Abstract
Background
Cardiogenic shock is a serious complication of a ST-segment elevation myocardial infarction (STEMI). We compared short- and long-term mortality among (1) STEMI patients with and without cardiogenic shock and (2) STEMI patients with cardiogenic shock with and without the use of an intra-aortic balloon pump (IABP).
Methods
From January 1, 2002 to December 31, 2010, all patients presenting with STEMI and treated with primary percutaneous coronary intervention (PCI) were identified. The hazard ratio (HR) for death was estimated using a Cox regression model, controlling for potential confounding.
Results
The study cohort consisted of 4293 STEMI patients: 286 (6.7%) with and 4007 (93.3%) without cardiogenic shock. Compared with patients without cardiogenic shock, patients with cardiogenic shock were older, and more likely to have diabetes mellitus, multi-vessel disease, anterior myocardial infarction (MI) or bundle-branch block MI and a reduced creatinine clearance.
Among patients with cardiogenic shock vs. without shock, 30-day cumulative mortality was 57.3% vs. 4.5% (p < 0.001), one-year cumulative mortality was 60.7% vs. 8.2% (p < 0.001) and five-year mortality was 65.0% vs. 18.9% (p < 0.001). STEMI with cardiogenic shock was associated with higher 30-day mortality (adjusted HR = 12.89 [95% CI: 9.72–16.66]), 1-year mortality (adjusted HR = 8.83 [95% CI: 7.06–11.05]) and five-year mortality (adjusted HR = 6.39 [95% CI: 5.22–7.80]). IABP was used in 71 (25%) patients with cardiogenic shock and was associated with improved 30-day outcome (adjusted HR = 0.48 [95% CI: 0.28–0.83]).
Conclusion
Patients with STEMI and cardiogenic shock had substantial short- and long-term mortality that may be improved with IABP implantation. More studies on use of IABP in such patients are warranted.
Keywords
ST-segment elevation myocardial infarction;Cardiogenic shock;Intra-aortic balloon pump
1. Introduction
Although the treatment of acute myocardial infarction (MI) has improved over the years, the mortality of patients in cardiogenic shock complicating MI remains high even with the use of primary percutaneous coronary intervention (PCI) and has not changed in decades [1] ; [2]. However, over the past decade, rates of cardiogenic shock developing during hospitalization as well as in-hospital mortality associated with shock have decreased; increased PCI rates for these critically ill patients may explain these secular trends [3].
Beyond the use of PCI, intra-aortic balloon pump (IABP) implantation has widely been used as an adjuvant treatment for cardiogenic shock in patients with acute MI based on the beneficial effect of aortic diastolic inflation and rapid systolic deflation, improving myocardial and peripheral perfusion and reducing afterload and myocardial oxygen consumption [4] ; [5]. The evidence base supporting IABP in cardiogenic shock use is mixed; in a recent meta-analysis [6], the use of IABP in patients with ST-segment elevation MI (STEMI) with cardiogenic shock treated was associated with a survival benefit [6]. However, on an individual level the data are less clearly supportive [7]. Notably, a randomized trial, the Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock (IABP-SHOCK II) study showed no improvement in survival using IABP in patients with MI and cardiogenic shock [8]. However, in this trial the patients represented a rather moderate-risk cohort with a substantially lower short-term mortality compared to those in other trials [2] ; [9]. Additionally, in IABP-SHOCK II, there was observed a favorable trend with IABP in younger patients and in those with a first MI. Accordingly, in an effort to examine this question in more detail, we used data from the Western Denmark Heart Registry (WDHR) to compare short- and long-term mortality among (A) STEMI patients with and without cardiogenic shock and (B) STEMI patients with cardiogenic shock with and without the use of IABP. It was our hypothesis that risk for mortality related to cardiogenic shock in this “real world” cohort of patients would be substantial, and that IABP use would reduce risk significantly compared to conservative management without IABP.
2. Patients and methods
2.1. Setting and design
The study was conducted using WDHR for patients treated at Odense University Hospital with a catchment population of 1.2 million inhabitants. A detailed description of the database has been reported previously [10].
