ, Barbagallo M, Paolisso G, Semba RD, and Ferrucci L. 2011. Magnesium and anabolic hormones in older men. International Journal of Andrology 34:E594-E600. 10.1111/j.1365-2605.2011.01193.x
Mallipattu SK, and He JC. 2015. The beneficial role of retinoids in glomerular disease. Frontiers in medicine 2.
Malpel S, Mendelsohn C, and Cardoso WV. 2000. Regulation of retinoic acid signaling during lung morphogenesis. DEVELOPMENT-CAMBRIDGE- 127:3057-3067.
Malpuech-Brugere C, Kuryszko J, Nowacki W, Rock E, Rayssiguier Y, and Mazur A. 1998. Early morphological and immunological alterations in the spleen during magnesium deficiency in the rat. Magnesium research 11:161-169.
Marshall RP. 2003. The pulmonary renin-angiotensin system. Current pharmaceutical design 9:715-722.
Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, Rosano GMC, and Caprio M. 2012. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Molecular and Cellular Endocrinology 350:281-288. 10.1016/j.mce.2011.09.011
Massion P, Feron O, Dessy C, and Balligand J-L. 2003. Nitric oxide and cardiac function. Circulation research 93:388-398.
Mazumdar J, O'Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, and Simon MC. 2010. O-2 regulates stem cells through Wnt/beta-catenin signalling. Nature Cell Biology 12:1007-1013. 10.1038/ncb2102
McGraw AP, Bagley J, Chen WS, Galayda C, Nickerson H, Armani A, Caprio M, Carmeliet P, and Jaffe IZ. 2013. Aldosterone Increases Early Atherosclerosis and Promotes Plaque Inflammation Through a Placental Growth Factor-Dependent Mechanism. Journal of the American Heart Association 2. UNSP e000018
10.1161/JAHA.112.000018
Milosevic VL, Stevanovic DM, Nesic DM, Sosic-Jurjevic BT, Ajdzanovic VZ, Starcevic VP, and Severs WB. 2010. Central effects of ghrelin on the adrenal cortex: a morphological and hormonal study. General Physiology and Biophysics 29:194-202. 10.4149/gpb_2010_02_194
Møller S, and Becker U. 1992. Insulin-like growth factor 1 and growth hormone in chronic liver disease. Digestive diseases 10:239-248.
Mount PF, and Power DA. 2006. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 187:433-446. 10.1111/j.1748-1716.2006.01582.x
Muellner MK, Schreier SM, Schmidbauer B, Moser M, Quehenberger P, Kapiotis S, Goldenberg H, and Laggner H. 2010. Vitamin C inhibits NO-induced stabilization of HIF-1 alpha in HUVECs. Free Radical Research 44:783-791. 10.3109/10715761003786172
Munoz FJ, Godoy JA, Cerpa W, Poblete IM, Huidobro-Toro JP, and Inestrosa NC. 2014. Wnt-5 alpha increases NO and modulates NMDA receptor in rat hippocampal neurons. Biochemical and Biophysical Research Communications 444:189-194. 10.1016/j.bbrc.2014.01.031
Murakami E, Ishii J, Muneta S, Hiwada K, and Kokubu T. 1989. Blood-Pressure Elevation Caused by Inhibition of Brain Glutathione-Reductase. Journal of Hypertension, Vol 7, Suppl 6:S24-S25.
Murholm M, Isidor MS, Basse AL, Winther S, Sorensen C, Skovgaard-Petersen J, Nielsen MM, Hansen AS, Quistorff B, and Hansen JB. 2013. Retinoic acid has different effects on UCP1 expression in mouse and human adipocytes. Bmc Cell Biology 14. Artn 41
10.1186/1471-2121-14-41
Nath B, and Szabo G. 2012. Hypoxia and hypoxia inducible factors: Diverse roles in liver diseases. Hepatology 55:622-633. 10.1002/hep.25497
Nejak-Bowen KN, Zeng G, Tan XP, Cieply B, and Monga SP. 2009. beta-Catenin Regulates Vitamin C Biosynthesis and Cell Survival in Murine Liver. Journal of Biological Chemistry 284:28115-28127. 10.1074/jbc.M109.047258
NIH-library-Mg. Magnesium. Available at [https:// https://]www.nlm.nih.gov/medlineplus/druginfo/natural/998.html.
Okado-Matsumoto A, and Fridovich I. 2001. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276:38388-38393. 10.1074/jbc.M105395200
Olivera WG, Ciccolella DE, Barquin N, Ridge KM, Rutschman DH, Yeates DB, and Sznajder JI. 2000. Aldosterone regulates Na, K-ATPase and increases lung edema clearance in rats. American journal of respiratory and critical care medicine 161:567-573.
