Summary
Objective
Esthesioneuroblastoma is an uncommon tumor that is described widely among the Caucasians. In Singapore, we see predominantly Asian patients with esthesioneuroblastomas. From our experience, we note significant and interesting differences between our data on Asian patients and the published ones on the Caucasian patients.
Methods
A retrospective review of all patients who underwent craniofacial resection for esthesioneuroblastomas was conducted from January 1997 to January 2010. Relevant data were collected and statistical analyses were carried out to determine factors that predicted mortality or complications.
Results
Out of a total of 48 patients who underwent craniofacial resections, half had esthesioneuroblastomas (50%). There was a peak age distribution at the sixth decade of life and 62% of our patients were male. Both local and regional recurrence rate was 50%.
Conclusion
Majority of our Asian patients who underwent craniofacial resections had esthesioneuroblastomas. There is a male predilection, and we do not see a bimodal age distribution that is commonly reported.
Keywords
craniofacial resection;esthesioneuroblastoma;olfactory neuroblastoma;skull base tumors
1. Introduction
Esthesioneuroblastoma is an uncommon tumor that was first reported in 1924 by Berger and Luc.1 With a reported incidence of 3% for sinonasal neoplasms, there have been more than 1000 cases reported in the literature, mostly in the last two decades, but mainly as single case reports or case series.2 Even large institutions have reported only relatively small case series over decades, and, as such, statistical analysis of data was limited. Moreover, most of these publications are on the Caucasian population. In our center, which sees predominantly Asian patients for craniofacial resections, we note significant and interesting differences between our data and the published ones.
2. Materials and methods
This was a retrospective review of all craniofacial resections performed in our center, Singapore General Hospital, from January 1997 to January 2010. Our center is the largest tertiary hospital in Singapore, which sees predominantly Asian patients comprising about 74% Chinese, 14% Malays, and 9% Indians of our Singapore population.3 Our unique ethnic distribution allows us to analyze craniofacial resection within the Asian ethnicity. We specifically focused our data analysis on patients diagnosed with esthesioneuroblastomas.
Preoperatively, most patients will have a computed tomography (CT) scan of the paranasal sinus to assess bony invasion to paranasal sinuses or intracranial spread. A magnetic resonance imaging (MRI) scan is also obtained, unless the tumor encountered is in an early stage (Kadish Stage A), so as to assess for intracranial spread accurately and also to help distinguish between fluid within sinuses and tumor involvement. Having both modalities of imaging (CT and MRI scans) will help in more accurate assessment of staging of tumor.
All patients with esthesioneuroblastomas were then treated with a dual modality of craniofacial resection (described below) and adjuvant radiotherapy, as the tumor is known to be radiosensitive and it improves preservation of the eye.4 ; 5 Chemotherapy is considered for advanced, high-grade (Hyams histopathological grade) lesion, as it has been shown to improve long-term survival.6 ; 7
We categorized postoperative complications into wound-related (infection, dehiscence, and flap necrosis), central nervous system (CNS) (cerebrospinal fluid leakage, meningitis, and pneumocephalus), orbital (nasolacrimal duct obstruction, diplopia, and blindness), and systemic complications. This categorization was also used by the International Collaborative Study on craniofacial resections and will help in comparing our data with the large series of data that has been published thus far.8
Institutional Review Board (IRB) approval was sought prior to starting this retrospective audit.
2.1. Surgical technique
Most patients were operated on by the same two surgeons (an otolaryngologist and a neurosurgeon) with the same open approach. Endoscopic craniofacial resection by a second surgeon was performed only on three patients. An extended lateral rhinotomy was used for the transfacial approach to access the tumor intranasally with a bicoronal incision with periosteal flap used by the neurosurgeons. After the tumor had been cleared, intraoperative frozen sections were routinely used for all patients to ensure that margins were clear prior to conclusion of the operations. Olfactory bulbs were routinely excised even if they were not clinically involved to minimize local recurrence rates. This was because this tumor was known to recur locally more than 13 years postoperatively despite clear margins of resections intraoperatively.7 With regard to reconstruction, neurosurgeons routinely used a vascularized pericranial flap to close small defects. A temporalis fascia graft was usually used to augment the closure and a bone calvarium graft used when the defect was larger. Surgicel and Tisseel glue were then used to reinforce the closure. All nasolacrimal ducts were routinely marsupialized to prevent postoperative epiphora. A flavine-soaked pack was then used to pack the nasal cavity for 5 days postoperatively. All patients were then monitored in the neurosurgical intensive care unit (NICU) postoperatively. They were also given prophylactic intravenous amoxicillin–clavulanate for the first 5 days postoperatively.