2.2. Patients and procedures
Primary PCI became the recommended treatment for STEMI in Denmark in 2003 after pivotal trials supported this approach [11]. To be eligible for primary PCI, patients must generally meet the following criteria: 1) symptoms present less than 12 h from onset of pain to time of catheterization, and 2) ST-segment elevation (at least 0.1 mV in two or more standard leads or v4–v6, or at least 0.2 mV in two or more contiguous precordial leads (v1–v3) or a presumed new left bundle-branch block. We used the WDHR to identify all primary PCIs performed from January 1, 2002 through December 31, 2010. A patient was considered in cardiogenic shock if systolic blood pressure was < 90 mm Hg with the need of infusion of catecholamines to maintain the blood pressure, had clinical signs of pulmonary congestion, and had impaired end-organ perfusion (cold, clammy skin, altered mental status) or the use of IABP within the first 24 h of admission. Primary PCI was performed according to the standard. A glycoprotein IIb/IIIa receptor blocker was administered at the operators discretion. The post-intervention antiplatelet regimen included lifelong acetylsalicylic acid (75 mg once daily) and clopidogrel with a loading dose of 300 mg followed by maintenance with 75 mg daily. The recommended duration of clopidogrel treatment was 12 months.
2.3. Endpoints
The endpoint was time to all-cause mortality. Data on mortality status were obtained from the Danish Civil Registration System; this system has data to support accuracy [12] ; [13].
The Danish National Health Service provides universal tax-supported health care, guaranteeing residents free access to general practitioners and hospitals. The Danish Civil Registration System has kept electronic records on gender, birth date, residence, emigration date, and vital status changes since 1968, with daily updates; the 10-digit civil registration number assigned at birth and used in all registries allows for accurate record linkage. The Civil Registration System provided vital status data for our study participants and minimized loss to follow-up.
2.4. Statistical methods
Continuous variables were presented as medians with inter quartile range (IQR 25th, 75th) or mean ± 1 standard deviations (SD). Medians were compared using the Mann–Whitney U test, and means were compared using the unpaired t test. Categorical variables were presented as numbers and percentages. Distributions of categorical variables were compared using the Chi-square test. We counted endpoint events that occurred during the follow-up period and compared rates for the two groups. Follow-up began on the date of primary PCI procedure and continued until date of death, December 31, 2010 or after 5 of years follow-up (to ensure at least 10% of the study population at risk), whichever came first.
We constructed Kaplan–Meier curves for patients with cardiogenic shock and without cardiogenic shock, and patients with cardiogenic shock were stratified according to treatment with an IABP or not. Cox proportional hazards regression analysis was used to estimate the hazard ratio (HR) for mortality. Crude and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were computed. Potential confounders associated with time to death in the univariable Cox regression analysis were included in the multivariable Cox regression model. Thus, in the final model, we adjusted for age, gender, diabetes mellitus, previous MI, creatinine clearance < 60 mL/min, treatment with glycoprotein IIb/IIIa receptor blocker, anterior MI/bundle-branch block MI, multivessel disease and procedure time. All data analyses were carried out using SPSS software version 20. A two-sided p value < 0.05 was considered significant.
3. Results
3.1. Study population
A total of 4601 consecutive patients were treated with primary PCI for STEMI or bundle-branch block MI at Odense University Hospital between January 1, 2002 and December 31, 2010. Mortality data were not available for 85 patients, who were foreign citizens and were excluded. Patients undergoing a later primary PCI for acute MI after the first index procedure (n = 223) were excluded. Thus, the final study cohort consisted of 4293 patients, of whom 286 (6.7%) had cardiogenic shock and 4007 (93.3%) were without cardiogenic shock.