Owen JL, Iragavarapu-Charyulu V, Gunja-Smith Z, Herbert LM, Grosso JF, and Lopez DM. 2003. Up-regulation of matrix metalloproteinase-9 in T lymphocytes of mammary tumor bearers: role of vascular endothelial growth factor. J Immunol 171:4340-4351.
Palmer BF, and Clegg DJ. 2015. An Emerging Role of Natriuretic Peptides: Igniting the Fat Furnace to Fuel and Warm the Heart. Mayo Clinic Proceedings 90:1666-1678. 10.1016/j.mayocp.2015.08.006
Pelosi M, Lazzarano S, Thoms BL, and Murphy CL. 2013. Parathyroid hormone-related protein is induced by hypoxia and promotes expression of the differentiated phenotype of human articular chondrocytes. Clinical Science 125:461-470. 10.1042/Cs20120610
Pereira-Terra P, Moura RS, Nogueira-Silva C, and Correia-Pinto J. 2015. Neuroendocrine factors regulate retinoic acid receptors in normal and hypoplastic lung development. Journal of Physiology-London 593:3301-3311. 10.1113/Jp270477
Petrovic V, Buzadzic B, Korac A, and Korac B. 2010. Antioxidative defense and mitochondrial thermogenic response in brown adipose tissue. Genes and Nutrition 5:225-235. 10.1007/s12263-009-0162-1
Porat O, Neumann D, Zamir O, Nachshon S, Feigin E, Cohen J, and Zamir N. 1996. Erythropoietin stimulates atrial natriuretic peptide secretion from adult rat cardiac atrium. J Pharmacol Exp Ther 276:1162-1168.
Pulkkinen K, Murugan S, and Vainio S. 2008. Wnt signaling in kidney development and disease. Organogenesis 4:55-59.
Pupilli C, Lasagni L, Romagnani P, Bellini F, Mannelli MR, Misciglia N, Mavilia C, Vellei U, Villari D, and Serio T. 1999. Angiotensin II stimulates the synthesis and secretion of vascular permeability factor vascular endothelial growth factor in human mesangial cells. Journal of the American Society of Nephrology 10:245-255.
Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, and Herzlinger D. 1999. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126:547-554.
Rodriguez JA, Nespereira B, Perez-Ilzarbe M, Eguinoa E, and Paramo JA. 2005. Vitamins C and E prevent endothelial VEGF and VEGFR-2 overexpression induced by porcine hypercholesterolemic LDL. Cardiovascular Research 65:665-673. 10.1016/j.cardiores.2004.08.006
Ross G, and Schlüter K-D. 2005. Cardiac-specific effects of parathyroid hormone-related peptide: Modification by aging and hypertension. Cardiovascular research 66:334-344.
Sanchez-Alavez M, Osborn O, Tabarean IV, Holmberg KH, Eberwine J, Kahn CR, and Bartfai T. 2011. Insulin-like Growth Factor 1-mediated Hyperthermia Involves Anterior Hypothalamic Insulin Receptors. Journal of Biological Chemistry 286:14983-14990. 10.1074/jbc.M110.188540
Santos M, Bastos P, Gonzaga S, Roriz J-M, Baptista MJ, Nogueira-Silva C, Melo-Rocha G, Henriques-Coelho T, Roncon-Albuquerque R, and Leite-Moreira AF. 2006. Ghrelin expression in human and rat fetal lungs and the effect of ghrelin administration in nitrofen-induced congenital diaphragmatic hernia. Pediatric research 59:531-537.
Savastano S, Di Somma C, Pizza G, De Rosa A, Nedi V, Rossi A, Orio F, Lombardi G, Colao A, and Tarantino G. 2011. Liver-spleen axis, insulin-like growth factor-(IGF)-I axis and fat mass in overweight/obese females. Journal of translational medicine 9:136.
Schlupf J, and Steinbeisser H. 2014. IGF antagonizes the Wnt/beta-Catenin pathway and promotes differentiation of extra-embryonic endoderm. Differentiation 87:209-219. 10.1016/j.diff.2014.07.003
Schooley JC. 1966. The effect of erythropoietin on the growth and development of spleen colony‐forming cells. Journal of Cellular Physiology 68:249-262.
Schrijvers BF, Flyvbjerg A, and De Vriese AS. 2004. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney international 65:2003-2017.
