Abstract
To evaluate the prevalence of infestation of ectoparasites of horses, a total of 894 horses (367 males and 527 females), aged 1–7 years old were examined. Horses were groomed for collection of ectoparasites. Out of the horses examined, 164 came from Fundong, 80 from Acha, 30 from Fongo-Tongo, 30 from Fokoue and 17 from Dschang, all of which were infested with ectoparasites. Five hundred and seventy-three (573) horses came from Banso and 99.48% were infested by ectoparasites. From the results obtained, three species of hard ticks (Boophilus decoloratus, Amblyomma hebraeum and Hyalomma rufipes) and one species of biting lice (Bovicola equi) were identified. Boophilus decoloratus (66%) had the highest prevalence per locality, followed by A. hebraeum (18%), H. rufipes (11%) and B. equi (5%). Boophilus decoloratus infested horses of all ages, sex or colour. Meanwhile, A. hebraeum and H. rufipes had a higher prevalence in adult male horses. Mono-parasitic infestation (69%) was more common than poly-parasitic infestation (31%). Only three horses were free from infestation with ectoparasites. Understanding the biology, epidemiology and economic impact on the equine industry is important for public health and disease prevention programmes.
Introduction
The main-stay of livelihood for 75% of Cameroonians is agriculture (Asongwe et al. 2014), and livestock plays a vital role in the farming system of Cameroon. The horse (Equus caballus) is considered as livestock pet or companion animal (Adda et al. 2001). Horses and humans interact in many ways from working activities such as police service, agriculture, entertainment and warfare to competitive and non-competitive leisure pursuits. Many important products are derived from horses including meat, milk, hide, bones, blood, hooves and pharmaceuticals. Quite often, horse neglect and abuse cases originate from lack of economic resources needed to adequately maintain the horses health (Ahern et al. 2006).
Ectoparasites of horses that include ticks, mites, flies and lice (Bob 2006; Kaufman et al., 2008) continue to cause a significant threat to the health of horses. They can cause decreased performance, unthriftness in horses and some can help proliferate the life cycle of some internal parasites (Brendal 2009). They can also act as vectors between domestic animals and humans, causing a number of diseases, some of which are zoonotic. To animals, they can cause diseases like babesiosis, anaplasmosis, theleriosis and heart water. To humans, they can transmit diseases like Lyme disease, Rocky Mountain spotted fever and of recent, emerging infectious diseases like Crimean–Congo haemorrhagic fever (CCHFV). Those who are at risk of zoonotic infection are mostly infants, pregnant women, the elderly, immune-compromised people, veterinarians, zoo-workers, breeders and other veterinary health care workers (Stephen & John 2003). Therefore, increased awareness of the risk of zoonotic infections, being spread by horses, is needed for adequate control and disease prevention (Anne & Gary 2006). Many of the ticks and tick-borne diseases usually occur in specific geographical areas but with globalization and climate change their range may be expanded and can even be spread inter-continentally (Hubalek 2004). Each species of tick is adapted to certain ranges of temperatures and moisture: some occur only in warm regions with a fair degree of humidity, while others are mostly active in dry climates (Randolph 2005).
The first effects of intensive tick control in endemic zones will be the reduction in size of the reservoirs of infection that reside in the ticks themselves. This will lead to a reduction in the numbers of animals that become carriers and the re-challenge of existing carriers will be lowered leading to a lower infection rates in ticks. The overall effect will be to reduce the numbers of animals that acquire immunity to tick borne infections through natural infection. These findings suggested that immune selection had generated higher levels of intrinsic virulence and that the changes were genetically ‘hard-wired’ (Mackinnon 2014).
