Summary
Noncardiac operations are being increasingly performed on patients with left ventricular assist devices (LVADs). However, little is known on the impact of continuous-flow LVADs on the vascular supply of the colon for anastomoses. In this case, a 67-year-old male supported on an LVAD underwent four successful noncardiac operations including two intestinal anastomoses; left colon and small bowel anastomosis. To the best of our knowledge, no existing literature has reported successful colonic anastomosis on a continuous-flow LVAD. This case illustrates the plausibility of performing colonic anastomoses with appropriately selected patients supported on an LVAD. A 67-year-old male with congestive heart failure underwent LVAD placement for decompensated heart failure while awaiting orthotopic transplantation. During his recovery, he developed a stage IV sacral decubitus ulcer which required a sigmoid loop colostomy placement and a rotational flap. Subsequent stoma closure with partial sigmoid colectomy and stapled anastomosis was performed, and healed without evidence of anastomotic leak. This case illustrates the potential for colonic anastomoses for patients on continuous-flow LVAD support. Although oxygenation is known to be an important aspect of healing, this patients outcome suggests that intestinal anastomoses can be performed on the induced pulseless environment of an LVAD. Further studies will be needed to further elucidate the success of longer segment resections and appropriate surgical candidates.
Keywords
anastomosis;heart failure;ventricular assist device;wound healing
1. Introduction
In the current armamentarium of advanced congestive heart failure, left ventricular assist devices (LVADs) are frequently used. Refinements in technology have allowed these devices to be used both as a bridge to orthotopic heart transplantation, as well as destination therapy.1 The devices have also recently been updated from pulsatile–flow devices to continuous–flow devices using centrifugal pumps, which have allowed them to become smaller and suitable for use outside of the inpatient environment. The development of continuous-flow devices improves their mechanical durability and functionality greatly, but differs from normal physiology; startlingly, many of these patients do not have a pulse. Currently, there are several small series of noncardiac operations and procedures on patients supported on LVADs, including a right hemicolectomy,2 but little is currently known about the healing of colonic and intestinal anastomoses, especially with continuous-flow LVADs. At our academic medical center, a single patient successfully underwent four major noncardiac operations while supported on a continuous-flow LVAD, two of which included intestinal anastomosis. The first anastomosis occurred during a loop sigmoid colostomy closure. This procedure was complicated by a small bowel obstruction requiring a second operation, and small bowel resection with enteroenterostomy. Both anastomoses healed without evidence of anastomotic leak, despite surgical concerns regarding intestinal perfusion with a continuous-flow LVAD.
2. Case report
The patient is a 67-year-old male with a past medical history of decompensated systolic congestive heart failure, New York Heart Association Stage IV, with complications of ventricular tachycardia. Due to decompensated heart failure progressing to acute renal failure on continuous veno–venous hemodialysis, a continuous-flow HeartMate II LVAD (Thoratec Corporation, Pleasonton, CA) was placed as a bridge to transplantation. During the patients prolonged postoperative course, he developed a Stage IV right sacral decubitus ulcer which precluded his placement on the orthotopic heart transplantation listing. After extensive multidisciplinary discussions with the transplantation committee, the patient was offered operative treatment in the hopes of qualifying for transplant relisting (Table 1).
| Operation | Days after LVAD | Estimated blood loss | Blood transfusions | Anticoagulation management |
|---|---|---|---|---|
| Loop sigmoid colostomy | 38 | 50 | 3 units packed red blood cells (pRBCs) intraoperatively, 2 pRBCs postoperatively | Hold heparin infusion |
| Right gluteal rotational flap | 46 | 100 | 5 units pRBCs postoperatively | Hold heparin infusion |
| Colostomy reversal | 219 | 20 | None | Hold heparin infusion |
| Laparotomy, lysis of adhesions | 234 | 100 | None | Prothrombin complex concentrate, vitamin K |
The patient underwent a laparotomy and loop sigmoid colostomy placement 38 days after his LVAD placement. Cardiac anesthesia specialists were used. Estimated blood loss was minimal at 20 mL. Perioperative anticoagulation was managed by discontinuation of an oral vitamin K antagonist and transition to a heparin continuous infusion which was held 8 hours before surgery and 24 hours afterwards. Eight days later, plastic and reconstructive surgery performed a right superior gluteal artery perforator myocutaneous rotational flap for soft-tissue coverage of the right sacral decubitus ulcer. The patient recovered uneventfully and was discharged to a rehabilitation facility several weeks later.
Six months later, the patient was reevaluated for sigmoid loop colostomy closure. This operation was considered high risk for morbidity and mortality, secondary to the concern for anastomotic complications. Given the unknown reliability of the left colonic vascular supply on a continuous forward-flow LVAD, the likelihood of an anastomotic leak was of specific concern. After an extensive discussion, the multidisciplinary transplant committee advocated colostomy reversal to minimize infectious concerns in support of orthotopic heart transplantation listing. The patient underwent a sigmoid loop colostomy closure locally through his ostomy site with a stapled side-to-side, functional end-to-end anastomosis on post-LVAD Day 219. A 100 mm blue-load linear GIA cutting stapler (Ethicon Endo-Surgery Inc., Blue Ash, OH) was used in two fires – one to create an anastomosis between the two limbs of bowel and the other as a transverse fire to close the enterotomies and resect the specimen. Again, cardiac anesthesiologists provided anesthesia services. Blood loss was 50 mL. Perioperative anticoagulation management involved transition to a continuous heparin infusion which was held 8 hours before and 24 hours following the operation. The patient had return of bowel function on postoperative Day 3 and was subsequently discharged to a rehabilitation facility on postoperative Day 5.