Shimizu H, Kiyohara Y, Kato I, Kitazono T, Tanizaki Y, Kubo M, Ueno H, Ibayashi S, Fujishima M, and Iida M. 2004. Relationship between plasma glutathione levels and cardiovascular disease in a defined population. Stroke 35:2072-2077.
Shimoda LA, and Semenza GL. 2011. HIF and the Lung Role of Hypoxia-inducible Factors in Pulmonary Development and Disease. American Journal of Respiratory and Critical Care Medicine 183:152-156. 10.1164/rccm.201009-1393PP
Shore AM, Karamitri A, Kemp P, Speakman JR, Graham NS, and Lomax MA. 2013. Cold-Induced Changes in Gene Expression in Brown Adipose Tissue, White Adipose Tissue and Liver. PloS one 8. ARTN e68933
10.1371/journal.pone.0068933
Silva-Filho JL, Souza MC, Ferreira-DaSilva CT, Silva LS, Costa MFS, Padua TA, das Graças Henriques M, Morrot A, Savino W, and Caruso-Neves C. 2013. Angiotensin II is a new component involved in splenic T lymphocyte responses during Plasmodium berghei ANKA infection. PloS one 8:e62999.
Slomiany MG, and Rosenzweig SA. 2004. IGF-1-induced VEGF and IGFBP-3 secretion correlates with increased HIF-1 alpha expression and activity in retinal pigment epithelial cell line D407. Investigative Ophthalmology & Visual Science 45:2838-2847. 10.1167/iovs.03-0565
Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, and Riley PR. 2007. Thymosin β4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445:177-182.
Sohmiya M, and Sohmiya Y. 2010. Effects of Recombinant Human Erythropoietin (rHuEPO) Treatment on Plasma Insulin-like Growth Factor-I (IGF-I) and Hemoglobin Concentra-tions in Patients with Type 2 Diabetes Mellitus Associated with Neph-ropathy and Anemia of Chronic Renal Failure. Biomedical Research 21.
Song YH, Li YX, Du J, Mitch WE, Rosenthal N, and Delafontaine P. 2005. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. Journal of Clinical Investigation 115:451-458. 10.1172/Jci200522324
Stirling D, Magness RR, Stone R, Waterman MR, and Simpson ER. 1990. Angiotensin-Ii Inhibits Luteinizing Hormone-Stimulated Cholesterol Side-Chain Cleavage Expression and Stimulates Basic Fibroblast Growth-Factor Expression in Bovine Luteal Cells in Primary Culture. Journal of Biological Chemistry 265:5-8.
Suh JG, Takai S, Yamanishi T, Kikuchi T, Folz RJ, Tanaka K, Oh YS, and Wada K. 1997. Sequence analysis, tissue expression and chromosomal localization of a mouse secreted superoxide dismutase gene. Molecules and Cells 7:204-207.
Sultanian R, Deng YM, and Kaufman S. 2001. Atrial natriuretic factor increases splenic microvascular pressure and fluid extravasation in the rat. Journal of Physiology-London 533:273-280. DOI 10.1111/j.1469-7793.2001.0273b.x
Suzuki M, Itoh T, Osada H, Rubin JS, Aaronson SA, Suzuki T, Koga N, Saito T, and Mitsui Y. 1993. Spleen-Derived Growth-Factor, Sdgf-3, Is Identified as Keratinocyte Growth-Factor (Kgf). Febs Letters 328:17-20. Doi 10.1016/0014-5793(93)80956-U
Tajima M, Kurashima Y, Sugiyama K, Ogura T, and Sakagami H. 2009. The redox state of glutathione regulates the hypoxic induction of HIF-1. European Journal of Pharmacology 606:45-49. 10.1016/j.ejphar.2009.01.026
Takase HM, Itoh T, Ino S, Wang T, Koji T, Akira S, Takikawa Y, and Miyajima A. 2013. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes & Development 27:169-181. 10.1101/gad.204776.112
Tan KS, Inoue T, Kulkeaw K, Tanaka Y, Lai MI, and Sugiyama D. 2015. Localized SCF and IGF-1 secretion enhances erythropoiesis in the spleen of murine embryos. Biology open 4:596-607.
Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, and Sata M. 2001. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. Journal of Histochemistry & Cytochemistry 49:121-129.
Tas N, Aricioglu A, Erbas D, and Ozcan S. 2002. The effect of calcitonin treatment on plasma nitric oxide levels in post-menopausal osteoporotic patients. Cell Biochemistry and Function 20:103-105. 10.1002/cbf.959
Teppala S, Shankar A, and Sabanayagam C. 2010. Association between IGF-1 and chronic kidney disease among US adults. Clinical and experimental nephrology 14:440-444.