In Cameroon, there is limited livestock and rangeland management, resulting in rangeland degradation and poor horse body condition, especially during the rainy season, therefore attention has to be taken on this aspect. Secondly, animals at risk need to be regularly treated with acaricides. Currently, prevalence data for the geographical areas studied are not available. However, reference has been made of cases published by the Center for Disease Control (CDC). Nevertheless, considering the great economic importance of certain ticks as vectors of diseases of domestic livestock, the study of the seasonal occurrence of ticks is of major importance in the control of ticks and tick-borne diseases. Therefore, there is urgent need to understand the current status of the prevalence of infestation of ectoparasites of horses especially in the Western High Land of Cameroon in order to establish a control programme to improve on their health and productivity.
Materials and methods
Area of study
This study was carried out in Menoua Division, Western Region of Cameroon (Fig. 1), with the following geographical characteristics; an altitude of 1500 m, at Latitude 20° N. Average temperature ranges from 20.7 to 21.1°C; annual rainfall ranges from 6.2 mm in January to 320.2 mm in August, the height of the rainy season. The relative humidity of this region ranges from 36.5 to 90.5% annually. The soil is predominantly lateritic with feralitic and volcanic areas. Menoua Division has a climate which is of the Sudano-guinean type, characterized by a long rainy season from mid-March to mid-November, and a short dry season from mid-November to mid-March.
|
|
|
Figure 1. Map of menoua division. |
Collection and preservation of ectoparasites
To evaluate the infestation prevalence of ectoparasites, 894 horses (367 males and 527 females) aged between 1 and 7 years were randomly selected and examined externally once per week every Tuesday at 6 am –10:00 am for 36 weeks. They were mostly on free range while grazing on pasture, together with other animals like cattle, sheep and goats. Tick collection was done during its parasitic phase on the animal. Hosts were properly restrained to stand on a white bed sheet of dimensions 2 × 4 m and groomed with the help of a hand brush. Ectoparasites that fell on the bed sheet were collected at the end of grooming, packed in plastic vials containing 70% ethanol and labelled immediately after whole body collection and quickly transported to the lab. The samples were labelled with the origin, age, sex and animal colour, date and month of collection. Ectoparasites were counted and identified in the laboratory using a stereomicroscope within 48 h. Identification was done based on morphological and structural differences in the species at different instars. The grouping to their genus and species was made according to the methods developed by Hoogstraal (1956) and Horak et al. (2002). After identification, ticks with all appendages were preserved in the laboratory while the others with imparted appendages were counted and discarded. A similar technique was used to collect and preserve lice from the poll region of the head.
Statistical analysis
The Chi square test was used to compare the prevalence of ectoparasites with respect to each locality, age, sex, colour of horses, predilection site and monthly variation. Data were considered significant at P < 0.05.
