Abstract
Background
The diagnosis of thrombus in the left atrium in patients with persistent atrial fibrillation (AF) and may be inconsistent because of variability in thrombus morphology. In some cases it is challenging and requires unusual approach. New Doppler-derived methods might be helpful to identify such thrombi. We evaluated quantitative differences in mechanical function of the left atrial appendage (LAA) basal segments using tissue Doppler imaging (TDI)and speckle tracking echocardiography (STE) in patients with non-valvular AF with and without LAA thrombus and compared them with SR patients.
Methods
A total of 80 patients with normal left ventricular ejection fraction underwent transesophageal echocardiography (40 patients with SR and 40 patients with AF on oral anticoagulants including patients with LAA thrombus). We analyzed the basal segments of LAA including left lateral ridge (LLR) and baso-medial appendage segment (BMAS). Quantitative analysis was used to calculate peak velocity, average velocity, strain, strain rate and deformation.
Results
In patients with AF the lower LLR strain rate was the sole new STE significant parameter differentiating patients with and without LAA thrombi: − 0.9(− 1.2; − 0.1)s− 1 vs. − 1.6(− 1.9; − 1.3)s− 1, (p = 0.004). Additionally, patients in SR demonstrated significantly better peak velocity, average velocity, strain, strain rate and deformation than those with AF (p < 0.001).
Conclusions
LLR appeared to be an appropriate site for measuring Doppler derived parameters. It is possible that the strain rate in LLR area may be a novel parameter correlating with the presence of thrombus in patients with AF.
Keywords
Strain;Strain rate;Appendage;Thrombus;Sinus rhythm;Atrial fibrillation
1. Introduction
Atrial mechanical activity plays a significant role in the disease course in many patients. Mechanical efficacy of the left atrial appendage LAA is considered an important factor in A-type natriuretic peptide (ANP) production, which stabilizes blood volume and pressure in the left atrium (LA) and may indirectly affect cardiac output [1]. The LAA is the most frequent cardiac source of thrombi in patients with AF [2]; [3]; [4] ; [5]. Also, the LAA flow velocity has been used to assess the propensity for thrombus formation [6]. Parameters of LA mechanics were initially directly evaluated by tissue Doppler imaging (TDI) and then by speckle tracking echocardiography (STE). In recent years atrial septal and wall motion have been most frequently analyzed by transthoracic echocardiography (TTE) [7]; [8]; [9]; [10]; [11] ; [12]. Quantitative analysis by strain imaging (SI) and strain rate imaging (SRI) most frequently includes evaluation of echocardiographic indices of left atrial filling and relaxation [13]; [14] ; [15]. Quantification of left atrial systolic function is another important component that describes atrial mechanics [10]; [11]; [12] ; [16]. In patients with AF atrial contraction is disorganized due to asynchronous atrial muscular activation [4]; [17] ; [18]. The LAA as a blind-ended pouch with the thinnest walls in the heart is most susceptible to consequences of abnormal atrial contraction which may lead to thrombus formation and arterial embolism with ischemic stroke as the most severe clinical presentation [2]; [3]; [19]; [20] ; [21]. In some cases thrombus formation in LAA is very likely but high bleeding risk requires consideration of alternative invasive treatment options [1]; [22]; [23] ; [24]. In such cases the detection of LAA thrombus was based on 2D or sometimes 3D imaging. An additional clue was reduced velocity flow in the LAA. So far no STE-based outcome prediction parameters have been proposed for use in clinical practice. Unfortunately, the possibilities of direct LAA assessment are very limited, especially by echocardiography. The LAA free wall thickness ranges from 1 to 2 mm, therefore it appears extremely difficult to be visualized for mechanical evaluation with currently available technology [25]. However, basal segments of the LAA such as left lateral ridge (LLR) and baso-medial appendage segment (BMAS) near the vestibule of the mitral valve are composed of over 4 mm thick myocardial tissue, which may be suitable for TEE assessment [26] ; [27]. This portion of the myocardium is thought to reflect to some degree LAA mechanical activity (Fig. 1). In patients in sinus rhythm (SR) LA contraction is delayed by about 50–60 ms from the onset of the P wave and lasts until the atrioventricular valves are closed [28]; [29] ; [30]. The electromechanical activation of the entire left atrium in healthy subjects lasts about 20–30 ms [10] ; [28]. In contrast, in patients with AF foci of reentrant electrical waves in the LA wall, including the LAA are found in the entire cardiac cycle between QRS complexes. An interval of 150 ms immediately before the QRS complex may be a suitable window for measuring systolic activity of the LA during AF. In some cases LAA thrombi may develop in spite of appropriate anticoagulation treatment. Therefore, medical treatment effects should be verified by TEE, mainly because the difference between the solid and sludge morphology of thrombi can be ambiguous. Moreover, the inside of the LAA is not always easily available for clot exclusion. Velocity measurements in the LAA could be helpful but it is only an indirect parameter and requires additional methods to confirm the diagnosis. Another parameter of thrombus detection would be of crucial importance.
|
|
|
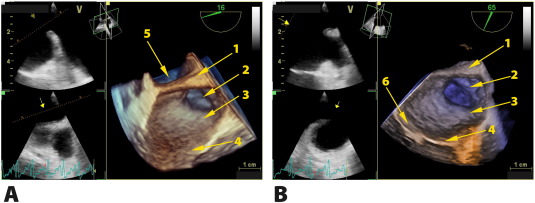
Fig. 1. Panel A. TEE: 3D superior view into left atrial cavity and LAA. 1-left lateral ridge, 2-left atrium appendage, 3- baso-medial appendage segment, 4-mitral posterior leaflet, 5-left common pulmonary veins. Panel B. TEE: 3D anterior view into left atrial cavity and LAA. 1-left lateral ridge, 2-left atrium appendage, 3-baso-medial appendage segment, 4-mitral posterior leaflet, 6-mitral anterior leaflet. |
2. Methods
2.1. Patients
A total of 80 patients aged from 18 to 80 years were included (40 with SR and 40 with AF for at least 3 months). The exclusion criteria were as follows: valvular heart defect (any form), rhythm other than SR or AF, prosthetic valve, the presence of shunt except patent foramen ovale, pulmonary arterial hypertension, any cardiac device implanted, all classes of heart failure and EF < 55%, arterial hypertension, atrioventricular conduction abnormalities, sinoatrial blocks and esophageal pouches or other abnormalities that make TEE difficult. ECG recording prior to TEE was performed in each patient to confirm the rhythm eligible for analysis. All patients had their height and weight measured to calculate body surface area (BSA) and body mass index (BMI). Anticoagulants were administered with INR maintained over 2.0 in patients treated with vitamin K antagonist or with novel oral anticoagulant dose titrated according to the glomerular filtration rate, respectively. This group included eight patients meeting the inclusion criteria and having LAA thrombi despite appropriate anticoagulation therapy. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki Informed consent was obtained from all patients. The study was approved by the Bioethics Committee of the Local Chamber of Physicians.
