Abstract
Background
Gastroesophageal reflux disease (GERD) is known as a common comorbidity of asthma and chronic cough. The impact of GERD symptoms on cough-specific quality of life (QoL) in patients with asthmatic cough is poorly understood. The aim of this study is to determine the association of GERD symptoms with cough-specific quality of life in patients with cough variant asthma (CVA) using the Leicester Cough Questionnaire (LCQ).
Methods
A total of 172 consecutive patients (121 females) with mean cough duration of 45.1 months (range 2–480 months) completed the Japanese version of the LCQ. The Frequency Scale for the Symptoms of Gastroesophageal reflux was administered to assess symptoms of acid-reflux and dysmotility. A range of clinical variables that may determine cough-specific QoL (LCQ) were estimated.
Results
The mean LCQ scores was 12.9 (SD 3.5), consistent with severe impairment in QoL. Female gender, symptoms of gastroesophageal dysmotility, sensitization to allergens (house dust and Japanese cedar pollen) and the number of sensitized allergens were associated with lower LCQ scores (i.e. impaired cough-specific QoL) in univariate regression analysis. Acid-reflux symptoms, airway hyperresponsiveness, fractional exhaled nitric oxide, and sensitization to molds were unrelated to the LCQ score. After adjustment for gender, symptoms of gastroesophageal dysmotility was the only significant determinant of impaired cough-specific QoL accounting for 23% of the variance.
Conclusions
Cough-specific QoL is severely impaired in patients with CVA. Symptoms of gastroesophageal dysmotility are an independent predictor of cough-specific QoL of patients with CVA.
Keywords
Chronic cough; Cough variant asthma; Gastroesophageal dysmotility; The Leicester Cough Questionnaire; Quality of life
Abbreviations
AHR, Airway hyperresponsiveness; CVA, Cough variant asthma; FeNO50, Fractional exhaled nitric oxide at 50 mL/s; FEV1, Forced expiratory volume in 1 s; FSSG, Frequency scale for the symptoms of gastroesophageal reflux; FVC, Forced vital capacity; GERD, Gastroesophageal reflux disease; IgE, Immunoglobulin E; LCQ, Leicester Cough Questionnaire; QoL, Quality of life; Rrs, Respiratory resistance; VAS, Visual analog scales
Introduction
Cough is a very common complaint for which patients seek medical attention. Although most cases are acute and self-limiting, some patients develop chronic cough that persists for 8 weeks or longer and are referred to specialists because chronic cough is associated with impairment of health-related quality of life (QoL).1 Cough variant asthma (CVA) is considered one of the most common causes of chronic cough worldwide.2, 3, 4, 5 and 6 The factors that contribute to the impairment of cough-specific QoL in patients with CVA or asthmatic cough are only partly understood. In studies of patients with asthma, cough-specific QoL assessed with the Leicester Cough Questionnaire (LCQ) was associated with objective cough counts,7 but not with pathophysiological parameters such as airway hyperresponsiveness (AHR), airway inflammation and pulmonary function.8 The impact of other potentially relevant factors on cough-specific QoL such as gastroesophageal reflux disease (GERD) and atopy have not been studied. The identification of important comorbidities may lead to a more targeted therapeutic approach.
GERD is another common cause of chronic cough.9 GERD co-exists frequently in difficult-to-treat patients with asthma and CVA.10 and 11 In a survey of a general population, symptoms of regurgitation and irritable bowel syndrome, but not heart burn, were significantly associated with the presence of chronic cough, suggesting that gastroesophageal dysmotility may be more importantly associated with chronic cough than acid reflux.12
The Frequency Scale for the Symptoms of GERD (FSSG) is a questionnaire which has been developed for not only diagnosis but also evaluation of the treatment response of GERD.13 It covers the 12 most common symptoms of GERD consisting of 2 domains: acid-reflux symptoms and dysmotility symptoms. Previous study has demonstrated association of endoscopic13 and manometric14 findings with FSSG scores. Thus, the FSSG could be a simple and useful tool to assess symptoms of GERD in clinical and research fields of respiratory medicine.15, 16, 17 and 18
The aim of this study was to determine the impact of GERD symptoms on cough-specific QoL in patients with CVA. This involved the development and validation of a Japanese version of the LCQ.