Baseline characteristics of patients with STEMI and cardiogenic shock and patients without cardiogenic shock are shown in Table 1. There were differences in several baseline characteristics and risk factors between STEMI patients with and without cardiogenic shock, as patients with STEMI and cardiogenic shock were older, and more likely to have diabetes mellitus, reduced creatinine clearance, previous MI and previous CABG. Also, patients with cardiogenic shock more often had multi-vessel disease, anterior MI or bundle-branch block MI, a lower pre and post intervention TIMI flow and a longer procedure time (Table 2).
| With cardiogenic shock | Valid cases | Without cardiogenic shock | Valid cases | p value (with vs. without cardiogenic shock) | With cardiogenic shock and without IABP | With cardiogenic shock and with IABP | p value (cardiogenic shock with versus without IABP) | ||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 286 | 4007 | 215 | 71 | |||||
| Male gender — no. (%) | 201 (70.3) | 286 | 2939 (73.3) | 4007 | 0.258 | 143 (66.5) | 58 (81.7) | 0.015 | |
| Age — years (inter-quartile range) | 71.0 (60.8–77.0) | 286 | 64.0 (55.0–74.0) | 4007 | < 0.001 | 73.0 (64.0–80.0) | 62.0 (53.0–69.0) | < 0.001 | |
| Family history — no. (%) | 53 (21.8) | 243 | 1433 (37.2) | 3853 | < 0.001 | 40 (21.9) | 13 (21.7) | 0.975 | |
| Smoking — no. (%) | 89 (50.0) | 178 | 1725 (46.6) | 3702 | 0.374 | 68 (54.4) | 21 (39.6) | 0.071 | |
| Body mass index — kg/m2 (inter-quartile range) | 25.7 (23.7–27.7) | 111 | 26.2 (24.0–29.1) | 2619 | 0.076 | 25.5 (23.0–28.3) | 25.8 (24.7–26.5) | 0.737 | |
| Diabetes mellitus — no. (%) | 37 (12.9) | 286 | 269 (9.2) | 4007 | 0.037 | 29 (13.5) | 8 (11.3) | 0.629 | |
| Hypertension — no. (%) | 83 (31.6) | 263 | 1316 (33.3) | 3956 | 0.569 | 63 (32.1) | 20 (29.9) | 0.727 | |
| Previous coronary artery bypass grafting — no. (%) | 8 (2.8) | 281 | 86 (2.1) | 4001 | 0.001 | 7 (3.3) | 1 (1.4) | 0.410 | |
| Previous percutaneous coronary intervention — no. (%) | 14 (5.3) | 264 | 277 (7.0) | 3940 | 0.284 | 10 (5.0) | 4 (6.2) | 0.698 | |
| Previous myocardial infarction — no. (%) | 48 (17.9) | 268 | 447 (11.3) | 3947 | 0.001 | 38 (18.8) | 10 (15.2) | 0.501 | |
| Lipid-lowering therapy — no. (%) | 64 (24.2) | 264 | 807 (20.4) | 3947 | 0.140 | 43 (21.6) | 21 (32.2) | 0.081 | |
| Glycoprotein IIb/IIIa receptor blocker — no (%) | 93 (34.0) | 269 | 1685 (45.2) | 3726 | 0.001 | 60 (29.6) | 33 (50.0) | < 0.001 | |
| Killip class — no. (%) | 279 | 3921 | < 0.001 | 0.003 | |||||
| I | 100 (35.8) | 3635 (92.7) | 85 (40) | 15 (21) | |||||
| II | 23 (8.2) | 188 (4.8) | 20 (10) | 3 (4) | |||||
| III | 27 (9.7) | 98 (2.5) | 20 (10) | 7 (10) | |||||
| IV | 129 (46.2) | 0 (0.0) | 84 (40) | 45 (64) | |||||
| Blood pressure — mm Hg (inter-quartile range) | |||||||||
| Systolic | 90.0 (80.0–105.5) | 228 | 120.0 (105.0–140.0) | 3027 | < 0.001 | 91.0 (80.0–105.5) | 80.0 (74.3–100.0) | 0.042 | |
| Diastolic | 60.0 (50.0–65.0) | 221 | 70.0 (60.0–80.0) | 3006 | < 0.001 | 60.0 (50.0–65.0) | 60.0 (50.0–70.0) | 0.144 | |
