Summary
A 65-year-old Taiwanese man presented with dark urine for 5 days before admission to hospital and with fever on the 2nd day of admission to hospital. Laboratory studies showed acute hepatitis with hyperbilirubinemia. Acute hepatitis with nontyphoidal salmonella and hepatitis E virus coinfection was diagnosed. The fever subsided after treatment with ceftriaxone and cefepime. His serum bilirubin reached its peak value on the 3rd week after admission to hospital and then gradually returned to the normal range. To the best of our knowledge, acute hepatitis E coinfection with nontyphoidal salmonella has not been reported previously.
Keywords
Hepatitis E ; Hepatitis E virus ; Nontyphoidal salmonella ; Salmonella
Introduction
Hepatitis E is an important public health issue in many developing countries, especially in Asia and Africa [1] . Hepatitis E virus (HEV) spreads mainly through the fecal contamination of water supplies or food; person-to-person transmission is uncommon. However, autochthonous hepatitis E in developed countries is far more common than previously recognized and might be more common than hepatitis A [2] . Hepatitis E is thought to be a zoonotic disease because animals are known to be a source of infection; both deer and pigs have been implicated as potential reservoirs of the virus. Outbreaks of acute hepatitis E have never occurred in Taiwan and only sporadic cases of acute hepatitis E have been reported to the Centers for Disease Control (CDC) Taiwan [3] .Nontyphoidal salmonella (NTS) species are important food-borne pathogens, with acute gastroenteritis being the most common clinical manifestation [4] . The most common manifestation of nontyphoidal salmonellosis is acute enterocolitis, but the organism can cause focal infection, bacteremia, and meningitis, as well as “enteric fever” that may be clinically indistinguishable from that caused by Salmonella typhi .
Typhoid fever has been reported to be associated with hepatitis E only sporadically [5] ; [6] ; [7] . To the best of our knowledge, acute hepatitis E coinfection with NTS has not been reported previously. We report here the case of a patient with acute hepatitis caused by NTS and HEV.
Case report
A 65-year-old man was generally healthy until 5 days before this admission to hospital, when dark urine developed. Malaise and decreased appetite were also found and his stools were firm and yellowish. There was no fever, chills, arthralgia, myalgia, abdominal pain, nausea, vomiting, diarrhea, night sweats, nor preceding weight loss.
The patient was a retired business executive living in Taiwan. No toxin exposure or history of drug use was found. He had stayed in Thailand for 5 days approximately 3 months before this admission to hospital. One month previously he had eaten raw fish, oysters, shrimp, and sea urchin while in Hokkaido, Japan. His wife, who had traveled with him, was well. He had also been to a local farm in southern Taiwan and eaten a fresh vegetable salad 10 days prior to this admission. He had not engaged in unprotected sexual behavior. No history of exposure to a cluster of infection or animals was reported.
On admission, his temperature was 36.8°C, pulse rate 70 beats per minute, respiratory rate 18 beats per minute, and blood pressure 125/80 mmHg. On physical examination icteric sclera with yellowish skin discoloration were noted. His liver and spleen were not palpable. Urinalysis showed a positive reaction for bilirubin and urobilinogen, and a hemogram showed a leukocyte count of 4500/μL, neutrophils 57%, and lymphocytes 29%. Laboratory studies showed increased serum levels of aspartate aminotransferase (AST) 1816 IU/L, alanine aminotransferase (ALT) 2684 IU/L, total bilirubin 5.85 mg/dL, and conjugated bilirubin 2.47 mg/dL. His prothrombin time was 10.5 seconds (international normalized ratio 1.02), and serum albumin 3.7 g/dL. He was admitted with a preliminary diagnosis of acute hepatitis.
Abdominal ultrasonography showed neither biliary tree dilatation nor space-occupying lesions. Viral markers were negative for hepatitis A, B, and C. Autoimmune profiles, including antinuclear antibodies, antimitochondrial antibodies, antineutrophil cytoplasmic antibodies, and antismooth muscle antibodies were negative. Two days after admission his temperature increased to 38.5°C and his pulse rate increased to 80 beats per minute; a blood specimen was drawn for culture. Doxycycline was administered empirically for a suspected atypical infection. A blood sample was also sent to the CDC, Taiwan to investigate the possibility of hepatitis E, leptospirosis, rickettsiosis, and Q fever.