Methods
Patients
Among patients who visited the Asthma and Chronic Cough Clinic of Kyoto University Hospital between July 2009 and December 2012, 260 patients were newly diagnosed as having CVA. This was according to the Japanese Respiratory Society guidelines for management of cough on the basis of isolated cough persisting for 8 weeks or longer without dyspnea or wheezing, no remarkable findings on chest X-ray and auscultation on the lung, presence of AHR to inhaled methacholine and improvement of coughing in response to inhaled short-acting β2 agonists (SABA).3 Double-blind controlled study conducted by Irwin et al. clearly demonstrated that improvement of cough with bronchodilators such as SABA was the essential diagnostic feature of CVA. 19 The patients had no history of typical asthma with wheezing, or upper respiratory infection within the previous 8 weeks. A total of 88 patients were excluded from the study because they were current smokers or ex-smokers who had smoked within 6 months or had a smoking history of more than 10 pack-years, failed to complete the LCQ or the FSSG or had been taking inhaled corticosteroids within 4 weeks prior to the first visit. As a result, the study involved 172 patients (121 females) in a cross-sectional design. This study was approved by the ethics committee of Kyoto University (Approval number E715), and was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government (23591117). Written informed consent was obtained from all participants.
Measurements of clinical indices
Patients underwent spirometry and measurement of AHR, fractional exhaled nitric oxide (FeNO), blood eosinophil counts and serum levels of total/specific IgE. They also filled in the LCQ and the FSSG questionnaires. Spirometry was performed using a ChestGraph HI-701 spirometer (Chest MI Corp, Tokyo, Japan) according to the current guidelines.20 AHR was examined by a continuous methacholine inhalation with simultaneous measurement of respiratory resistance (Rrs) (cmH2O/L/s) (Astograph; Chest, Tokyo, Japan) as described previously.21 FeNO levels were measured at an expiratory flow rate of 50 mL/s with a chemiluminescence analyzer (NOA 280; Sievers, Boulder, CO, USA) according to current guidelines.22 Specific IgE titers were measured against nine allergens: house dust, Dermatophagoides pteronyssinus, Japanese cedar pollen, mixed gramineae pollens (orchard grass, sweet vernal grass, bermuda grass, timothy, reeds), mixed weed pollens (ragweed, mugwort, goldenrod, dandelion, oxeye daisy), mixed moulds (Penicillium, Cladosporium, Aspergillus, Candida, Alternaria), cat dander, dog dander, and Trichophyton rubrum) (ImmunoCAP®; Phadia K.K., Tokyo, Japan). Specific IgE levels ≥0.35 UA/mL were considered positive. If patients had one or more sensitized allergens, they were considered as having atopic predisposition.
Measurement of cough-specific QoL and cough severity
Cough-specific QoL was evaluated using the LCQ, which was translated into Japanese. The Leicester Cough Questionnaire (LCQ) is a validated questionnaire that evaluates the degree of chronic cough and reflects cough-specific QoL.23 It consists of 19 questions covering 3 subdomains (physical, social, and psychological); the total scores range from 3 to 21, with the higher scores indicating better QoL.23 We performed forward and backward translation according to The International Quality of Life Assessment protocol.24 Briefly, two Japanese specialists of cough, both of who are familiar with English, produced the Japanese version of the LCQ (first forward translation). Followed by first backward translation conducted by a professional English editor who belongs to Forte corporation (Tokyo, Japan), the original developer (SSB) checked it and pointed out problems. Based on the original developers comments, some parts of the original questionnaire were retranslated into Japanese (second version of the forward translation). After this version was re-translated into English again, original developer rechecked it and confirmed that intended questions did not change.23 and 24 The Japanese translators including one of the present authors (AN) were authorized to do the translation of the LCQ and to have the copyright for the Japanese version of LCQ by the original author.23 Previous studies demonstrated significant correlations between the LCQ and objective cough monitoring.7 and 25
The use of LCQ questionnaire was permitted by the original developer (SSB)
In addition to the LCQ, cough visual analog scales (VAS) and numeric cough scores,26 both of which have been used to measure the severity and frequency of cough, have been also determined since January 2012. Cough VAS is a 100 mm-line scale where its length represents the severity of cough7 and 25 (i.e. 0 mm = no cough, 100 mm = worst cough imaginable). Numeric cough scores were developed by Hsu et al. 26 ranging from 0 to 5 reflect the cough frequency and severity in the daytime and nighttime. 7, 25 and 26 Lower score indicates less severe or less frequent coughing.