| With cardiogenic shock | Valid cases | Without cardiogenic shock | Valid cases | p value (with vs. without cardiogenic shock) | With cardiogenic shock and without IABP | With cardiogenic shock and with IABP | p value (cardiogenic shock with versus without IABP) | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 286 | 4007 | 215 | 71 | ||||
| Infarct related artery — no. (%) | 275 | 3901 | < 0.001 | 0.021 | ||||
| Left anterior descending artery — no. (%) | 110 (40.0) | 1679 (43.0) | 81 (39.3) | 29 (42.0) | ||||
| Left circumflex artery — no. (%) | 30 (10.9) | 552 (14.2) | 25 (12.1) | 5 (7.2) | ||||
| Right coronary artery — no. (%) | 78 (28.4) | 1605 (41.1) | 65 (31.3) | 13 (18.8) | ||||
| Left main — no. (%) | 57 (20.7) | 65 (1.7) | 35 (17.0) | 22 (31.9) | ||||
| Anterior STEMIa or BBBMIb — no. (%) | 155 (56.6) | 275 | 1687 (43.8) | 4366 | < 0.001 | 108 (52.4) | 47 (69.1) | 0.016 |
| Multivessel disease — no. (%) | 184 (67.4) | 273 | 1722 (44.1) | 3903 | < 0.001 | 142 (48.3) | 42 (64.6) | 0.519 |
| Pre-intervention TIMI flow — no. (%) | 273 | 4038 | 0.010 | 0.016 | ||||
| Grade 0 | 170 (62.3) | 2134 (54.8) | 124 (60.8) | 46 (66.7) | ||||
| Grade 1 | 21 (7.7) | 243 (6.2) | 11 (5.4) | 10 (14.5) | ||||
| Grade 2 | 28 (10.3) | 674 (17.3) | 22 (10.8) | 6 (8.7) | ||||
| Grade 3 | 54 (19.8) | 846 (21.7) | 42 (23.0) | 7 (10.1) | ||||
| Final TIMI flow — no. (%) | 273 | 3897 | < 0.001 | 0.156 | ||||
| Grade 0 | 22 (8.1) | 111 (2.8) | 18 (8.8) | 4 (5.8) | ||||
| Grade 1 | 11 (4.0) | 42 (1.1) | 11 (5.4) | 0 (0.0) | ||||
| Grade 2 | 11 (11.4) | 225 (5.8) | 21 (10.3) | 10 (14.5) | ||||
| Grade 3 | 209 (76.6) | 3519 (90.3) | 154 (75.5) | 55 (79.7) | ||||
| Lesion length — mm | 15.0 (10.0–20.0) | 270 | 15.0 (10.0–20.0) | 4001 | 0.359 | 15.0 (10.0–20.0) | 15.0 (10.0–20.0) | 0.485 |
| Reference segment — mm | 3.4 (3.0–3.8) | 269 | 3.2 (3.0–3.6) | 3965 | 0.084 | 3.2 (3.0–3.6) | 3.5 (3.1–3.8) | 0.001 |
| Sapheneous vein graft — no. (%) | 0 (0.0) | 274 | 14 (0.4) | 3902 | 0.321 | 0 (0.0) | 0 (0.0) | – |
| Stent length — mm | 18.0 (13.0–23.0) | 238 | 18.0 (14.0–23.0) | 3621 | 0.737 | 18.0 (13.0–24.0) | 18.0 (15.0–23.0) | 0.280 |
| Type of stent — no. (%) | 285 | 3952 | 0.203 | 0.005 | ||||
| POBA only | 49 (17.2) | 417 (10.6) | 40 (18.6) | 9 (12.7) | ||||
| Bare metal stent | 103 (36.1) | 1229 (31.1) | 86 (40.2) | 17 (23.9) | ||||
| Drug eluting stent | 133 (46.7) | 2306 (58.4) | 88 (41.1) | 45 (63.9) | ||||
| Max balloon pressure — atm | 16.0 (14.0–18.0) | 261 | 16.0 (14.0–18.0) | 3786 | 0.614 | 16.0 (14.0–18.0) | 16.0 (14.0–18.0) | 0.792 |
| Max balloon diameter — mm | 3.5 (3.2–3.8) | 261 | 3.5 (3.2–3.8) | 3784 | 0.944 | 3.4 (3.2–3.8) | 3.7 (3.3–4.0) | 0.004 |
| Procedure time — minutes (inter-quartile range) | 21.0 (15.0–41.0) | 285 | 16.0 (10.0–26.0) | 3991 | < 0.001 | 20.0 (13.0–34.0) | 30.5 (19.0–51.0) | < 0.001 |
| Flouro time — minutes (inter-quartile range) | 8.4 (5.5–15.1) | 273 | 6.6 (4.0–11.5) | 3954 | < 0.001 | 7.9 (5.1–14.9) | 10.1 (6.4–16.1) | 0.048 |
| Contrast — mL (inter-quartile range) | 110.0 (79.0–200.0) | 4731 | 100 (75.0–175.0) | 3937 | 0.115 | 100.0 (70.0–200.0) | 135.0 (100.0–200.0) | 0.052 |
a. ST-segment elevation myocardial infarct.