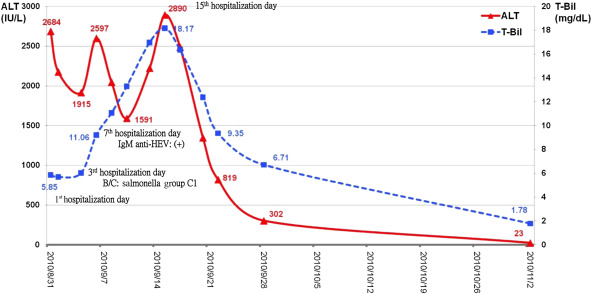
Salmonella group C1 was isolated from his blood on the 5th day of admission to hospital. Doxycycline was then replaced by ceftriaxone and the fever subsequently decreased. Ultrasonography and a computed tomography scan of his abdomen showed neither a mycotic aneurysm nor biliary tree dilatation. The laboratory tests for leptospirosis, rickettsiosis, and Q fever were negative, whereas the serum immunoglobulin antibodies, both IgM and IgG, against hepatitis E were positive on the 7th day of admission to hospital. HEV of genotype 4 was finally identified by the CDC, Taiwan. A transient increase in the serum bilirubin level with fluctuating transaminase levels was noted. The hyperbilirubinemia reached a peak on the 15th day of admission to hospital and his general condition, including serum bilirubin and transaminase levels, then improved (Fig. 1 ). The patient was discharged in a satisfactory condition after a stay in hospital of 24 days.
|
|
|
Figure 1. Schematic representation of serum liver enzyme concentrations in our patient. Fever developed 2 days after admission and salmonella group C1 was isolated from his blood on the 5th day after admission to hospital. The fever decreased after treatment with ceftriaxone. IgM and IgG against hepatitis E were positive on the 7th day after admission to hospital. Serum liver enzymes reached a peak on the 15th day after admission to hospital. ALT = alanine aminotransferase; HEV = hepatitis E virus; T-Bil = total bilirubin; IgM = Immunoglobulin M; IgG = Immunoglobulin G; BC = blood culture. |
Discussion
HEV is a nonenveloped virus with a positive-stranded RNA genome of approximately 7.2 kb in length. It occurs all over the world and is responsible for large epidemics of acute hepatitis and a proportion of sporadic cases of hepatitis in southeast and central Asia, the Middle East, parts of Africa, and Mexico [8] . In developed regions, hepatitis E is far more common than previously recognized and may have a zoonotic source. Sporadic cases of acute HEV infection with the production of anti-HEV IgM have been occasionally reported in Taiwan, despite no reported outbreaks [9] .
The clinical features of hepatitis E infection range from asymptomatic infection, to mild hepatitis, to subacute liver failure. The incubation period ranges from 2 to 10 weeks. The level of serum liver enzymes increased, peaking at approximately 6 weeks after exposure before decreasing to normal levels by week 10 [2] . Acute icteric hepatitis, the most common recognizable form of the illness, is usually insidious in onset and has an initial prodromal phase lasting a few days with a variable combination of flu-like symptoms, fever, abdominal pain, anorexia, nausea, vomiting, clay-colored stools, dark or tea-colored urine, diarrhea, arthralgia, and asthenia. The illness is usually self-limiting and typically lasts for 1–4 weeks [8] . In our reported case, HEV of genotype 4 was identified by the CDC, Taiwan and was categorized as sporadic acute hepatitis E. This patient had jaundice for 5 days before admission and fever on the 2nd day of admission to hospital. The travel history, incubation period, and clinical course implicated acute HEV infection [2] ; [8] . However, the coinfection of HEV with salmonella made the clinical diagnosis more complicated.