2.2. Transthoracic echocardiography
All patients underwent TTE for confirmation of study inclusion criteria using the General Electric Vivid E9 device (version 112; upgrade BT12) and a sector array M5S (2.5–3.5 MHz) transducer. The following parameters were analyzed: LA diameter, LA area, left atrial volume index (LAVI) and left ventricular ejection fraction (LVEF). The size of left atrial cavity was measured at end diastole in the parasternal long axis view (PLAX) and then indexed to the patients BSA. LA area was measured in the apical four chamber and the apical two chamber views at end diastole and then LA volume was calculated. The left ventricular ejection fraction was calculated with the biplane Simpsons method using the apical four chamber and the apical two chamber views.
2.3. Transesophageal echocardiography
TEE was performed using the same device and a TEE omniplane 6Tc (6–8 MHz) transducer. LAA images were obtained in the long axis view at the level including the appendage itself, LA cavity, left upper pulmonary vein draining into the LA, mitral leaflets and basal segments of the left ventricle. Moreover, thickness and length of LLR in the long axis of LAA were measured. Flow velocity in the LAA was measured. The view was obtained by positioning the transducer in a range of 50–110°. The LAA was also inspected for the presence of thrombi. HR was recorded in all patients.
2.4. Tissue Doppler imaging
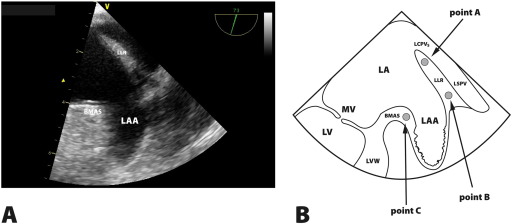
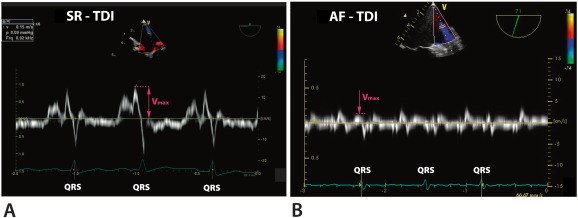
TDI was performed at a frame rate of over 100 frames per second (fps). During a TEE procedure the patients ECG was recorded. TDI echocardiograms included potential sites for STE analysis within the region of interest and at least three QRS complexes. Measurements were made within LLR near its top (area A) and near its bottom (area B). BMAS was the third point of measurement (area C) (Fig. 2). In order to acquire images at fps > 100 in some cases it was not possible to visualize simultaneously all measurement areas, i.e. A, B and C. For this reason the region of interest (ROI) showing A and B and separately the ROI that included C were selected for analysis. The peak atrial velocity in the middle of LLR was also measured using pulse-wave TDI (PW-TDI) (Fig. 3). This measurement served as the basis for calculating TDI-derived peak velocities. The beam-to-flow deviation in PW-TDI was established. Peak velocities in patients with SR and AF were measured in the same beam positions. In patients with SR and AF peak positive LLR values were measured at 150 ms before the QRS complex. In AF patients measurements were performed when the duration between QRS complexes exceeded 800–900 ms.
|
|
|
Fig. 2. Panel A. LAA structure with its basic segments in TEE (2D). LLR-left lateral ridge, BMAS-baso-medial appendage segment. Panel B. Left atrial appendage longitudinal view in transesophageal echocardiography and regions of interests A, B and C. MVC-mitral valve closure, LA-left atrium, LAA-left atrium appendage, MV-mitral valve, LV-left ventricle, LVW-left ventricle wall, VMV-mitral valve vestibule, LLR-left lateral ridge, LSPV-left superior pulmonary vein, LCPV-left common pulmonary veins. |
|
|
|
Fig. 3. Left lateral ridge maximal velocity in pulse wave tissue Doppler imaging in patient with sinus rhythm (SR, Panel A) and atrial fibrillation (AF, Panel B). |
2.5. Post-processing analysis
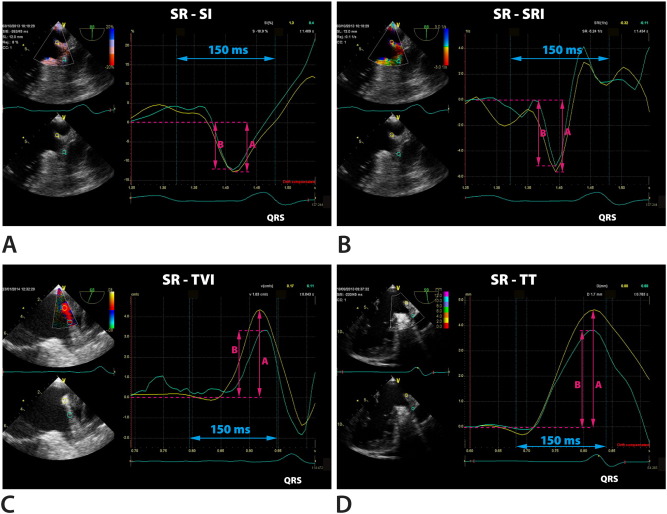
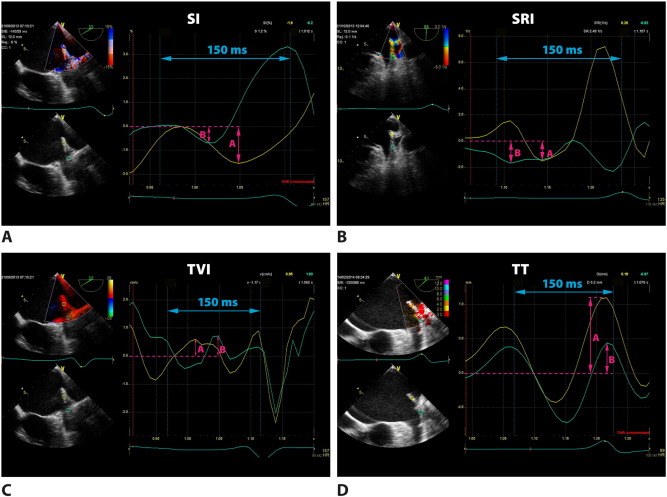
Off-line analysis was performed with the Q-Analysis software (General Electric EchoPac workstation, version 112; upgrade BT12). The TDI recording at 150 ms before the QRS complex was selected for analysis of the systolic phase in all segments. In TEE in the view enabling visualization of the LAA in the longitudinal axis the TDI sector width was selected that included LLR or BMAS. In patients in SR a fragment of the wave at least at 800–900 ms between the QRS complexes before was chosen for analysis. In patients with AF, due to the lack of P wave the TDI sector width was selected in the same period between QRS complexes. Then we analyzed average tissue velocity imaging (TVI), strain imaging (SI), strain rate imaging (SRI) and deformation (tissue tracking, TT) in each area A, B and C in all patients. Atrial systolic parameters could be measured in the late diastolic