b. Left bundle branch block.
Among STEMI patients with cardiogenic shock, IABP was used in 71 (25%) patients. STEMI patients with cardiogenic shock treated with IABP were younger, more often male, and had a lower systolic blood pressure compared to patients with cardiogenic shock without IABP treatment (Table 1).
3.2. Mortality
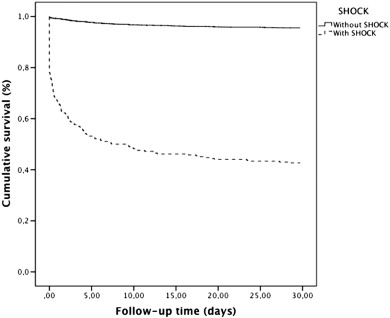
The median follow-up interval was 3.3 years (25th–75th percentile: 1.4–5.0 years), with a 30-day cumulative mortality of 8.0% (n = 495), one-year mortality of 495 (11.7%) and five-year mortality of 22.0% (n = 768). Among patients with STEMI with cardiogenic shock and without cardiogenic shock, 30-day cumulative mortality was 57.3% (n = 164) and 4.5% (n = 179), respectively (log-rank p < 0.001); one-year cumulative mortality was 60.7% (n = 173) and 8.2% (n = 322), respectively (log-rank p < 0.001); five-year cumulative mortality was 65.0% (n = 181) and 18.9% (n = 587), respectively (log-rank p < 0.001). Short and long term cumulative survival are shown in Fig. 1. After adjustment for covariates associated with mortality (see Statistical methods), STEMI with cardiogenic shock was associated with increased mortality compared to STEMI without cardiogenic shock after 30 days [adjusted HR = 12.89, 95% CI: 9.97–16.66], one-year [adjusted HR = 8.83, 95% CI: 7.06–11.05] and five-year [adjusted HR = 6.39, 95% CI: 5.22–7.81] (Table 3).
|
|
|
Fig. 1. Kaplan–Meier survival curves for consecutive patients with STEMI with and without cardiogenic shock. |
| Valid cases | Crude hazard ratio (95% CI) | p value | Adjusted hazard ratio (95% CI) | p value | |
|---|---|---|---|---|---|
| Cardiogenic shock | 13.04 (10.82–15.72) | < 0.001 | 8.80 (7.06–11.05) | < 0.001 | |
| Female gender | 4116 | 1.50 (1.25–1.81) | < 0.001 | 0.96 (0.78–1.06) | 0.683 |
| Creatinine clearance < 60 mL/min | 4116 | 4.83 (4.05–5.76) | < 0.001 | 1.90 (1.53–2.37) | < 0.001 |
| Age — years | 4116 | 1.07 (1.06–1.08) | < 0.001 | 1.05 (1.04–1.06) | < 0.001 |
| Diabetes mellitus | 4116 | 2.30 (1.83–2.87) | < 0.001 | 1.90 (1.47–2.46) | < 0.001 |
| Previous myocardial infarction | 4043 | 1.91 (1.52–2.40) | < 0.001 | 1.56 (1.19–2.05) | 0.001 |
| Glycoprotein IIb/IIIa receptor blocker | 3826 | 0.46 (0.38–0.57) | < 0.001 | 0.66 (0.53–0.82) | < 0.001 |
| Multivessel disease | 4011 | 2.50 (2.07–3.01) | < 0.001 | 1.44 (1.15–1.79) | 0.001 |
| Anterior MI/BBBMI | 4011 | 1.35 (1.13–1.62) | < 0.001 | 1.23 (1.03–1.53) | 0.022 |
| Procedure time — minutes | 4100 | 1.01 (1.01–1.02) | < 0.001 | 1.00 (1.00–1.01) | 0.166 |
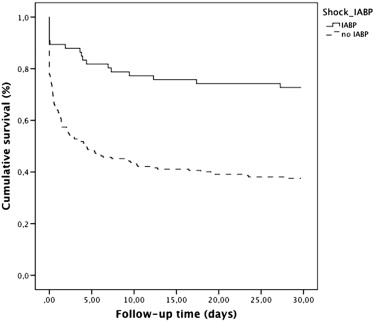
When stratifying patients with STEMI and cardiogenic shock into two groups with (1) treatment with IABP and (2) no treatment with IABP cumulative mortality rates were: 30-day cumulative mortality was 32.4% (n = 23) and 65.6% (n = 141), respectively (log-rank p < 0.001); one-year cumulative mortality was 33.8% (n = 24) and 69.9% (n = 149), respectively (log-rank p < 0.001); and five-year cumulative mortality was 48.8% (n = 26) and 73.4% (n = 155), respectively (log-rank p < 0.001). Short term cumulative survival is shown in Fig. 2. After adjustment for covariates, treatment with IABP in patients with STEMI and cardiogenic shock was associated with a lower mortality compared to STEMI with cardiogenic shock without IABP support after 30 days [adjusted HR = 0.45, 95% CI: 0.26–0.79], one-year [adjusted HR = 0.48, 95% CI: 0.28–0.83] and five-year [adjusted HR = 0.5, 95% CI: 0.30–0.85].