3. Results
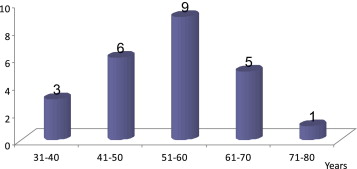
Over a period of 13 years, we had a total of 48 patients who underwent craniofacial resections. The majority of our patients had esthesioneuroblastomas (50%) (Table 1). The rest of the histological diagnoses were a heterogeneous mix of tumors. Among the patients with esthesioneuroblastomas, 22 were Chinese, one was Malay, and one was Indian. There was a peak incidence in the sixth decade of life (Fig. 1). We did not see the bimodal peak in age distribution commonly described in the second and sixth decade of life.9 A slight male predilection (62% males, 38% females) was present. The top three commonest symptoms of epistaxis, blocked nose, and rhinorrhea were observed in other series as well10 (Table 2). One of our patient was asymptomatic. His condition was diagnosed incidentally while performing a CT scan of the head for a stable head injury. We had seven patients with Kadish Stage A, 10 patients with Kadish Stage B, and seven patients with Kadish Stage C. Hyams histopathological grade was present in 15 out of 24 patients. Majority (10 out of 15 patients) had high-grade tumors (Hyams histopathological grade 3 or 4). It was interesting to note that none of our patients had cervical lymph nodal metastases at the initial presentation.
| Tumor | Number |
|---|---|
| Esthesioneuroblastoma | 24 (50%) |
| Squamous cell carcinoma | 3 |
| Adenocarcinoma | 3 |
| Meningioma | 3 |
| Clival chordoma | 2 |
| Sinonasal undifferentiated carcinoma | 2 |
| Adenoid cystic carcinoma | 2 |
| Nasopharyngeal carcinoma | 1 |
| Basosquamous carcinoma | 1 |
| Schwannoma | 1 |
| Inverted papilloma | 1 |
| Neuroblastoma | 1 |
| Recurrent giant cell tumor | 1 |
| Leiomyoma | 1 |
| Fibrous dysplasia maxillary sinus | 1 |
| Renal cell carcinoma metastases (adenocarcinoma) | 1 |
| Total | 48 |
|
|
|
Figure 1. Age distribution of patients with esthesioneuroblastomas. |
| Clinical symptoms | Number of patients |
|---|---|
| Epistaxis | 19 |
| Blocked nose | 11 |
| Rhinorrhea | 8 |
| Hyposmia | 7 |
| Headache | 1 |
| Blood-stained sputum | 1 |
| Asymptomatic | 1 |
Postoperative mortality occurred in one patient (2%). This patient had multiple comorbidities, with a recent coronary artery bypass graft 6 months prior to the craniofacial resections. He unfortunately sustained a massive acute myocardial infarction (AMI) on Postoperative Day 9 and passed away later. In our series, two more patients died from causes unrelated to the tumor. One patient died from pneumonia 2 years after the initial operation and the other from a urinary tract infection 5 years after the initial surgery. Both patients were free of esthesioneuroblastomas at the time of their death.
Postoperative complication rate occurred in 42% of patients (Table 3). Wound complications occurred in four patients (17%). One patient had transient epistaxis on Postoperative Day 6 after packs were removed, which resolved spontaneously without intervention. Another one had sinusitis with mucopus noted in the bilateral middle meatus, which was treated successfully with antibiotics. This was possibly from the nasal packing. One patient had a stitch granuloma that was noted after stitches were removed on Postoperative Day 8, which also resolved spontaneously. Another patient had a nasal valve collapse from the lateral rhinotomy incision that was placed too internal in the nares, which was noted 3 months after the operation. As this was not causing too great a discomfort, this was not treated further.
| Complications (number of patients) | Details of complications |
|---|---|
| Wound (n = 4) | Epistaxis |
| Sinusitis | |
| Stitch granuloma | |
| Nasal valve collapse | |
| Central nervous system (n = 3) | Meningitis |
| Frontal lobe abscess | |
| Scar epilepsy | |
| Systemic (n = 2) | Urinary tract infection |
| Atelectasis | |
| Orbital (n = 1) | Epiphora |
CNS complication occurred in three patients (13%). One patient had a frontal lobe abscess that subsequently required drainage by the neurosurgeons. This patient recovered well after the procedure. Another patient sustained meningitis, but, fortunately, this resolved with intravenous antibiotics. The third patient developed frontal lobe scar epilepsy that occurred 2 years after the operation. This patients condition was controlled well with antiepileptic medications.