phase of the mechanical ventricular cycle. Therefore such systolic strain reflecting the extent of myocardial fiber shortening was evaluated as the lowest value below the baseline. For correct presentation the tracking start was placed at the top of the P wave, whereas the tracking end immediately after the QRS complex. Similarly, in SRI we analyzed the lowest value at 150 ms before the QRS complex. The last parameter to measure was deformation TT, showing the highest deflection of the curve at the same time interval. Then we compared peak values obtained in PW-TDI as well as TVI, SI, SRI and TT measured at A, B and C in groups with SR and AF. In patients with AF those with and without LAA thrombus were analyzed separately. Fig. 4 ; Fig. 5 show an example of measurements at A, B and C in patients in SR and AF, respectively. Differences in TVI, SI, SRI and TT between A, B and C in patients in SR and AF were then submitted for statistical analysis. Additional analyses of subgroups with and without thrombus in LAA were also made.
|
|
|
Fig. 4. Q-analysis in patient with sinus rhythm (SR). A sample of measurements (arrows) in the area of left lateral ridge in region A (yellow curves) and in region B (green curves). Panel A: strain imaging (SI). Panel B: strain rate imaging (SRI). Panel C: tissue velocity imaging (TVI). Panel D: tissue tracking (TT). |
|
|
|
Fig. 5. Q-analysis in patient with atrial fibrillation (AF). A sample of measurements (arrows) in the area of left lateral ridge in region A (yellow curves) and in region B (green curves). Panel A: strain imaging (SI). Panel B: strain rate imaging (SRI). Panel C: tissue velocity imaging (TVI). Panel D: tissue tracking (TT). |
2.6. Statistical analysis
Data were evaluated with Statistica 10 software. For continuous variables the distributions were assessed using the Shapiro-Wilk test. If variables had normal distributions they were presented as mean values ± standard deviation (SD), otherwise as medians and the lower and upper quantile (Q1–Q3). The categorical data were presented as the percentage and number of patients. Differences between two independent groups (SR vs. FA) for continuous variables were analyzed using a parametric t-test or Mann–Whitney test, as applicable. Comparisons between the three points (A,B,C) in relation to TEE-derived parameters were performed using nonparametric ANOVA (the Friedman test). In case of a statistically significant difference multiple comparisons were performed using the Bonferroni correction. A p value of < 0.05 for two-sided tests was accepted as statistically significant.
3. Results
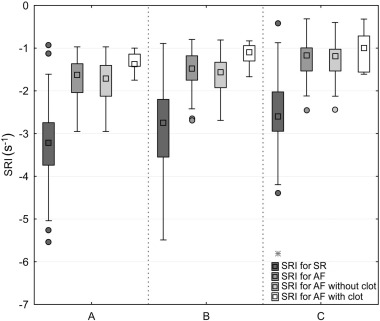
Patient subgroups in the present study were similar with respect to the proportion of men and women, their BMI and BSA. Patients with AF were slightly older and significantly more often had an increased heart rate than those in SR (p = 0.068). In the entire study population of 80 patients TTE revealed no significant differences in left atrial size and volume and no differences in the remaining parameters, including the left ventricular ejection fraction. We demonstrated significant differences in maximal systolic PW velocity in the LAA between SR and AF patients (p < 0.001) and between both AF subgroups (p < 0.001). Thrombi in the AF subgroup were more frequent in women than men (p = 0.043). Moreover, the remaining physical and TEE parameters in AF patients with and without thrombi were similar, too. In PW-TDI peak velocities at LLR and BMAS, differentiating patients in SR and AF (p < 0.001) did not show any significant differences in AF subjects with and without LAA thrombi. Analysis of acoustic markers demonstrated significant differences between patients in SR and AF with respect to all study parameters, i.e. average velocity (p < 0.001), strain (p < 0.001), strain rate (p < 0.001) and deformation (p < 0.001) in favor of SR patients (Table 1). In contrast, in patients with known thrombi the SRI value was lower, especially at LLR top (A) (p = 0.016) and bottom (B) (p = 0.004) (Table 2 and Fig. 6). Furthermore, the value of deformation in those places at LLR was lower, too but the differences were less significant (p = 0.068, p = 0.042). The sole significant parameter differentiating patients with AF from those without LAA thrombi was the lower LLR strain rate (B) (p = 0.004).