|
|
|
Fig. 2. Kaplan–Meier survival curves for consecutive patients with STEMI and cardiogenic shock with and without the use of intra-aortic balloon pump. |
4. Discussion
In studies of cardiogenic shock complicating STEMI, rates of mortality vary widely [4]. Additionally, reported efficacy of widely used therapies such as IABP in these studies has been variable [4] ; [14]. However, current guidelines of the European Society of Cardiology have downgraded IABP use in patients with cardiogenic shock complicated by myocardial infarction (class 2B) [5]. The downgrading is primarily due to the results from the IABP-SHOCK II trial [8]. Despite this fact, IABP use remains in wide use, driven by substantial anecdotal evidence as well as meta-analytic results [6].
Our study indicates that among STEMI patients treated with primary PCI in a real world clinical setting, the presence of cardiogenic shock was associated with increased short-and long-term mortality compared to STEMI patients without cardiogenic shock. Importantly, use of IABP support in STEMI patients with cardiogenic shock was associated with significantly reduced mortality.
The incidence of cardiogenic shock was 6.7% in our registry, which included all consecutive STEMI patients treated with primary PCI, was similar to the results of several previous studies [1] ; [15]. Additionally, overall 30-day and 5-year mortality rate of ~ 60% is in accordance with other studies [1]; [2]; [9] ; [15], and in line with the assumption that the highest mortality of cardiogenic shock is during the first weeks after the shock appears. Importantly, 30-day mortality rate of 57% in our study was considerably higher compared to the results from IABP-SHOCK II trial, which had 30-day mortality of ~ 40% [8]. The higher mortality in our study may reflect a high risk cohort e.g. there was a high number of patients with left main disease in cardiogenic shock needing IABP. IABP-SHOCK II was the first adequately powered randomized controlled study of 600 patients with cardiogenic shock complicating acute MI. In this randomized multicenter study, the patients in cardiogenic shock underwent early revascularization, best medical therapy and were randomly assigned to IABP. In the IABP-SHOCK II trial [8], there was no difference in 30-day mortality, renal function or attenuation in lactate or C-reactive protein levels between patients treated with IABP compared to those without [8]. However, important caveats regarding differences between our study and IABP-SHOCK II exist. For example, a quarter of the patients in IABP-SHOCK II had serum lactate < 2.0 mmol/L which indicates that the patients represented a moderate-risk cohort and ~ 10% of the patients randomized to optimal medical therapy had a cross-over and were treated with an IABP.