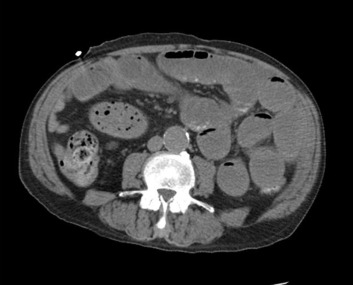
Fifteen days later, he represented with nausea, vomiting and was diagnosed with a high-grade small bowel obstruction (Fig. 1). He underwent a laparotomy with lysis of adhesions and small bowel resection and anastomosis for an adhesion of the bowel to the anterior abdominal wall creating a nonviable segment of small intestine. A two-staple technique, as described, was used with a 100 mm blue-load linear cutting stapler. At the time of this operation, the colonic anastomosis was visualized and noted to be healing and without leak. Estimated blood loss was 100 mL. Due to the operative urgency, the patients pharmacologically elevated international normalized ratio was quickly corrected with prothrombin concentrate complex and vitamin K. A continuous heparin infusion was started 24 hours following the operation. Postoperatively, the patient had return of bowel function on Day 3 and was discharged to a rehabilitation facility on Day 22.
|
|
|
Figure 1. Computed–tomography scan image demonstrating high grade small bowel obstruction. |
3. Review of the literature
Congestive heart failure represents a common clinical problem in the United States as well as around the world. In the United States, 550,000 people are diagnosed with the condition each year and it is estimated that 5.8 million patients are currently diagnosed.3 Since its approval in the early 1990s, LVADs have emerged as an effective treatment in improving survival rates of patients with New York Heart Association Stage IV disease.4 In 2001, the REMATCH trial showed a 48% mortality reduction which further popularized the LVADs use in advanced heart failure.5 More recent studies have widened the application of LVADs to a final treatment modality, or destination therapy, in addition to serving as a temporary measure to those awaiting suitable organ availability for orthotopic heart transplantation.6 ; 7
As LVADs have become increasingly present amongst congestive heart failure patients and survival rates continue to improve,8 the need for noncardiac surgery and procedures has become more prevalent.9 One study suggested that 18% of their LVAD population required a noncardiac operation.10 Several small-series studies have been published since 1994 regarding the outcomes of noncardiac operations amongst patients supported on ventricular assist devices.11 ; 12 Schmid et al13 described 20 procedures in 14 LVAD patients which included several cecostomies, cholecystectomies, and a small bowel resection. Garatti et al14 described 12 procedures in 11 patients including a right hemicolectomy. Brown et al10 described 27 procedures. To date, the largest series published is Barbara et al15 which reports the outcomes of 33 patients undergoing 67 procedures. To our knowledge, no report of colo-colonic anastomosis currently exists.
Multiple factors affect the healing of intestinal anastomoses. Oxygenation of the anastomosis has been demonstrated in several animal studies to be an important factor in predicting successful healing versus leaking.16; 17 ; 18 Similarly, vasoactive medications which constrict the blood supply have also been shown to significantly increase the likelihood of an anastomotic leak in a clinical trial.19 LVAD devices have shifted from pulsatile-flow to continuous-flow models. Continuous-flow devices lack a pulse and instead maintain a constant systolic pressure. However, little is known regarding the physiologic impact and end-organ oxygen delivery in this pulseless environment.20 While contemplating sigmoid colostomy closure, the unreliability of the colonic vascular supply on a continuous-flow device was a specific concern as the left side of the colon has been shown to be less vascular than the small intestine or the right colon.
In terms of overall complications observed in LVAD patients, the available literature suggests that bleeding is the most common complication, ranging from 36% in one series to 90.9% requiring perioperative transfusions.10 ; 21 The perioperative management of anticoagulation remains varied amongst the available literature. Discontinuation of a vitamin K antagonist to a continuous heparin infusion that is held several hours before and after the operation along with aspirin has been described.21 Less aggressive anticoagulation strategies are continuing to be investigated.22 Infections are also common at 33%.10 Mortality rates vary in the available studies. The immediate perioperative mortality rates are low. The 28-day mortality rates vary from 0% to 26%,2 ; 10 although the lower mortality rate series included endoscopic procedures.
4. Conclusion
With the inclusion of a multidisciplinary team of providers, mounting evidence supports the increasing safety of performing noncardiac operations on LVAD patients. In our case report, a single patient underwent four major elective operations with two successful intestinal anastomoses. Although little is known regarding the reliability of the blood supply of the colon on continuous-flow LVADs, this case supports that a primary anastomosis can be considered in this setting and may not mandate a colostomy or ileostomy. Our patient received a small-segment partial colectomy to reverse a loop sigmoid colostomy and it is unknown whether larger colonic resections can undergo anastomosis, although the physiology would be expected to be the same. Further reports can further elucidate the feasibility of this approach.
