Summary
We report herein a case of an 80-year-old female patient who presented with hemosputum and a left chest tumor. Owing to her medical history and imaging findings, we strongly suspected chronic expanding hematoma (CEH). Arteries feeding the mass were embolized prior to surgery to control intraoperative bleeding. We successfully inhibited the massive bleeding from the wall of the tumor and performed surgery rapidly and with minimal blood loss. Thus, preoperative embolization followed by palliative therapy is effective for controlling a CEH both intra- and post-operatively and should be considered an alterative treatment, especially for patients unable to undergo complete removal.
Keywords
arterial embolization;chronic expanding hematoma;palliative surgery;thoracoplasty
1. Introduction
Chronic expanding hematoma (CEH) is a rare disease, and most patients have a history of surgery for tuberculosis.1 The usual treatment for CEH is complete surgical removal, because the CEH can have caused continuous bleeding from the capsule.2 Removal of the CEH often leads to massive intraoperative bleeding and disseminated intravascular coagulation (DIC).1 However, complete extirpation is limited to patients who can tolerate the operation. We encountered a patient with CEH with a decline in physical performance. Therefore, we performed palliative removal in combination with preoperative arterial embolization. In this article, we discuss the clinical implications of such treatments.
2. Case report
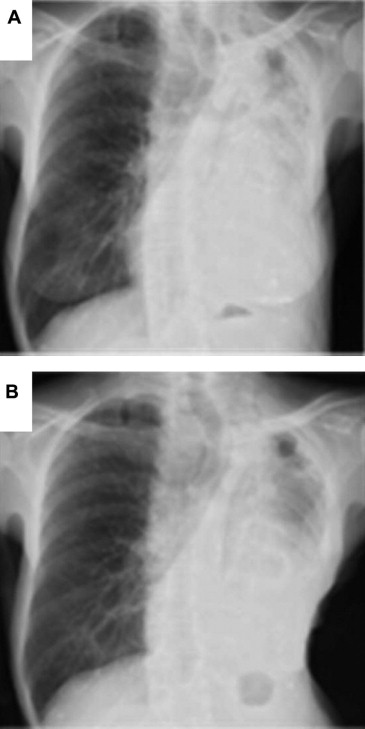
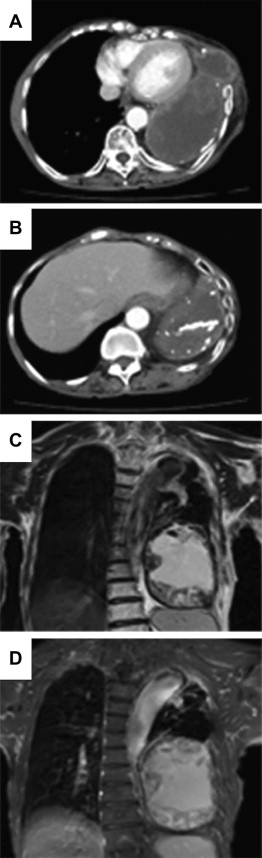
An 80-year-old Japanese female was referred to our department with hemosputum and a progressive mass on the left chest wall. She had received left thoracoplasty owing to tuberculosis at the age of 28. Her respiratory performance status was grade 2 according to the Hugh–Jones classification system. Chest X-ray revealed a huge mass shadow in the left field (Fig. 1A). Computed tomography (CT) revealed that a huge mass was present beyond the left thorax. The fifth–seventh ribs were penetrated by the progressive mass (Fig. 2A). Calcification was detected at the peripheral lesions of the mass (Fig. 2B). Magnetic resonance imaging (MRI) revealed an inhomogeneous mass containing fluid and solid components compressing the left lung (Fig. 2C and D). Hemosputum presented in the left main bronchus, and washing cytology showed neither malignancy nor tuberculosis bacilli. We strongly suspected that the patient had a CEH based on her past history and the imaging findings as described. She underwent surgery after we obtained her informed consent.
|
|
|
Figure 1. (A) Chest X-rays revealed a huge mass shadow in the left field and an infiltrative shadow in the left upper field; (B) chest X-ray on postoperative Day 22 showed that the heart was located in a more normal position and that the left upper lobe had expanded. |
|
|
|
Figure 2. (A) Chest CT revealed a huge mass beyond the left thorax; (B) calcification was detected at the peripheral lesions of the mass; (C) the mass revealed high signal intensity on T1-weighted images; (D) the mass revealed a mosaic of various signal intensities on partially T2-weighted images. |
We judged that complete removal would be difficult to achieve because of her advanced age and relatively poor general health. Therefore, arterial embolization in preparation for intraoperative bleeding prior to surgery was scheduled. Arteriography showed that the inferior phrenic, bronchial, internal thoracic, lateral thoracic, and intercostal arteries fed the mass. These arteries were embolized with gelform particles 1 day before the operation. We performed a left posterolateral thoracotomy through the lateral position. The tumor adhered completely to the pericardium and left diaphragm, with a tough capsule. The inner fluid in the CEH was aspirated to facilitate the surgery with stay sutures and to confirm that there was no persistent bleeding from the tumor after the embolization. Approximately 200 mL of dark-brown fluid was removed from the inner space.