The mortality reduction of IABP in our study is in line with a recent meta-analysis which included prospective and retrospective cohort studies [6]. In contrast, analyzing data from the Euro Heart Survey Programme (EHS PCI) from the European Society of Cardiology including 653 patients with STEMI and non-STEMI, Zeymer et al. reported higher in-hospital mortality in the 25% of cardiogenic shock patients treated with IABP in comparison to the non-IABP group (56.9% versus 36.1%, p = 0.0004). In multivariate analysis including parameters such as age, gender, mechanical ventilation, severity of coronary artery disease, diabetes, renal failure and history of prior myocardial infarction, IABP use was not independently associated with mortality, although the corresponding p-value of 0.07 can be interpreted as a trend [16]. This is in contrast to our registry results, the opposite was found with a significant lower mortality rate in cardiogenic shock patients supported with IABP. Of course, important selection bias may be present in both registries e.g. operators discretion for using IABP, the definition of cardiogenic shock, and difference in baseline characteristics of the patients.
It is worthwhile to discuss 30-day and 1 year mortality of patients with and without cardiogenic shock in our analysis. In-hospital mortality after STEMI in patients without cardiogenic shock has been reduced to < 10% in the last decades [17]. This is mainly attributable to optimal interventional and drug treatment in the acute and subacute phase; indeed we observed quite similar results. In contrast to the clinical outcome in STEMI patients with hemodynamic stability, 30-day mortality of cardiogenic shock complicating STEMI remains high with rates of approximately 50% [1]. Thus, mortality related to cardiogenic shock has not changed substantially in nearly two decades [2], and studies focused on improved myocardial support, reperfusion, and protection in this population are critically needed. One option is alternative modes of support, such as the use of percutaneous left ventricular assist devices (LVAD). The prospective, randomized, open-label, multicenter, controlled Danish Cardiogenic Shock Trial (DanShock) [ClinicalTrials.gov identifier: NCT01633502] is ongoing and will assess whether the Impella cVAD™ LVAD treatment is beneficial for the treatment of cardiogenic shock.
4.1. Limitations
The validity of our findings depends on data quality and the ability to control for potential confounding. Like all observational studies, our study is prone to biases related to unmeasured factors. Bias due to unknown variables cannot be eliminated e.g. peripheral vascular disease or smaller peripheral vasculature. Our design is based on computerized registries with complete coverage, allowing study of a well-defined, large population with complete follow-up. That said, within the context of these limitations, it would appear that selection of patients to receive IABP support in our dataset led to more favorable outcomes, indeed a finding worth pointing out. We did not have systematic access to duration of inotropic support, serum lactate or information about left ventricular ejection fraction. We also lacked data on causes of mortality, however, in a previous STEMI cohort from Western Denmark Heart Registry, we found early causes of death were typically due to a cardiac reason: the 1-year mortality reason was cardiac in the great majority [18].
5. Conclusion
In STEMI patients, cardiogenic shock was associated with increased short- and long-term mortality compared to STEMI patients without cardiogenic shock. IABP balloon support in STEMI patients with cardiogenic shock was associated with improved outcome. Given that the mortality rate of those suffering cardiogenic shock after STEMI has not changed substantially over the past 2 decades, more studies focused on the optimal care of this high-risk patient population are warranted.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- [1] R.J. Goldberg, N.A. Samad, J. Yarzebski, J. Gurwitz, C. Bigelow, J.M. Gore; Temporal trends in cardiogenic shock complicating acute myocardial infarction; N Engl J Med, 340 (1999), pp. 1162–1168