| Sinus rhythm n = 40 | Atrial fibrillation n = 40 | p value | |
|---|---|---|---|
| Age, years | 57.5 ± 13.3 | 62.5 ± 6.7 | 0.038 |
| Males, % | 55 | 68 | 0.251 |
| BMI, kg/m2 | 29.0 ± 3.0 | 29.8 ± 2.8 | 0.235 |
| BSA, m2 | 1.9 ± 0.2 | 2.0 ± 0.1 | 0.095 |
| HR, min− 1 | 80.7 ± 14.1 | 90.2 ± 10.4 | 0.068 |
| LA, mm/m2 | 23.5 ± 4.2 | 25.3 ± 2.3 | 0.075 |
| LA area, cm2 | 23 (22–24) | 26 (23–28) | 0.064 |
| LAVI, cm/m2 | 26 (24–28) | 29 (26–32) | 0.081 |
| LVEF, % | 57.7 ± 5.9 | 55.7 ± 5.3 | 0.120 |
| LLR width, mm | 4.6 ± 0.6 | 4.3 ± 0.2 | 0.581 |
| LLR length, mm | 18 ± 3.6 | 20 ± 4.5 | 0.283 |
| PW-neck LLA, cm/s | 85.1 ± 25.2 | 33.05 ± 12.6 | < 0.001 |
| TDI-PW angle, ° | 30.0 (17.5–42.5) | 30.0 (20.0–40.0) | 0.749 |
| TDI-PW, cm/s | 11 (8.5–13.0) | 4 (3–4) | < 0.001 |
| V-A, cm/s | 6.7 (5.9–7.4) | 1.5 (1.0–2.3) | < 0.001 |
| V-B, cm/s | 5.2 (4.3–6.3) | 1.6 (1.0–2.3) | < 0.001 |
| V-C, cm/s | 6.0 (4.7–6.7) | 1.5 (0.8–2.0) | < 0.001 |
| SI-A, % | − 12.4 (− 13.3; − 9.8) | − 3.7 (− 4.7; − 2.9) | < 0.001 |
| SI-B, % | − 11.2 (− 12.3; − 9.9) | − 3.2 (− 4.0; − 2.4) | < 0.001 |
| SI-C, % | − 9.9 (− 11.4; − 8.9) | − 2.6 (− 3.8; − 2.3) | < 0.001 |
| SRI-A, s− 1 | − 3.2 (− 3.7; − 2.7) | − 1.6 (− 2.0; − 1.4) | < 0.001 |
| SRI-B, s− 1 | − 2.8 (− 3.6; − 2.2) | − 1. 5 (− 1.8; − 1.2) | < 0.001 |
| SRI-C, s− 1 | − 2.6 (− 2.9; − 2.0) | − 1.2 (− 1.5; − 1.0) | < 0.001 |
| TT-A, mm | 4.3 (3.3–4.8) | 1.4 (1.1–1.9) | < 0.001 |
| TT-B, mm | 3.6 (2.7–4.4) | 1.4 (0.9–1.7) | < 0.001 |
| TT-C, mm | 3. 5 (3.1–4.2) | 1.3 (1.0–1.9) | < 0.001 |
Data are expressed as mean ± standard deviation or mean and interquartile range. BMI – body mass index, BSA – body surface area, HR – heart rate, LA – left atrium, LAVI – left atrial volume index, LVEF – left ventricular ejection fraction, TDI-PW – tissue Doppler imaging-pulse wave, V – velocity, SI – strain imaging, SRI – strain rate imaging, TT – tissue tracking, A, B and C – region of interest as depicted in Fig. 2B (also see Methods).
| Without thrombus n = 32 | With thrombus n = 8 | p value | |
|---|---|---|---|
| Age, years | 63.5 (60–67) | 59 (56.5–68.5) | 0.589 |
| Males, % | 75 | 38 | 0.043 |
| BMI, kg/m2 | 29.7 (27.9–31.8) | 28.5 (27.8–29.4) | 0.279 |
| BSA, m2 | 2 (1.9–2.1) | 2 (1.8–2.1) | 0.589 |
| HR, min− 1 | 88.3 ± 12.5 | 93.6 ± 10.4 | 0.132 |
| LA, mm/m2 | 25.3 ± 2.3 | 26.2 ± 3.3 | 0.253 |
| LA area, cm2 | 26 (23–28) | 26 (24–28) | 0.682 |
| LAVI, cm/m2 | 29 (26–32) | 29 (27–31) | 0.872 |
| LLR width, mm | 4.5 ± 0.4 | 4.2 ± 0.1 | 0.863 |
| LLR length, mm | 20 ± 1.8 | 19 ± 2.9 | 0.792 |
| PW-neck LLA, cm/s | 33.4 ± 18.1 | 18.2 ± 8.5 | < 0.001 |
| LVEF, % | 55.5 (50.5–59.5) | 58.5 (53–61.5) | 0.230 |
| TDI-PW angle, ° | 25 (15–35) | 37.5 (27.5–42.5) | 0.079 |
| TDI-PW, cm/s | 4 (3–4) | 3.8 (2.5–4) | 0.310 |
| V-A, cm/s | 1.5 (1.2–2.3) | 1.3 (0.6–2.4) | 0.488 |
| V-B, cm/s | 1.7 (1.1–2.4) | 1.1 (0.5–1.9) | 0.108 |
| V-C, cm/s | 1.5 (0.9–1.9) | 1.5 (0.7–2.1) | 0.933 |
| SI-A, % | − 3.6 (− 4.9; − 2.9) | − 4 (− 4.4; − 2.8) | 0.710 |
| SI-B, % | − 3 (− 4.0; − 2.4) | − 3.2 (− 4.0; − 2.6) | 0.488 |
| SI-C, % | − 2.6 (− 3.5; − 2.2) | − 2.6 (− 3.6; − 2.1) | 0.987 |
| SRI-A, s− 1 | − 1.7 (− 2.1; − 1.4) | − 1.4 (− 1.4; − 1.1) | 0.016 |
| SRI-B, s− 1 | − 1.6 (− 1.9; − 1.3) | − 0.9 (− 1.2; − 0.1) | 0.004 |
| SRI-C, s− 1 | − 1.2 (− 1.5; − 1.0) | − 1 (− 1.6; − 0.7) | 0.327 |
| TT-A, mm | 1.5 (1.1–2.0) | 1.1 (0.7–1.5) | 0.068 |
| TT-B, mm | 1.5 (1.1–1.8) | 0.96 (0.5–1.4) | 0.042 |
| TT-C, mm | 1.2 (1–1.9) | 1.4 (0.9–1.8) | 0.892 |
Abbreviations as in Table 1.