Systemic complication occurred in two patients (8%). One patient had a urinary tract infection that occurred on Postoperative Day 6, and was possibly precipitated by immobility and the urine catheter present in situ postoperatively. Antibiotics aborted the infection successfully. The other patient had pneumonia that occurred on Postoperative Day 2. This also resolved successfully with antibiotic therapy.
Orbital complication occurred in one patient (4%). This patient had epiphora despite marsupialization of the nasolacrimal duct intraoperatively. This was possibly due to postoperative edema that compressed the nasolacrimal duct; fortunately, this resolved spontaneously.
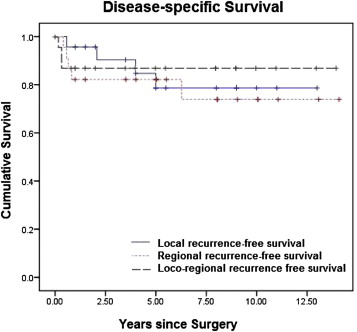
In our series, recurrences (local and regional) occurred in 12 patients. Patients with regional recurrence all had cervical lymph nodal involvement, and these patients subsequently went on to receive a neck dissection. All patients with local recurrences were fortunately resectable, as they were picked up promptly on postoperative surveillance; they all underwent a revision craniofacial resection. Fig. 2 shows the disease-specific survival depending on local, regional, or loco-regional recurrences. Most of the recurrences occurred within the first year after the initial operation. Some recurrences (either local or regional) occurred more than 5 years after the initial diagnosis. Two out of three patients with both local and regional recurrences had Hyams histopathological grade 3, while one of them had Hyams histopathological grade 4 esthesioneuroblastoma.
|
|
|
Figure 2. Disease-specific survival. |
4. Discussion
Esthesioneuroblastomas are uncommon tumors. As a result, individual institutions often do not have a significant number of patients to report meaningful results regarding survival outcomes and postoperative mortality and complications. A collaborative study for esthesioneuroblastomas across multiple centers will be useful in achieving meaningful statistical analyses to increase our understanding of this disease. Our retrospective study is primarily a descriptive study within a single institution that aims at analyzing our experience with esthesioneuroblastomas and comparing our results with overseas institutions.
In our series of craniofacial resections on Asian patients, 50% were diagnosed with esthesioneuroblastomas compared with 3–12.6% quoted in other institutions' series of craniofacial resections.2 ; 8 This is suggestive of a possibly higher incidence of esthesioneuroblastomas, although a population-based study would be necessary to confirm this. Another reason could be that our hospital is a tertiary center that sees patients from the south-east Asia region, and this could potentially result in a selection bias. There was also a male predilection in our series, in contrast with the female preponderance in several literature published on the Caucasian population.2 Also, there is a lack of a bimodal age distribution that is commonly described for esthesioneuroblastomas.2 Our patients afflicted with this disease are older, as we do not see patients in the second decade of life with esthesioneuroblastomas. These differences may suggest there could be a genetic basis for the differences between Asian and Caucasian patients suffering from esthesioneuroblastomas. Preliminary genetic studies on the tumors have already shown that different chromosomal alterations may lead to different biological behavior.4 It could also be extrapolated that Asian patients may have different genetic subtypes of this disease, although further genetic studies are needed to confirm this. Also, a multicenter study within Asia would be helpful in achieving larger numbers for statistical significance in the clinical and genetic study of this relatively uncommon tumor worldwide.
Our postoperative mortality rate of 2% was lower than the 4.7% quoted for craniofacial resections.8 One patient passed away from an AMI. He was a high-risk patient who went for a coronary artery bypass graft just 6 months prior to his craniofacial resection and, despite preoperative optimization by the anesthetist, the AMI could not be prevented. Our low mortality rate can be attributed to a dedicated combined specialty team that is familiar with patients who undergo neurosurgical treatment. After the operation, which is just the first step, all patients are transferred to the NICU whereby dedicated doctors and nurses trained in monitoring these patients take over. They are usually kept in the NICU for 5 days postoperatively and moved to a step-down or high-dependency unit only after assessment by the two primary surgeons. We had two other patients who passed away from causes unrelated to esthesioneuroblastomas. This tumor seems to have a fairly good prognosis, with no tumor-related mortality in our series thus far despite either local or regional recurrences. Most patients can further be treated and can have good survival subsequently. Two of our patients died of other diseases and not from the primary disease itself.