Results
Prevalence of ectoparasites by locality
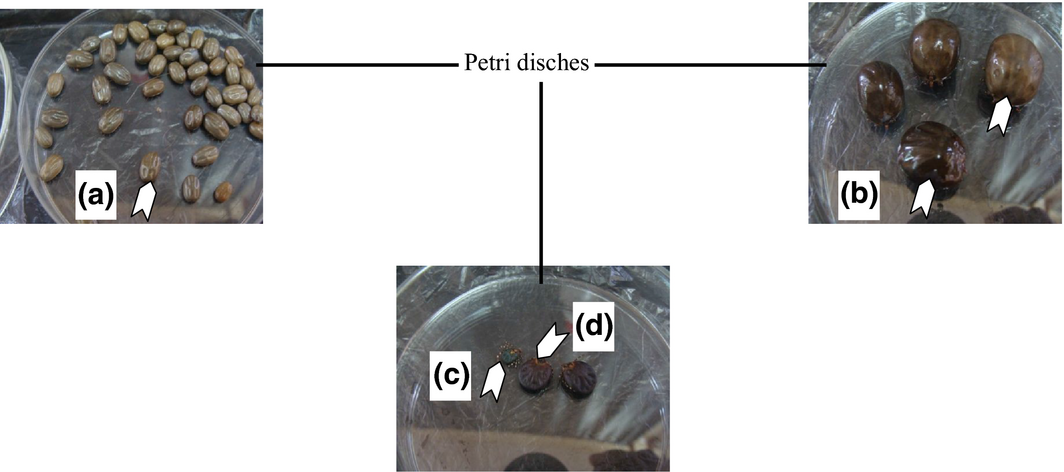
Three species of hard ticks (Boophilus decoloratus, Amblyomma hebraeum and Hyalomma rufipes) and one species of biting lice (Bovicola equi) were identified. The prevalence of ectoparasites of horses per locality showed that all the four parasites identified were present in all study areas (Table 1). Independent of locality, the most prevalent species was Boophilus decoloratus [(66%), Fig. 2a] followed by Amblyomma habraeum [(18%), Fig. 2b], Hyalomma rufipes [(11%), Fig. 2c and d] and a species of lice Bovicola equi (5%). Out of the ectoparasites encountered, B. decoloratus had the highest prevalence in horses from Fokoue (78.38%), as compared to those from Banso (73.39%) and Acha (72.07%), and A. habraeum was most prevalent in horses from Fongo-Tongo (25.58%) as compared to those from Fundong (23.73%), Dschang (19.35%) and Banso (15.46%). The species H. rufipes had its highest prevalence in Dschang (16.13%) as compared to those from Fundong (15.85%), Acha (10.81%) and Banso (9.69%). Bovicola equi was most prevalent in horses from Dschang (9.68%) as compared to those from Fokoue (8.11%), Fongo-Tongo (4.65%), Fundong (4.32%) and Acha (3.6%).
| Localities | Prevalence (%) | |||
|---|---|---|---|---|
| Boophilus decoloratus | Amblyomma habraeum | Hyalomma rufipes | Bovicola equi | |
| Banso | 73.39 | 15.46 | 9.69 | 1.44 |
| Fundong | 56.11 | 23.73 | 15.83 | 4.32 |
| Acha | 72.07 | 13.51 | 10.81 | 3.6 |
| Fongo-Tongo | 60.46 | 25.58 | 9.29 | 4.65 |
| Fokoue | 78.38 | 10.81 | 2.7 | 8.11 |
| Dschang | 54.83 | 19.35 | 16.13 | 9.68 |
|
|
|
Figure 2. Boophilus decoloratus (a, Females), Amblyomma hebraeum (a, Females) and Hyalomma rufipes male left (c)-females right (d). |
Effect of age on prevalence
The four ectoparasties encountered were found in all the age groups except for lice that were not present at 2, 6 and 7 years (Table 2). Irrespective of age group, the highest prevalence was recorded in B. decoloratus (69.8%), followed by A. habraeum (17.6%), then H. rufipes (10.8%) and B. equi (8%). Horses were more commonly infested with A. habraeum. Boophilus decoloratus was more prevalent in horses of all ages, while A. habraeum and H. rufipes preferred older horses of 6 and 7 years old. Meanwhile, lice were observed more on horses of 3 years (5.47%) and 4 years (3.62%) old.
| Age of horses (year) | Prevalence (%) | |||
|---|---|---|---|---|
| Boophilus decoloratus | Amblyomma habraeum | Hyalomma rufipes | Bovicola equi | |
| 1 | 92.58 | 4.45 | 1.84 | 1.48 |
| 2 | 72.34 | 14.89 | 12.76 | 0.00 |
| 3 | 70.89 | 13.83 | 9.80 | 5.47 |
| 4 | 67.77 | 17.43 | 11.19 | 3.62 |
| 5 | 69.91 | 17.26 | 10.85 | 1.98 |
| 6 | 62.89 | 23.27 | 13.83 | 0.00 |
| >7 | 52.13 | 13.91 | 15.96 | 0.00 |
The prevalence of ectoparasites in relation to sex of horses is illustrated in Table 3. Independent of sex, B. decoloratus had the highest prevalence (68.78%), followed by A. habraeum (17.4%), H. rufipes (11.1%), and B. equi (2.8%). Female horses were more commonly infested with B. decoloratus (83.2%) and B. equi (4.4%) than the males. On the other hand, A. habraeum (26.6%) and H. rufipes (17.4%) were more prevalent in males than females.