Macroscopic observations showed that the resected mass contained a large encapsulated mass with necrotic tissue and hemorrhagic materials with calcification. We resected the mass with a tough capsule as completely as possible. Intraoperative frozen diagnosis of the mass indicated that it was compatible with CEH, with no malignancy. The surgery lasted only 135 minutes, and the total blood loss was 280 mL. Pathological examinations showed that the mass had dense hyalinization, calcification, focal inflamed granulation tissue with hematoma formation, and an organization representative of CEH.
The postoperative course of the patient was uneventful expect for mild delirium on postoperative Day 1. The defective chest wall was compressed by sand gall and gauze for 3 weeks to fit the thoracic cavity and to avoid fluid collection. The heart shifting to the right was improved, and the left upper lobe expanded well (Fig. 1b). She was discharged from the hospital on the 17th postoperative day. Up to 7 months postoperation, no relapse had occurred.
3. Discussion
It has been suggested that during the expansion of CEH, increased permeability of the vascular wall causing bleeding from dilated microvessels under the fibrous capsule can occur.3 Therefore, complete resection, including the capsule, is the preferred surgical procedure, as incomplete resection might result in a recurrence of the hematoma owing to intermittent episodes of bleeding, probably caused by respiratory motion and coughing.3 However, complete removal of the CEH requires highly invasive surgery, which leads to a long recovery period for patients, especially those with a decline in physical performance. Furthermore, the lung compressed by the CHE is often nonfunctional because it is destroyed by complete atelectasis and pneumonia. Therefore, an additional complete pneumonectomy is often necessary.4
Therefore, the complete removal of CEH and the optimal surgical procedure has remained a controversial issue. Recently, a few clinicians have reported the usefulness of preoperative embolization of the arteries feeding the CEH.5 ; 6 Moreover, new vascularization beneath the capsule and rigid adhesion to surrounding tissues often cause massive bleeding in the perioperative and postoperative periods. Thus, palliative procedures, such as removal of as much as practicable of both the inner substance and subcapsular lesion without whole-capsule excision, might be recommended because of the difficulty of surgical resection or the risk of possible complications. In this case, we performed careful removal in combination with preoperative arterial embolization as a palliative procedure. This strategy was safe and less invasive, and led to good recovery of a patient of advanced age.
In conclusion, we performed a palliative removal in combination with preoperative arterial embolization for a patient deemed to be unable to tolerate a complete removal. Although longer follow-up is needed, and additional cases will need to be observed, this strategy appears to represent an alterative treatment for CEH, especially for patients who are unable to tolerate complete removal. Future clinical studies, including the indications for embolization by angiographic signs of the blood supply to the tumor, and the optimal timing of embolization and surgery, are needed.
References
- 1 H. Uramoto, R. Nakanishi, R. Eifuku, et al.; Chronic expanding hematoma in the chest; J Cardiovasc Surg, 41 (2000), pp. 143–146
- 2 C.L. Roper, J.D. Cooper; Chronic expanding hematoma of the thorax; J Thorac Cardiovasc Surgeon, 122 (2001), pp. 1046–1048
- 3 E.L. Labadie, D. Glover; Physiopathogenesis of subdural hematomas; J Neurosurg, 45 (1976), pp. 382–392
- 4 S. Endo, T. Hasegawa, Y. Sato, Y. Sohara; Thoraco-pleuropneumonectomy for rupture of a huge chronic hematoma of the thorax; Jpn J Thorac Cardiovasc Surg, 53 (2005), pp. 162–164
- 5 M. Kuriyama, N. Mitsukawa; Preoperative arterial embolisations of a huge, chronic, expanding haematoma to inhibit intra-operative massive bleeding: a case report; J Plast Reconstr Aesthet Surg, 62 (2009), pp. 203–205
- 6 T. Sakuragi, Y. Sakao, M. Natsuaki, T. Itoh; Arterial embolization to preoperatively manage pulmonary disease associated with inflammation; Jpn J Thorac Cardiovasc Surg, 50 (2002), pp. 125–128
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?