Thompson MD, and Monga SP. 2007. WNT/β‐catenin signaling in liver health and disease. Hepatology 45:1298-1305.
Tichelaar JW, Lu W, and Whitsett JA. 2000. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 275:11858-11864.
Torsello A, Bresciani E, Rossoni G, Avallone R, Tulipano G, Cocchi D, Bulgarelli I, Deghenghi R, Berti F, and Locatelli V. 2003. Ghrelin plays a minor role in the physiological control of cardiac function in the rat. Endocrinology 144:1787-1792. 10.1210/en.2002-221048
Touyz RM, and Schiffrin EL. 1993. The effect of angiotensin II on platelet intracellular free magnesium and calcium ionic concentrations in essential hypertension. J Hypertens 11:551-558. Doi 10.1097/00004872-199305000-00011
Troncoso R, Ibarra C, Vicencio JM, Jaimovich E, and Lavandero S. 2014. New insights into IGF-1 signaling in the heart. Trends in Endocrinology and Metabolism 25:128-137. 10.1016/j.tem.2013.12.002
Tsao CS, Leung PY, and Young M. 1987. Effect of Dietary Ascorbic-Acid Intake on Tissue Vitamin-C in Mice. Journal of Nutrition 117:291-297.
Tsuji K, Kitamura S, and Makino H. 2014. Hypoxia-inducible factor 1 alpha regulates branching morphogenesis during kidney development. Biochemical and Biophysical Research Communications 447:108-114. 10.1016/j.bbrc.2014.03.111
Ueno H, Yamaguchi H, Kangawa K, and Nakazato M. 2005. Ghrelin: a gastric peptide that regulates food intake and energy homeostasis. Regulatory Peptides 126:11-19. 10.1016/j.regpep.2004.08.007
van Deel ED, Lu Z, Xu X, Zhu G, Hu X, Oury TD, Bache RJ, Duncker DJ, and Chen Y. 2008. Extracellular superoxide dismutase protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radical Biology and Medicine 44:1305-1313.
van der Meer P, Voors AA, Lipsic E, van Gilst WH, and van Veldhuisen DJ. 2004. Erythropoietin in cardiovascular diseases. European Heart Journal 25:285-291.
Vesely DL, Overton RM, McCormick MT, and Schocken DD. 1997. Atrial natriuretic peptides increase calcitonin gene-related peptide within human circulation. Metabolism-Clinical and Experimental 46:818-825. Doi 10.1016/S0026-0495(97)90129-3
Vlahakos DV, Balodimos C, Papachristopoulos V, Vassilakos P, Hinari E, and Vlachojannis JG. 1995. Renin-Angiotensin System Stimulates Erythropoietin Secretion in Chronic-Hemodialysis Patients. Clinical Nephrology 43:53-59.
Voelkel NF, Vandivier RW, and Tuder RM. 2006. Vascular endothelial growth factor in the lung. American Journal of Physiology-Lung Cellular and Molecular Physiology 290:L209-L221. 10.1152/ajplung.00185.2005
Walczak C, Gaignier F, Gilet A, Zou F, Thornton SN, and Ropars A. 2011. Aldosterone increases VEGF-A production in human neutrophils through PI3K, ERK1/2 and p38 pathways. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1813:2125-2132.
Wang GL, Jiang BH, Rue EA, and Semenza GL. 1995. Hypoxia-Inducible Factor-1 Is a Basic-Helix-Loop-Helix-Pas Heterodimer Regulated by Cellular O-2 Tension. Proceedings of the National Academy of Sciences of the United States of America 92:5510-5514. DOI 10.1073/pnas.92.12.5510
Wang L, Teng RF, Di LJ, Rogers H, Wu H, Kopp JB, and Noguchi CT. 2013. PPAR alpha and Sirt1 Mediate Erythropoietin Action in Increasing Metabolic Activity and Browning of White Adipocytes to Protect Against Obesity and Metabolic Disorders. Diabetes 62:4122-4131. 10.2337/db13-0518
Welniak LA, Karas M, Yakar S, Anver MR, Murphy WJ, and LeRoith D. 2004. Effects of organ-specific loss of insulin-like growth factor-I production on murine hematopoiesis. Biology of Blood and Marrow Transplantation 10:32-39.