| Sex | Prevalence (%) | |||
|---|---|---|---|---|
| Boophilus decoloratus | Amblyomma habraeum | Hyalomma rufipes | Bovicola equi | |
| Male | 54.7 | 26.6 | 17.4 | 1.2 |
| Female | 83.2 | 7.9 | 4.5 | 4.4 |
The four parasites considered were found in horses of brown, white, black and grey colours, with B. decoloratus (61.98%) having the highest prevalence, followed by A. habraeum (20.41%), H. rufipes (13.95%) and B. equi (3.66%) (Table 4). Boophilus decoloratus showed higher preference for brown horses (71.69%) while Amblyomma showed higher prevalence in black horses (24.14%). The prevalence of H. rufipes (16.94%) was highest on grey horses as compared to brown horses (9.98%). Bovicola equi prevalences were 4.71% and 2.42% on grey and brown horses, respectively.
| Coat colour | Prevalence (%) | |||
|---|---|---|---|---|
| Boophilus decoloratus | Amblyomma habraeum | Hyalomma rufipes | Bovicola equi | |
| Brown | 71.69 | 16.00 | 9.89 | 2.42 |
| White | 59.91 | 21.95 | 14.34 | 3.80 |
| Black | 58.62 | 24.14 | 13.79 | 3.45 |
| Grey | 59.29 | 19.06 | 16.94 | 4.71 |
Monthly variation of ectoparasites
Irrespective of the month, the highest prevalence was seen in B. decoloratus, H. rufipes and lice (Table 5). Amblyomma habraeum (41.67%), H. rufipes (21.97%) and lice (9.84%) were more prevalent in the month of March, while B. decoloratus seemed to occur mostly in April, June, July and September (100%). The prevalence of A. habraeum dropped from 28.16% in April to 14.29% in September, with another peak in October (22.41%). Hyalomma rufipes had two peaks (16.88% and 17.24%) in the months of July and October, respectively. A peak in lice infestation was observed in the month of August (7.55%).
| Month | Prevalence (%) | |||
|---|---|---|---|---|
| Boophilus decoloratus | Amblyomma habraeum | Hyalomma rufipes | Bovicola equi | |
| March | 96.96 | 41.67 | 21.97 | 9.84 |
| April | 100 | 28.16 | 19.41 | 2.81 |
| May | 95.95 | 26.26 | 13.13 | 3.03 |
| June | 100 | 25.27 | 15.38 | 1.1 |
| July | 100 | 21.69 | 16.88 | 1.2 |
| August | 99.05 | 17.92 | 10.38 | 7.55 |
| September | 100 | 14.29 | 12.38 | 1.9 |
| October | 94.82 | 22.41 | 17.24 | 5.17 |
| November | 88.03 | 18.8 | 13.68 | 0.85 |
Predilection site of infestation
Boophilus decoloratus had no specific site and was found on every part of the horse. Biting lice were collected on the poll region of the head where grooming was difficult. Amblyomma habraeum and H. rufipes, were collected from the groin at the level of prepuce and testes or udder region.