|
|
|
Fig. 6. Strain rate imaging (SRI) in regions A, B, and C in patients with sinus rhythm (SR), atrial fibrillation (AF) and in subgroups with and without thrombus. |
4. Discussion
The left atrial appendage is the most frequent site of thrombus formation due to stagnation of blood flow. Additionally, LAA segmental anatomy, which is most commonly seen, increases the likelihood of thrombi [31]; [32]; [33] ; [34]. Di Baise et al. demonstrated that non-chicken wing LAA morphology may precipitate thrombus formation [35]. Left atrial appendage blood flow velocity is another echocardiographic parameter in assessment of LAA function. It is recorded even in patients with AF, but only reduced blood flow below 25 cm/s may be associated with thrombus formation [36]; [37] ; [38]. In contrast, in patients in sinus rhythm blood flow velocity in the LAA is much higher than in those with AF [38]; [39] ; [40]. Direct imaging shows various types of morphologically altered blood such as spontaneous echocardiographic contrast or echogenic blood, sludge or organized thrombus [33]; [36]; [37] ; [41]. Visualization of morphologically less organized clots in the LAA is challenging, therefore all possible parameters should be taken into account when differentiating blood clots. Providencia et al. provided a review of various studies in this area [38]. Assessment of muscle deformation includes several parameters such as velocity, strain, strain rate and displacement. However, strain and strain rate are the most important measurements. Analysis of systolic deformation in patients with atrial fibrillation is rather difficult because the magnitude of strain is very small and requires extreme precision in the assessment of TDI-derived images. It applies both to strain and strain rate at late diastole. In patients in sinus rhythm the measurements are quite easy because left atrial depolarization leading to atrial contraction is clearly visible as the P wave in the ECG. Some investigators estimate that in patients in sinus rhythm the left atrial free wall strain at late diastole ranges from − 15 to − 30%, whereas the strain rate from − 3 s− 1 to − 5 s− 1[18] ; [42]. In the present study we assessed basal LAA segments which were slightly smaller (strain − 12% and strain rate ok. − 3 s− 1). On the other hand, other investigators obtained similar results [28]. Unfortunately, only some of the investigators decided to undertake a challenging analysis of LA deformation at late diastole in patients with atrial fibrillation. The results obtained by Schneider et al. showed that left atrial strain rate at late diastole in patients in sinus rhythm and sustained atrial fibrillation was similar to the findings in the current study, although other atrial muscle segments had been assessed. Strain rate for patients in sinus rhythm was − 2,9 s− 1 and with AF − 1,9s− 1[28]. Similar strain rate values at late diastole in patients with sustained atrial fibrillation were reported by other investigators [42]. Unfortunately, none of them assessed deformation parameters in patients with LAA thrombus. In the clinical practice, assessment of the risk of thrombus formation in the LAA based on measurements of atrial muscle deformity, which appear to best reflect LA and LAA mechanical function, is not common. A large amount of data based on STE, TDI and 2D images of atrial muscle demonstrates significant effects of various clinical states on atrial mechanical function [43] ; [44]. Some of the investigators made an attempt to compare LA deformation measurements in patients with non-rheumatic atrial fibrillation based on the CHADS2 Risk Criteria [44]. Obokata et al. demonstrated that a significant decrease in peak positive strain in the reservoir phase of LA significantly correlated with the high CHA2DS2VASc score, however late diastole was excluded from analysis [45]. The results obtained by Sasaki et al. and other investigators show that the peak positive strain in the filling phase (the reservoir phase) below 19%, even 15.5% in patients with AF may be associated with a high risk of systemic embolus and correlates with the high CHA2DS2VASc score [45] ; [46]. Despite the predictive value of the reduced peak positive strain of LA in the filling phase in patients with atrial fibrillation it appears that there are other technical possibilities of expanding diagnostic options to include other parameters of mechanical contraction in patients with this arrhythmia. The study of Limantoro et al. demonstrated that atrial fibrillatory wall motion velocity can be used to evaluate atrial function [47]. The investigators evaluated the amplitude of velocity and cycle length of AF wave between the end of E' and start of S' waves obtained from the lateral wall of the LA in the basal segment, which was 1 cm/s for most patients, however only when the heart rate was below 100 bpm. In the current study the mean tissue velocity in the basal LAA segments in patients with AF was about 1.5 cm/s being several times lower than in patients in sinus rhythm. Bearing in mind a possibility of evaluating wall motion velocity we measured the remaining parameters of systolic LA deformation. Although there are few similar analyses in the literature, we can compare the findings of single studies on this issue. One of the first investigators who studied tissue velocity was Tamura et al. They analyzed the left atrial appendage wall velocity (LAAWV) obtained by TDI from a modified TTE projection showing the LAA. The LAVWV < 8.7 cm/s was found to be a predictor of stroke in patients with AF [48] ; [49]. In the current study we also used TDI only to measure tissue velocity in the LLR. For patients in sinus rhythm it was about 11 cm/s and for those with AF about 4 cm/s. However, STE provided much lower measurements for both basal segments (for SR about 6 cm/s and for AF about 1.5 cm/s). Unfortunately, in patients with AF and LAA thrombus the wall velocity was the parameter that would differentiate those without LAA clot (4 cm/s vs. 3.8 cm/s). The left atrial appendage is made of