As HEV and salmonella have a common route of transmission via the fecal contamination of water supplies or food, coinfection of HEV and salmonella has been reported sporadically [5] ; [6] ; [7] . Patients with salmonella infection typically present with an acute onset of fever, diarrhea, and cramping. The incubation period is dependent on the host and inoculum, but is generally 6–72 hours. In salmonella hepatitis, a high fever and bradycardia are detected more often than in viral hepatitis E. Moreover, the serum aminotransferase levels rarely increase to more than 1000 IU/L and AST levels are usually higher than ALT levels in salmonella hepatitis. It has also been reported that the ALT/lactate dehydrogenase ratio may be the best discriminator of the two conditions as it is usually <4.0 in salmonella hepatitis, but >5.0 in acute viral hepatitis [10] ; [11] . The high AST/ALT level in our patient suggested acute hepatitis E, whereas his high fever, leucopenia, and relative bradycardia implicated salmonella hepatitis. Thus the clinical course of our patient could not be fully explained by salmonella hepatitis or hepatitis E alone. In this instance, contaminated food, such as the raw fish in Japan or fresh vegetable salad in southern Taiwan, may have been the source of HEV and salmonella infection.
In summary, hepatitis E should be considered in the differential diagnosis in patients with jaundice and increased serum transaminase, especially if they have a history of travel to endemic areas or have consumed raw food. These patients usually present with an acute onset of fever, especially high fever and abdominal discomfort. Other microorganisms such as NTS may coinfect a patient with hepatitis E because of the common oral–fecal route of infection.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] R. Aggarwal, S.R. Naik; Epidemiology of hepatitis E: past, present and future; Trop Gastroenterol, 18 (1997), pp. 49–56
- [2] H.R. Dalton, R. Bendall, S. Ijaz, M. Banks; Hepatitis E: an emerging infection in developed countries; Lancet Infect Dis, 8 (2008), pp. 698–709
- [3] C.H. Wang; Hepatitis E virus infection in Taiwan: prevalence of neutralizing anti-HEVne positive serum; Epidemiol Bull, 17 (2001), pp. 281–306
- [4] A. Dhanoa, Q.K. Fatt; Non-typhoidal Salmonella bacteraemia: epidemiology, clinical characteristics and its association with severe immunosuppression; Ann Clin Microbiol Antimicrob, 8 (2009), p. 15
- [5] A.Y. Phadke, H.G. Desai; Hepatitis E virus and Salmonella paratyphi A coinfection; Indian J Gastroenterol, 16 (1997), pp. 115–116
- [6] C.K. Pandey, N. Singh, V. Kumar, A. Agarwal, P.K. Singh; Typhoid, hepatitis E, or typhoid and hepatitis E: the cause of fulminant hepatic failure – a diagnostic dilemma; Crit Care Med, 30 (2002), pp. 376–378
- [7] S. Ohnishi, K. Hatanaka, M. Nakanishi, S. Hige, M. Asaka, Y. Takizawa; Acute hepatitis with Salmonella paratyphi A and hepatitis E virus coinfection; J Clin Gastroenterol, 37 (2003), pp. 350–351
- [8] R. Aggarwal, K. Krawczynski; Hepatitis E: an overview and recent advances in clinical and laboratory research; J Gastroenterol Hepatol, 15 (2000), pp. 9–20
- [9] K.T. Wu, K.M. Chung, I.C. Feng, M.J. Sheu, H.T. Kuo, L.B. Koay, et al.; Acute hepatitis E virus infection in Taiwan 2002–2006 revisited: PCR shows frequent co-infection with multiple hepatitis viruses; J Med Virol, 81 (2009), pp. 1734–1742
- [10] H.M. El-Newihi, M.E. Alamy, T.B. Reynolds; Salmonella hepatitis: analysis of 27 cases and comparison with acute viral hepatitis; Hepatology, 24 (1996), pp. 516–519
- [11] C. Pramoolisinsap, V. Viranuvatti; Salmonella hepatitis; J Gastroenterol Hepatol, 13 (1998), pp. 745–750
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?