Prevalence of ectoparasitic associations
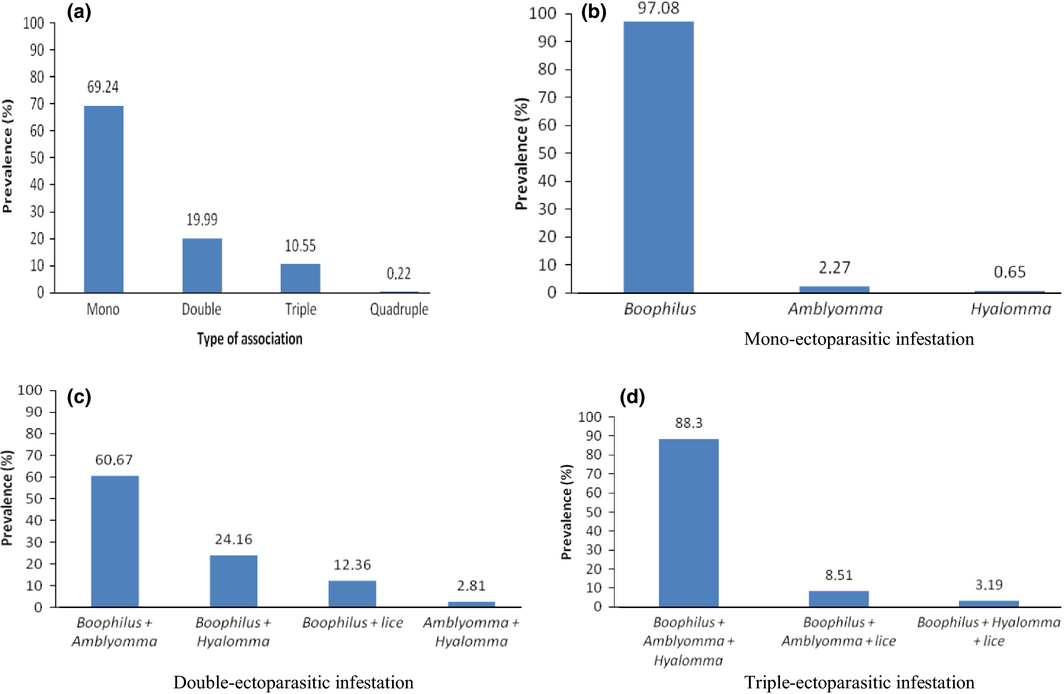
Four types of ectoparasitic prevalence associations were identified. Fig. 3(a–d) shows the combinations of different parasitic associations. Mono-parasitic infestation (69.24%, Fig. 3a) was the most frequent as compared to double (19.99%), triple (10.55%) and quadruple (0.22%) infestations. In mono-parasitic infestation (Fig. 3b), B. decoloratus (97.08%) had the highest prevalence, followed by A. habraeum (2.27%) and H. rufipes (0.65%). For double infestation, the most frequent was B. decoloratus + A. habraeum (60.67%). In triple associations (Fig. 3d), three types of combinations were observed. The highest prevalence recorded being B. decoloratus + A. habraeum + H. rufipes (88.3%), B. decoloratus + A. habraeum + B. equi (8.51%) and finally B. decoloratus + H. rufipes + B. equi (3.1%). Only two horses with poly-parasitic associations of four combinations (B. decoloratus + A. habraeum + H. rufipes + B. equi) were observed.
|
|
|
Figure 3. Prevalences of ectoparasitic associations: Mono (b), double (c) and triple association (d). |
Discussion and conclusion
Parasites continue to be a significant threat to the health of horses. From the results obtained, three species of hard ticks and one species of lice were identified using methods developed by Hoogstraal (1956) and Horak et al. (2002). However, other ectoparasites may have been present. Other parasites like mange mites may be present on the hooves of the horses. But provisions were not made to restraint the animals and collect these parasites.
Horses from all the localities infested with the parasites were identified as having a high prevalence, irrespective of age, sex, colour of horse, predilection site or month of the year. This may be due to the fact that horse breeders do not follow best practice guidelines. Generally, the prevalence of the species B. decoloratus was highest in all the localities followed by A. habraeum, H. rufipes and B. equi. This may be due to the fact that (1) B. decoloratus is a one host tick which parasitizes large mammals especially horses and cattle in the Neotropical, Afrotropical and Australian regions of the world (Abebaw 2004) (2) other ruminants such as cattle, sheep and goats are most of the time on free range and also pulled by ropes together to the markets by the shepherds or herds men. Due to its cosmopolitan nature, it may equally be found on other domestic and wild animals like cattle, sheep and goats as these potential host species are mostly managed free range and pulled by ropes to markets by shepherds. All three stages (adults, nymphs, larvae) engorge on the same animal and the two ecdyses also take place on the same host. Furthermore, B. decoloratus has a short basic capitulum, mouth parts and palps. When it attaches itself to feed on the thin skin of the horse, it buries its mouth parts deeply into the tissues of the host and remains attached until it is fully engorged. Boophilus decoloratus has a short life span, and it can have six generations per year. In Sub-Saharan Africa, their high rate of reproduction permits this tick to infest many animals.