a very thin muscle wall, therefore the currently available tools cannot be used reliably for deformation assessment [26] ; [50]. However, the opposite basal segments of the LAA have a different wall morphology, the muscular layer is significantly thicker, making the assessment feasible [27]; [33] ; [51]. Some investigators demonstrated the anatomic evaluation of the LLR dimensions with its length and thickness reaching 25–27 mm and 4.5–5.5 mm, respectively [26,26]. Although it was a small amount of tissue, the reduction of the area of interest to 4 mm provided a possibility of making an attempt to measure at least longitudinal deformation. In the current study the LLR dimensions were similar: length 18–20 mm and width 4.5 mm. Left atrial appendage flow velocity obtained using PW Doppler is another parameter that can help determine the factors that trigger thrombus formation in the LAA. The reduced LAA flow velocity to below 25 cm/s has been shown to increase the risk of developing blood clots [52]. In the present study LAA flow velocity obtained using PW Doppler significantly differentiated patients in sinus rhythm from those with atrial fibrillation (p < 0.001) similar to the significantly reduced blood flow velocity in patients with LAA thrombus, which confirmed only the available results. Calemi et al. demonstrated that the morphology of the left atrial appendage, especially multi-lobar atrial appendage may favor blood stasis [4]. However, this assessment is possible only when using other imaging tools such as CT or NMR [27]. Providentia et al. used TTE in patients with LAA sludge who were not receiving anticoagulation treatment and demonstrated that the peak negative strain rate was below − 1.07 ± 0.22 s− 1 m at early diastole (conduit phase) and could serve as an independent predictive factor [7]. At the same time, in patients with AF the peak negative strain rate was − 1.45 ± 0.42 s− 1. However, strain rate at early diastole corresponds to the E wave of mitral inflow and is dependent on left ventricular diastolic function [53]. In the current study we measured strain rate at late diastole based on TEE images. The values of LLR and BMAS were similar, however in patients with LAA thrombus the value of LASRA (ƐA) was smaller i.e. − 0.9 (− 1.2; − 0.1) s− 1 vs. − 1.6 (− 1.9; − 1.3) s− 1 (p = 0.004). Possibly, the imaging of the atrial muscle with the TEE transducer located at a smaller distance from the tissue provided a possibility of obtaining more precise measurements. However, the most difficult part of our analysis was deformation assessment at late diastole.
For patients in sinus rhythm an interval of 150 ms right before the QRS complex in the ECG was definitely late diastole corresponding to mechanical contraction. In contrast, late diastole is diminished and difficult, however possible to assess in patients with AF. Assessment of deformation parameters in the basal segment, but only the medial LAA segment in patients with AF before and after electrical cardioversion has already been described by Kaya et al., but only with respect to reservoir phase, and strain and strain rate in passive early diastolic (conduit) phase [54]. The investigators did not take into account end-diastolic phase (pump) parameters, assessed in the current study. In our patients in sinus rhythm and with atrial fibrillation with and without LAA thrombus, analogically as for LA wall and atrial septum we analyzed all four parameters related to deformation at late diastole: velocity, strain, strain rate and deformation. Velocity, strain and strain rate values were similar to those obtained by other investigators, however in patients with AF and LAA thrombus there were significant differences in strain rate which was below 1 s− 1.
4.1. Study limitations
The main limitations of the study result from the fact that there are no analyses of STE-derived parameters regarding basal LAA segments except the analysis by Kaya et al. It is only possible to compare them with parameters relating to the LA wall. Secondly, STE technique in TEE study has certain limitations resulting from technical possibilities of image analysis systems. For this reason using the Vivid E9 system it is not possible to carry out direct analysis of distal segments of the LAA, with the wall thickness being 1–2 mm. Equally difficult was the positioning of the sample (in segments 4 mm thick) because most probably multidirectional movement of these segments (not only longitudinal) often caused displacement of the sampling area beyond atrial muscle. The positioning of STE sampling area is a tedious process but necessary to eliminate artifacts making post-processing analysis difficult. Fourthly, because of differences in the construction of ultrasound transducers at the tip of TEE probes the STE-derived measurements cannot be of the same quality as in TTE examinations. It applies to TEE transducers range of frequencies which in the Vivid E9 system slightly exceeds 100, even for the smallest angle in the TDI range. Furthermore, a possible TDI angle of recording using the TEE probe cannot be so narrow as that in TTE examination.
5. Conclusions
The new techniques of tracking the acoustic markers provide a valid information regarding mechanical properties of basal LAA segments. The atrial mechanical activity can be indirectly assessed from strain and strain rate of basal LAA segments. We demonstrated that TEE-derived parameters such as average velocity, strain, strain rate and displacement were significantly better in patients with SR than in those with AF. Mechanical parameters of basal LAA segments indicate that LLR is an appropriate site of measurement as it is the part of left atrium and simultaneously forms the part of LAA base, and importantly, it has appropriate thickness. It is possible that due to these morphological properties the LLR may be the site where we obtained a result correlating with the presence of thrombus in patients with AF with respect to such a sensitive parameter as strain rate (SRI).
Conflict of interests
None declared.