- [2] J.S. Hochman, L.A. Sleeper, J.G. Webb, T.A. Sanborn, H.D. White, J.D. Talley, et al.; Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock; N Engl J Med, 341 (1999), pp. 625–634
- [3] R.V. Jeger, D. Radovanovic, P.R. Hunziker, M.E. Pfisterer, J.C. Stauffer, P. Erne, et al.; Ten-year trends in the incidence and treatment of cardiogenic shock; Ann Intern Med, 149 (2008), pp. 618–626
- [4] H. Thiele, B. Allam, G. Chatellier, G. Schuler, A. Lafont; Shock in acute myocardial infarction: the Cape Horn for trials?; Eur Heart J, 31 (2010), pp. 1828–1835
- [5] P.G. Steg, S.K. James, D. Atar, L.P. Badano, C. Blomstrom-Lundqvist, M.A. Borger, et al.; ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation; Eur Heart J, 33 (2012), pp. 2569–2619
- [6] A. Bahekar, M. Singh, S. Singh, R. Bhuriya, K. Ahmad, S. Khosla, et al.; Cardiovascular outcomes using intra-aortic balloon pump in high-risk acute myocardial infarction with or without cardiogenic shock: a meta-analysis; J Cardiovasc Pharmacol Ther, 17 (2012), pp. 44–56
- [7] R. Prondzinsky, H. Lemm, M. Swyter, N. Wegener, S. Unverzagt, J.M. Carter, et al.; Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome; Crit Care Med, 38 (2010), pp. 152–160
- [8] H. Thiele, U. Zeymer, F.J. Neumann, M. Ferenc, H.G. Olbrich, J. Hausleiter, et al.; Intraaortic balloon support for myocardial infarction with cardiogenic shock; N Engl J Med, 367 (2012), pp. 1287–1296
- [9] M.G. Lindholm, L. Kober, S. Boesgaard, C. Torp-Pedersen, J. Aldershvile; Cardiogenic shock complicating acute myocardial infarction; prognostic impact of early and late shock development; Eur Heart J, 24 (2003), pp. 258–265
- [10] L.O. Jensen, M. Maeng, A. Kaltoft, P. Thayssen, H.H. Hansen, M. Bottcher, et al.; Stent thrombosis, myocardial infarction, and death after drug-eluting and bare-metal stent coronary interventions; J Am Coll Cardiol, 50 (2007), pp. 463–470
- [11] H.R. Andersen, T.T. Nielsen, K. Rasmussen, L. Thuesen, H. Kelbaek, P. Thayssen, et al.; A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction; N Engl J Med, 349 (2003), pp. 733–742
- [12] C.B. Pedersen, H. Gotzsche, J.O. Moller, P.B. Mortensen; The Danish Civil Registration System. A cohort of eight million persons; Dan Med Bull, 53 (2006), pp. 441–449
- [13] L. Frank; Epidemiology. When an entire country is a cohort; Science, 287 (2000), pp. 2398–2399
- [14] H.V. Barron, N.R. Every, L.S. Parsons, B. Angeja, R.J. Goldberg, J.M. Gore, et al.; The use of intra-aortic balloon counterpulsation in patients with cardiogenic shock complicating acute myocardial infarction: data from the National Registry of Myocardial Infarction 2; Am Heart J, 141 (2001), pp. 933–939
- [15] D.R. Holmes Jr., E.R. Bates, N.S. Kleiman, Z. Sadowski, J.H. Horgan, D.C. Morris, et al.; Contemporary reperfusion therapy for cardiogenic shock: the GUSTO-I trial experience. The GUSTO-I Investigators. Global utilization of streptokinase and tissue plasminogen activator for occluded coronary arteries; J Am Coll Cardiol, 26 (1995), pp. 668–674
- [16] U. Zeymer, T. Bauer, C. Hamm, R. Zahn, F. Weidinger, R. Seabra-Gomes, et al.; Use and impact of intra-aortic balloon pump on mortality in patients with acute myocardial infarction complicated by cardiogenic shock: results of the Euro Heart Survey on PCI; EuroIntervention, 7 (2011), pp. 437–441
- [17] F. Schiele, M. Hochadel, M. Tubaro, N. Meneveau, W. Wojakowski, M. Gierlotka, et al.; Reperfusion strategy in Europe: temporal trends in performance measures for reperfusion therapy in ST-elevation myocardial infarction; Eur Heart J, 31 (2010), pp. 2614–2624
- [18] L.O. Jensen, M. Maeng, P. Thayssen, H.H. Tilsted, C.J. Terkelsen, A. Kaltoft, et al.; Influence of diabetes mellitus on clinical outcomes following primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction; Am J Cardiol, 109 (2012), pp. 629–635
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?