Westenbrink BD, Ruifrok WPT, Voors AA, Tilton RG, van Veldhuisen DJ, Schoemaker RG, van Gilst WH, and de Boer RA. 2010. Vascular endothelial growth factor is crucial for erythropoietin-induced improvement of cardiac function in heart failure. Cardiovascular Research 87:30-39. 10.1093/cvr/cvq041
Weydert CJ, and Cullen JJ. 2010. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nature Protocols 5:51-66. 10.1038/nprot.2009.197
Willis RJ, and Kratzing CC. 1976. Extracellular Ascorbic-Acid in Lung. Biochimica Et Biophysica Acta 444:108-117. Doi 10.1016/0304-4165(76)90228-2
Wilson KM, and Fregly MJ. 1985. Angiotensin Ii-Induced Hypothermia in Rats. Journal of Applied Physiology 58:534-543.
Wu GY, Fang YZ, Yang S, Lupton JR, and Turner ND. 2004. Glutathione metabolism and its implications for health. Journal of Nutrition 134:489-492.
Xia Q, Pang W, Pan H, Zheng Y, Kang JS, and Zhu SG. 2004. Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regulatory Peptides 122:173-178. 10.1016/j.regpep.2004.06.016
Yoshimoto A, Mori K, Sugawara A, Mukoyama M, Yahata K, Suganami T, Takaya K, Hosoda H, Kojima M, and Kangawa K. 2002. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. Journal of the American Society of Nephrology 13:2748-2752.
Zanjani ED, Ascensao JL, McGlave PB, Banisadre M, and Ash RC. 1981. Studies on the liver to kidney switch of erythropoietin production. J Clin Invest 67:1183-1188.
Zelko IN, and Folz RJ. 2005. Extracellular superoxide dismutase functions as a major repressor of hypoxia-induced erythropoietin gene expression. Endocrinology 146:332-340. 10.1210/en.2004-1007
Zhu XJ, Liu Y, Dai ZM, Zhang X, Yang X, Li Y, Qiu M, Fu J, Hsu W, Chen Y, and Zhang Z. 2014. BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet 10:e1004687. 10.1371/journal.pgen.1004687
Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, Pilc K, Suchanek R, Pierzchala K, Namyslowski G, Misiolek M, Sodowski K, Kato I, Kuwahara A, and Zabielski R. 2007. Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol 58 Suppl 1:53-64.
Table 1 Groups of signaling molecules
| Φ1 | Φ2 | Φ3 | |
| Heart Group | IGF | Ang | Mg |
| Spleen Group | ANP | aldosterone,
retinoic acid |
ghrelin |
| Lung Group | FGF7, VEGF | ascorbic acid | HIF |
| Kidney Group | calcitonin, PTHrP | Wnt | NO |
| Liver Group | EPO, HGF | renin, SOD, AKR | GSH |
Figure Legends
Figure 1. The interrelationships of five group of signaling molecules with organs. The small square indicates the closed-loop pathways of signaling molecules within the same group. Along the outer circle, the thick red arrow shows that the group of molecules assists the following organ, and it follows heart-spleen-lung-kidney-liver-heart arrangement. In the diagonal direction, the thick green arrow shows that the group of molecules assists the following organ, and it follows heart-lung-liver-spleen-kidney-heart arrangement. Arrow means assisting/increasing, solid circle means inhibiting/decreasing, and arrow and circle together indicate regulating.
Figure 2. The pathways of signaling molecules among the five groups of molecules. Along the outer circle, the pathways follow the order of heart-spleen-lung-kidney-liver-heart groups. In the diagonal arrangement, the pathways follow the order of heart-lung-liver-spleen-kidney-heart groups. Arrow means increasing, solid circle means decreasing, and arrow and circle together indicate regulating.
Figure 1. The interrelationships of five group of signaling molecules with organs. The small square indicates the closed-loop pathways of signaling molecules within the same group. Along the outer circle, the thick red arrow shows that the group of molecules assists the following organ, and it follows heart-spleen-lung-kidney-liver-heart arrangement. In the diagonal direction, the thick green arrow shows that the group of molecules assists the following organ, and it follows heart-lung-liver-spleen-kidney-heart arrangement. Arrow means assisting/increasing, solid circle means inhibiting/decreasing, and arrow and circle together indicate regulating.
Figure 2. The pathways of signaling molecules among the five groups of molecules. Along the outer circle, the pathways follow the order of heart-spleen-lung-kidney-liver-heart groups. In the diagonal arrangement, the pathways follow the order of heart-lung-liver-spleen-kidney-heart groups. Arrow means increasing, solid circle means decreasing, and arrow and circle together indicate regulating.
Document information
Published on 30/06/17
Submitted on 30/06/17
Licence: CC BY-NC-SA license
Share this document
claim authorship
Are you one of the authors of this document?