References
- 1 K. Lietz, J.W. Long, A.G. Kfoury, et al.; Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection; Circulation, 116 (2007), pp. 497–505
- 2 M. Ahmed, H. Le, J.M. Aranda Jr., C.T. Klodell; Elective noncardiac surgery in patients with left ventricular assist devices; J Card Surg, 27 (2012), pp. 639–642
- 3 C. Norton, V.V. Georgiopoulou, A.P. Kalogeropoulos, J. Butler; Epidemiology and cost of advanced heart failure; Progr Cardiovasc Dis, 54 (2011), pp. 78–85
- 4 D.J. Goldstein, M.C. Oz, E.A. Rose; Implantable left ventricular assist devices; N Engl J Med, 339 (1998), pp. 1522–1533
- 5 E.A. Rose, A.C. Gelijns, A.J. Moskowitz, et al.; Long-term use of a left ventricular assist device for end-stage heart failure; New Engl J Med, 345 (2001), pp. 1435–1443
- 6 M.J. Jurmann, Y. Weng, T. Drews, M. Pasic, E. Hennig, R. Hetzer; Permanent mechanical circulatory support in patients of advanced age; Eur J Cardiothorac Surg, 25 (2004), pp. 610–618
- 7 M.S. Slaughter, J.G. Rogers, C.A. Milano, et al.; Advanced heart failure treated with continuous-flow left ventricular assist device; N Engl J Med, 361 (2009), pp. 2241–2251
- 8 J.K. Kirklin, D.C. Naftel, R.L. Kormos, et al.; Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients; J Heart Lung Transplant, 32 (2013), pp. 141–156
- 9 S.H. McKellar, D.S. Morris, W.J. Mauermann, S.J. Park, S.P. Zietlow; Evolution of general surgical problems in patients with left ventricular assist devices; Surgery, 152 (2012), pp. 896–902
- 10 J.B. Brown, W.M. Hallinan, H.T. Massey, et al.; Does the need for noncardiac surgery during ventricular assist device therapy impact clinical outcome?; Surgery, 146 (2009), pp. 627–633 discussion 633–624
- 11 D.J. Goldstein, S.L. Mullis, E.S. Delphin, et al.; Noncardiac surgery in long-term implantable left ventricular assist-device recipients; Ann Surg, 222 (1995), pp. 203–207
- 12 T.V. Votapka, D.G. Pennington, L.R. McBride, D.L. Kaminski, C.H. Andrus, M.T. Swartz; Noncardiac operations in patients supported with mechanical circulatory support devices; J Am Coll Surg, 179 (1994), pp. 318–320
- 13 C. Schmid, M. Wilhelm, K.H. Dietl, C. Schmidt, D. Hammel, H.H. Scheld; Noncardiac surgery in patients with left ventricular assist devices; Surgery, 129 (2001), pp. 440–444
- 14 A. Garatti, G. Bruschi, T. Colombo, et al.; Noncardiac surgical procedures in patient supported with long-term implantable left ventricular assist device; Am J Surg, 197 (2009), pp. 710–714
- 15 D.W. Barbara, D.R. Wetzel, J.N. Pulido, et al.; The perioperative management of patients with left ventricular assist devices undergoing noncardiac surgery; Mayo Clin Proc, 88 (2013), pp. 674–682
- 16 J.A. Attard, M.J. Raval, G.R. Martin, et al.; The effects of systemic hypoxia on colon anastomotic healing: an animal model; Dis Colon Rectum, 48 (2005), pp. 1460–1470
- 17 M. Millan, E. Garcia-Granero, B. Flor, S. Garcia-Botello, S. Lledo; Early prediction of anastomotic leak in colorectal cancer surgery by intramucosal pH; Dis Colon Rectum, 49 (2006), pp. 595–601
- 18 W.G. Sheridan, R.H. Lowndes, H.L. Young; Tissue oxygen tension as a predictor of colonic anastomotic healing; Dis Colon Rectum, 30 (1987), pp. 867–871
- 19 T. Zakrison, B.A. Nascimento Jr., L.N. Tremblay, A. Kiss, S.B. Rizoli; Perioperative vasopressors are associated with an increased risk of gastrointestinal anastomotic leakage; World J Surg, 31 (2007), pp. 1627–1634
- 20 K.G. Soucy, S.C. Koenig, G.A. Giridharan, M.A. Sobieski, M.S. Slaughter; Defining pulsatility during continuous-flow ventricular assist device support; J Heart Lung Transplant, 32 (2013), pp. 581–587
- 21 J.A. Morgan, G. Paone, H.W. Nemeh, et al.; Non-cardiac surgery in patients on long-term left ventricular assist device support; J Heart Lung Transplant, 31 (2012), pp. 757–763
- 22 M.S. Slaughter, Y. Naka, R. John, et al.; Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy; J Heart Lung Transplant, 29 (2010), pp. 616–624
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?