The species A. habraeum prefers a hot climate where humidity is low (Kettle 2000). It was observed that H. rufipes (16.3%) and B. equi (9.68%) had a high prevalence in horses from Dschang as many animals transit through Dschang to the Littoral and Southwest regions dropping these parasites on the way and rendering other animals and the general public at risk. With respect to sex, we observed that A. hebraeum and H. rufipes preferred adult male horses because of predilection sites (prepuce and testes) where they are presumably hidden from predator birds. In females, they also prefer the udder for the same reasons as above. It is likely for the same reason that A. hebraeum was more prevalent in black horses compared to brown, white and grey horses. The increase in prevalence of B. decoloratus in March corroborates the findings of (Mangold et al. 1987), who observed that March being a transition period between the dry and rainy seasons offers conducive climatic conditions, which favour growth and multiplication of these ticks. A decrease in tick population in the months of April, May and June was reported in Rwanda (Nshimiyimana & Mutandwa 2002). They attributed this to the absence of conducive temperatures and relative humidity which favour the development of tick species.
Poor or primitive methods of horse management could have been responsible for some of the results obtained. These horses graze permanently on free range and pasture which is rarely treated thereby exposing them to frequent infestation. The high prevalence of these ectoparasites shows that very little or no veterinary services are rendered to these horses either for preventive or curative treatment. Furthermore, the high prevalence of H. rufipes (29.41%) and lice (17.65%) in Dschang indicates that the parasite came from animals transiting through Dschang. This implies that these horses have to be properly managed during their transit. Although tick load on cattle is usually low in the dry season, it tends to increase after the first rains, reaching its highest abundance 1 month after the heavy rains (i.e. from July to September), when all tick species are present (Okpala 1978).
The four ecto-parasites discussed are of veterinary importance because they are present in sufficiently large numbers to cause a pathogenic effect on the host thereby bringing economic loss to the breeder, either through stock loss or financing preventive measures such as dipping or showering (Hall 1986). To the animal, these parasites perforate the skin, predisposing the animal to secondary bacterial infections thus causing weight loss, skin deterioration, anaemia, anorexia and even pyrexia.
From this study, for some parasites, male horses were more infested than females, meanwhile horses between 3 and 4 years old were more infested than those between 6 and 7 years old. The prevalence of infestation is influenced by the origin of the animals, age, sex and colour. The most prevalent species were B. decoloratus, followed by A. habraeum, H. rufipes and B. equi. With respect to parasitic associations, infestation by one parasite was more prevalent compared to double, triple or quadruple infestation.
Strategic tick control using acaricides during the hot-wet and post-rainy seasons to avert major losses caused by high tick loads, especially in the Western High land of Cameroon is recommended.
Based on these findings, horse breeders are advised to regularly consult veterinarians for prophylactic and curative treatment of their animals and also consider pasture management.
Emerging infectious diseases (e.g. CCHFV) might originate from ticks, therefore, a disease like CCHFV is of public health importance and the Ministries of Public Health and Livestock, Fisheries and Animal Industries of Cameroon should take the responsibility of educating the public and the breeders of the importance of this and even other diseases. Current efforts to repopulate urban areas with improved breeds of horses require determination of the breeds performance and resistance to ticks and tick-borne diseases (TBDs) in urban areas. This information can also be useful in advising urban farmers on the selection and rearing of appropriate breeds that are tolerant to ticks and TBDs.