References
- [1] G. Di Salvo, P. Caso, R. Lo Piccolo, et al.; Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation — a color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study; Circulation, 112 (2005), pp. 387–395
- [2] T. Cianciulli, M. Saccheri, J. Lax, A. Bermann, D. Ferreiro; Two-dimensional speckle tracking echocardiography for the assessment of atrial function; World J. Cardiol., 2 (2010), pp. 163–170
- [3] L. Thomas, T. Mckay, K. Byth, T. Marwick; Abnormalities of left atrial function after cardioversion: an atrial strain rate study; Heart, 93 (2007), pp. 89–95
- [4] M. Cameli, M. Caputo, S. Mondillo, et al.; Feasibility and reference values of left atrial longitudinal by two-dimensional speckle tracking; Cardiovasc. Ultrasound, 7 (2009), p. 6
- [5] G. Di Salvo, M. Drago, G. Pacileo, et al.; Atrial function after surgical and percutaneous closure of atrial sepatal defect: a strain rate study imaging study; J. Am. Soc. Echocardiogr., 18 (2005), pp. 930–933
- [6] M. Quintana, P. Lindell, S.K. Saha, et al.; Assessment of atrial regional and global electromechanical function by tissue velocity echocardiography: a feasibility study on health individuals; Cardiovasc. Ultrasound, 3 (2005), p. 4
- [7] R. Providencia, A. Faustino, M.J. Ferreria, et al.; Evaluation of left atrial deformation to predict atrial stasis in patients with non-valvular atrial fibrillation — a pilot study; Cardiovasc. Ultrasound, 11 (2013), p. 44
- [8] R. Vianna-Pinton, C.A. Moreno, C.M. Baxter, K.S. Lee, T.S. Tsang, C.P. Appelton; Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects; J. Am. Soc. Echocardiogr., 22 (2009), pp. 299–305
- [9] Z. Jing, C. Jianchang, X. Weiting, G. Lan, F. Shaikh, W. Yanni; Comparision of left atrial function in healthy individuals versus patients with non-ST-segment evevation myocardial infarction using two-dimensional speckle tracking echocardiography; Cardiovasc. J. Afr., 24 (2013), pp. 154–160
- [10] A. D'Andrea, P. Caso, S. Romano, et al.; Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischaemic dilated cardiomyopathy: a two-dimensional speckle strain study; Int. J. Cardiol., 132 (2009), pp. 354–363
- [11] R. Ancona, S.C. Pinto, P. Caso, et al.; Left atrium by echocardiography in clinical practice: from conventional methods to new echocardiographic techniques; Sci. World J., 2014 (2014), p. 451042
- [12] A. D'Andrea, G. De Corato, R. Scarafile, et al.; Left atrial myocardial function in either physiological left ventricular hypertrophy: two-dimensional speckle strain study; Br. J. Sports Med., 42 (2008), pp. 696–702
- [13] A. Shaikh, A. Maan, U.A. Khan, et al.; Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: a prospective study; Cardiovasc. Ultrasound, 10 (2012), p. 48
- [14] M.C. Todaro, I. Choudhuri, M. Belohlavek, et al.; New echocardiographic techniques for evaluation of left atrial mechanisms; Eur. Heart J. Cardiovasc. Imaging, 13 (2012), pp. 973–984
- [15] T.S. Kim, H.J. Youn; Role of echocardiography in atrial fibrillation; J. Cardiovasc. Ultrason., 19 (2011), pp. 51–61
- [16] M. Cameli, M. Lisi, F.M. Righini, S. Mondillo; Novel echocardiographic techniques to assess left atrial size, anatomy and function; Cardiovasc. Ultrasound, 10 (2012), pp. 1–13
- [17] V. Markides, R.J. Schilling, S.Y. Ho, A.W. Chow, D.W. Davies, N.S. Peters; Characterization of left atrial activation in the intact human heart; Circulation, 107 (2003), pp. 733–739
- [18] C. Sirbu, L. Herbots, J. D'hooge, P. Claus, A. Marciniak, T. Langeland; Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in normal subjects; Eur. J. Echocardiogr., 7 (2006), pp. 199–208
- [19] A. Dabrowska-Kugacka, E. Lewicka-Nowak, P. Rucinski, P. Zagozdzon, G. Raczak, A. Kutarski; Atrial electromechanical sequence and contraction synchrony during single and multisite atrial pacing in patients with Brady-tachycardia syndrome; Pacing Clin. Electrophysiol., 32 (2009), pp. 591–603
- [20] K. Wakami, N. Ohte, K. Asada, H. Fukuta, T. Goto, S. Mukai; Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole; J. Am. Soc. Echocardiogr., 22 (2009), pp. 847–851
- [21] D.G. Kim, K.J. Lee, S. Lee, S.Y. Jeong, Y.S. Lee, C. Yj; Feasibility of two-dimensional global longitudinal strain and strain rate imaging for the assessment of left atrial function: a study in subjects with a low probability of cardiovascular disease and normal exercise capacity; Echocardiography, 26 (2009), pp. 1179–1187
- [22] Y. Inaba, S. Yuda, N. Kobayashi, A. Hashimoto, K. Uno, T. Nakata; Strain rate imaging for noninvasive functional quantification of the left atrium: comparative studies in controls and patients with atrial fibrillation; J. Am. Soc. Echocardiogr., 18 (2005), pp. 729–736
- [23] G.Y. Cho, S.H. Jo, M.K. Kim, H.S. Kim, W.J. Park, Y.J. Choi; Left atrial dyssynchrony assessed by strain imaging in predicting future development of atrial fibrillation in patients with heart failure; Int. J. Cardiol., 134 (2009), pp. 336–341
- [24] S. Mondillo, M. Cameli, M.L. Caputo, M. Lisi, E. Palmerini, M. Padeletti; Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size; J. Am. Soc. Echocardiogr., 24 (2011), pp. 898–908
- [25] S.S. Kuppahally, N. Akoum, N.S. Burgon, T.J. Badger, E.G. Kholmovski, S. Vijayakumar; Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI; Circ. Cardiovasc. Imaging, 3 (2010), pp. 231–239
- [26] J.A. Cabrera, S.Y. Ho, V. Climent, D. Sanchez-Quintana; The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation; Eur. Heart J., 29 (2008), pp. 356–362
- [27] J.L. Lopez-Minguez, R. Gonzalez-Fernandez, C. Fernandez-Vegaz, et al.; Anatomical classification of left atrial appendages in specimens applicable to CT imaging techniques for implantation of Amplatzer cardiac plug; J. Cardiovasc. Electrophysiol., 25 (2014), pp. 976–984
- [28] C. Schneider, R. Malisius, K. Krause, F. Lampe, E. Bahlmann, S. Boczor; Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation; Eur. Heart J., 29 (2008), pp. 1397–1409