Our postoperative morbidity of 42% was comparable to that quoted in the literature, which varies from 30% to 54%.5; 6; 7; 10 ; 11 Similar to the classification of postoperative morbidity by the International Collaborative Study for craniofacial resections, we classified it into wound-related, CNS-related, systemic, and orbital-related complications, as these were the significant complications that could have happened in our series8 (Table 3). The commonest complication in our series was wound-related complication, followed by CNS-related, systemic, and orbital-related complications. This finding was similar to that of the International Collaborative Study as well.8
Esthesioneuroblastoma is a disease that is known to possibly have late recurrences. Therefore, recurrence rate in any series depends on the duration of follow-up. Our recurrence rate of 50% over a 13-year period is comparable to that of data from University of Virginia, which quoted an overall recurrence rate of 51.3% over a 20-year period.11 The mean follow-up for our patients was 7.3 years. For esthesioneuroblastomas, the longer the follow-up, the higher the recurrence rate. The important point to note is that, despite the recurrences, patients can be treated adequately to have a good survival prospect subsequently. None of our patients in this series died from tumor-related causes despite the recurrences.
5. Conclusion
Our study on Asian patients with esthesioneuroblastomas indicated that the patients tend to be male and in the older age group. This is different from the female preponderance in the Caucasian population, where the patients tend to have a bimodal age distribution, with a significant proportion being affected in the second decade of life as well. We also know that craniofacial resection is a safe procedure for esthesioneuroblastomas with a low mortality rate. As recurrences can occur several years after the initial surgery, lifelong postoperative surveillance of patients is important, and when recurrences happen, adequate salvage treatment can ensure good survivability of patients.
Conflict of interest statement
No financial or material support was received for this study. The authors have no conflicts of interest to declare.
References
- 1 L. Berger, R. Luc; L'esthesioneuroepitheliome olfactif; Bull Assoc Franc Etude Cancer, 13 (1924), pp. 410–421
- 2 I. Broich, A. Pagliari, F. Ottaviani; Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924; Anticancer Res, 17 (1997), pp. 2683–2706
- 3 Singapore Census of Population; Statistical Release 1: Demographic Characteristics, Education, Language and Religion; (2010) http://www.singstat.gov.sg/pubn/catalogue.html#sib
- 4 P.A. Levine, W.C. McLean, R.W. Cantrell; Esthesioneuroblastoma: the University of Virginia Experience 1960–1985; Laryngoscope, 96 (1986), pp. 742–746
- 5 W.S. McCary, P.A. Levine, R.W. Cantrell; Preservation of the eye in the treatment of sinonasal malignant neoplasms with orbital involvement. A confirmation of the original treatise; Arch Otolaryngol Head Neck Surg, 122 (1996), pp. 657–659
- 6 J.M. Sheehan, J.P. Sheehan, J.A. Jane, R.S. Polin; Chemotherapy for esthesioneuroblastoma; Neurosurg Clin N Am, 11 (2000), pp. 693–701
- 7 E.A. McElroy, J.C. Buckner, J.E. Lewis; Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience; Neurosurgery, 42 (1998), pp. 1023–1028
- 8 I. Ganly, S.G. Patel, B. Singh, et al.; Complications of craniofacial resection for malignant tumors of the skull base: report of an International Collaborative Study; Head Neck, 27 (2005), pp. 445–451
- 9 A. Morita, M.J. Ebersold, K.D. Olsen, R.L. Foote, J.E. Lewis, L.M. Quast; Esthesioneuroblastoma: prognosis and management; Neurosurgery, 32 (1993), pp. 706–715
- 10 J. Constantinidis, H. Steinhart, M. Koch, et al.; Olfactory neuroblastoma: the University of Erlangen-Nuremberg Experience 1975–2000; Otolaryngol Head Neck Surg, 130 (2004), pp. 567–574
- 11 R.J. Oskouian Jr., J.A. Jane Sr., A.S. Dumont, J.M. Sheehan, J.J. Laurent, P.A. Levine; Esthesioneuroblastoma: clinical presentation, radiological, and pathological features, treatment, review of the literature, and the University of Virginia experience; Neurosurg Focus, 12 (2002), p. e4 [Review 2002]
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?