Acknowledgements
The authors express their sincere thanks to all the horse breeders, butchers, technician of the Laboratory of Animal Health (LASAN) and veterinarians who contributed to the realization of this work. They also thank Professor Zoli Pagnah Chief of LASAN of the Faculty of Agronomy and Agricultural Sciences (FASA) of the University of Dschang for his support.
Source of funding
None.
Conflict of Interest
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Contributions
The authors have no additional contributions to declare.
References
- Abebaw G.K. (2004) Seasonal dynamics of ticks (Amblyomma cohaerens, Boophilus decoloratus) and development of a management plan for tick and tick borne disease on cattle in Jimma zone (South Western Ethiopia), 147 p.
- Adda Q., Judy B., Judy T. & Sigrid W. (2001) Horse Talk. Avaliable at: www.bayquest.com/horsetalk/livestock.htm (Accessed December 30, 2014).
- Ahern J.J., Anderson D.P., Bailey D., Baker L.A., Arden Colette W., Shannon Neibergs J., North M.S., Potter G.D. & Stull C.L. (2006). The unintended consequenses of a ban on the humane slaughter (processing) of horses in the United States. Animal Welfare Council, Inc. May 15, 2006. April 30, 2014.
- Anne M.Z., Gary A.C. (2006) Veterinary clinical parasitology, 7th ed, p. 382. American Association of Veterinary Parasitologists (AAV), Blackwell Publishing.
- Asongwe G.A., Yerima B.P.K. & Tening A.S. (2014) Vegetable production and the livelihood of farmers in Bamenda Municipality, Cameroon. International Journal of Current Microbiology and Applied Sciences3, 682–700.
- Bob J. (2006) External Parasite Control in Horses. pp 1. Veterinary Information Network, Inc: Texas Farm Bureau Network.
- Brendal M. (2009) Identification, Prevention and control of external parasites on horses and Helium Pets and Animals, horses. External Parasite prevention.
- Hall H.T.B. (1986) Diseases and Parasites of Livestock in the Tropics. Intermediate Tropical Agriculture Series. Longman, London and New York, 2nd Ed. Pp. 327.
- Hoogstraal H. (1956). African Ixodoidea. I. Ticks of the Sudan. Pp. 1101. Research Report NM, Bureau of Medicine and Surgery, US Department of the Navy, Washington, DC.
- Horak I.G., Camicas J.L. & Kierans J.E. (2002) The argasidae, ixodidae and nuttalliellidae (Acari: Ixodida): a world list of valid tick names. Experimental and Applied Acarology28, 27–54.
- Hubalek Z. (2004) An annotated checklist of pathogenic microorganisms associated with migratory birds. Journal of Wildlife Diseases40, 639–659.
- Kaufman E., Koehler G. & Butler J.F. (2008) External Parasites on Horses. Cooperative Extension Service, University of Florida: US Department of Agriculture.
- Kettle D.S. (2000) Medical and Veterinary Entomology, CABI Publishing Series 2nd edn, pp. 690.
- Mackinnon M.J. (2014) The role of immunity in mosquito-induced attenuation of malaria virulence. Malaria Journal13, 25.
- Mangold A. J., Aguirre D.H., Gaido A.B., Guglielmone A.A. (1987) Seasonal variation of Ticks (Ixodidae) in Bos Taurus and Bos indicus cattle under rational grazing in forested and deforested habitats in Northwestern Argentina.
- Nshimiyimana J. & Mutandwa E. (2002) Seasonal dynamics and distribution of ticks in rwanda: implications for tick control strategy in rwanda. International Journal of Animal and Veterinary Advances2, 21–25.
- Okpala I. (1978) Studies on the ectoparasitic fauna of Nigerian livestock II: seasonal infestation rates. Bulletin of Animal Health Production in Africa16, 351–358.
- Randolph S. (2005) Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology129(S1), 37–65.
- Stephen J.G. & John R. (2003) Conservation biology livestock breeds and their conservation. A Global Overview7, 815–825.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?