- [29] S.K. Saha, P.L. Anderson, G. Caracciolo, A. Kiotsekoglou, S. Wilansky, S. Govind; Global left atrial strain correlates with CHADS2 risk score in patients with atrial fibrillation; J. Am. Soc. Echocardiogr., 24 (2011), pp. 506–512
- [30] G. Acar, A. Akcay, A. Sokmen, et al.; Assessment of atrial electromechanical delay, diastolic functions, and left atrial mechanical functions in patients with type 1 diabetes mellitus; J. Am. Soc. Echocardiogr., 22 (2009), pp. 732–738
- [31] I. Meissner, J. Whisnant, B. Khandheria, et al.; Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study Mayo clinic proceedings; 74 (1999), pp. 862–869
- [32] J. Lacomis, O. Goitein, C. Deible, P. Moran, G. Mamone; Dynamic multidimensional imaging of the human left atrial appendage; Europace, 9 (2007), pp. 1134–1140
- [33] R. Beigel, N. Wunderlich, S. Ho, R. Arsanjani, R. Siegel; The left atrial appendage: anatomy, function, and noninvasive evaluation; J. Am. Coll. Cardiol. Img., 7 (2014), pp. 1251–1265
- [34] D.S. Beutler, D. Richard, R.D. Gerkin, A.I. Loli; The morphology of left atrial appendage lobes: a novel characteristic naming scheme derived through three-dimensional cardiac computed tomography; World J. Card. Surg., 4 (2014), pp. 17–24
- [35] L. Di Biase, P. Santangeli, M. Anselmino, et al.; Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation?; J. Am. Coll. Cardiol., 60 (2012), pp. 531–538
- [36] R.W. Troughton, C.R. Asher, A.L. Klein; The role of echocardiography in atrial fibrillation and cardioversion; Heart, 11 (2003), pp. 1447–1454
- [37] M. Zabalgoitia, J.L. Halperin, L.A. Pearce, J.L. Blackshear, R.W. Asinger, R.G. Hart; Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke prevention in atrial fibrillation III investigators; J. Am. Coll. Cardiol., 11 (1998), pp. 1622–1626
- [38] R. Providencia, J. Trigo, L. Paiva, S. Barra; The role of echocardiography in thromboembolic risk assessment of patients with nonvalvular atrial fibrillation; J. Am. Soc. Echocardiogr., 26 (2013), pp. 801–812
- [39] T. Tabata, T. Oki, N. Fukuda, et al.; Influence of aging on left atrial appendage flow velocity patterns in normal subjects; J. Am. Soc. Echocardiogr., 9 (1996), p. 274e280
- [40] R.A. Mikael Kortz, B.J. Delemarre, J.M. van Dantzig, H. Bot, O. Kamp, C.A. Visser; Left atrial appendage blood flow determined by transesophageal echocardiography in healthy subjects; Am. J. Cardiol., 71 (1993), p. 976e981
- [41] B.S. Lowe, V. Brandon, K. Shrestha, C. Whitman, A.L. Klein; Prognostic significance of left atrial appendage “sludge” in patients with atrial fibrillation: a new transesophageal echocardiographic thromboembolic risk factor; J. Am. Soc. Echocardiogr., 27 (2014), pp. 1176–1183
- [42] W.-C. Tsai, C.-H. Lee, C.-C. Lin, et al.; Association of left atrial strain and strain rate assessed by speckle tracking echocardiography with paroxysmal atrial fibrillation; Echocardiography, 26 (2009), pp. 1188–1194
- [43] M. Rosca, B.A. Popescu, C.C. Beladan; Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy; J. Am. Soc. Echocardiogr., 23 (2010), pp. 1090–1098
- [44] Y. Liu, K. Wang, D. Su, et al.; Noninvasive assessment of left atrial phasic function in patients with hypertension and diabetes using two-dimensional speckle tracking and volumetric parameters; Echocardiography, 31 (2014), pp. 727–735
- [45] M. Obokata, K. Negishi, K. Kurosawa, et al.; Left atrial strain provides incremental value for embolism risk stratification over CHA(2)DS(2)-vasc score and indicates prognostic impact in patients with atrial fibrillation; J. Am. Soc. Echocardiogr., 27 (2014), pp. 709–716
- [46] S. Sasaki, T. Watanabe, H. Tamura, et al.; Left atrial strain as evaluated by two-dimensional speckle tracking predicts left atrial appendage dysfunction in patients with acute ischemic stroke; BBA Clin., 40–47 (2014)
- [47] I. Limantoro, C. de Vos, T. Delhaas, et al.; Clinical correlates of echocardiographic tissue velocity imaging abnormalities of the atrial wall during atrial fibrillation Europace; 16 (2014), pp. 1546–1553
- [48] H. Tamura, T. Watanabe, O. Hirono, et al.; Low Wall velocity of left atrial appendage measured by trans-thoracic echocardiography predicts thrombus formation caused by atrial appendage dysfunction; J. Am. Soc. Echocardiogr., 23 (2010), pp. 545–552
- [49] H. Tamura, T. Watanabe, S. Nishlyama, et al.; Prognostic value of left atrial appendage wall velocity in patients with ischemic stroke and atrial fibrillation; J. Am. Soc. Echocardiogr., 25 (2012), pp. 576–583
- [50] T. Saito, K. Tamura, D. Uchida, T. Saito, M. Togashi, T. Nitta, Y. Sugisaki; Histopathological features of the resected left atrial appendage as predictors of recurrence after surgery for atrial fibrillation in valvular heart disease; Circ. J., 71 (1) (2007 Jan), pp. 70–78
- [51] B. Schmidt, S. Ernst, F. Ouyang, K.R. Chun, T. Broemel, D. Bansch, K.H. Kuck, M. Antz; External and endoluminal analysis of left atrial anatomy and the pulmonary veins in three-dimensional reconstructions of magnetic resonance angiography: the full insight from inside; J. Cardiovasc. Electrophysiol., 17 (2006), pp. 957–964
- [52] A1. Mügge, H. Kühn, P. Nikutta, J. Grote, J.A. Lopez, W.G. Daniel; Assessment of left atrial appendage function by biplane transesophageal echocardiography in patients with nonrheumatic atrial fibrillation: identification of a subgroup of patients at increased embolic risk; J. Am. Coll. Cardiol., 23 (1994), pp. 599–607
- [53] Q. Zhang, G.W.-K. Yip, C.-M. Yu; Approaching regional left atrial function by tissue Doppler velocity and strain imaging; Europace, 10 (2008), pp. iii62–iii69
- [54] E.B. Kaya, L. Tokgozoglu, K. Aytemir, U. Kocabas, E. Tulumen, O.S. Deveci; Atrial myocardial deformation properties are temporarily reduced after cardioversion for atrial fibrillation and correlate well with left atrial appendage function; Eur. J. Echocardiogr., 9 (2008), pp. 472–477
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?