The GERD questionnaire
GERD symptoms were evaluated using the original version of self-reporting FSSG questionnaire originally developed in Japan,13 which was validated on the basis of endoscopic findings. It consists of the most prevalent 12 symptoms chosen from 50 symptoms of GERD. Each symptom was scored according to its frequency as follows: 0 = never, 1 = occasionally, 2 = sometimes, 3 = often, and 4 = always.13 The maximum scores of the acid-reflux symptoms domain (comprised of 7 items), dysmotility symptoms domain (comprised of 5 items), and the total of both were 28, 20, and 48 points, respectively.13 A previous study demonstrated that esophageal peristaltic pressures during dry swallow which were measured using a manometry catheter via a transnasal endoscope showed a negative correlation with FSSG scores.14 Permission for use of the FSSG was obtained from Dr. Kusano, the original developer of the FSSG.
Statistical analysis
Statistical analyses were performed using JMP 9.0 (SAS Institute Inc., Tokyo, Japan). Data are presented as means (SD). The effect of clinical variables on the LCQ scores was estimated using univariate regression analysis. When data were not distributed normally, they were log-transformed. Sex, smoking status, atopic predisposition, and sensitized specific antigens were analyzed as dichotomous variables. Multivariate regression analysis was used to examine whether individual factors contributed independently to the LCQ. Variables with p ≤ 0.10 on the univariate analysis were involved in the multivariate analysis. Total score of the FSSG was excluded from multivariate analysis because of strong correlation with dysmotility-like dyspepsia symptoms score of the FSSG. Correlations between the LCQ and cough visual analog scales or numeric cough scores were conducted using Pearsons correlation test in a subset of patients (n = 54). A p value ≤ 0.05 was considered significant. To confirm the reliability of the LCQ translated into Japanese, Cronbachs α coefficient, an index of reliability, was calculated. 27 Alpha coefficient ranges from 0 to 1 and its value represents the degree to internal consistency within an instrument. Cronbachs α coefficient of 0.7 or greater is generally considered to guarantee an acceptable reliability.27
Results
Patients' characteristics
The characteristics of the 172 patients with CVA are presented in Table 1. Overall, 70% of patients were female, and 30 patients were ex-smokers with a median smoking history of 3.9 (3.3) pack-years. Cough duration ranged from 2 to 480 months. The results of spirometry, AHR, and FeNO are also shown in Table 1.
| Gender, females/males | 121/51 |
| Age, years old | 51.6 ± 17.5 |
| Disease duration, months | 45.1 ± 81.6 |
| Body mass index, kg/m2 | 22.5 ± 3.4 |
| Smoking, never/ex-smoker | 142/30 |
| FEV1, L | 2.73 ± 0.9 |
| %FEV1, % predicted | 109.6 ± 20.6 |
| FEV1/FVC, % | 81.5 ± 7.8 |
| Fractional exhaled nitric oxide at 50 ml/s, ppb | 25.4 ± 17.1 |
| AHR (Dmin†, unit) | 8.6 ± 11.7 |
| Blood eosinophil, μL | 179 ± 163 |
| Total IgE, IU/ml | 65 (0–5400) |
| Antigen specific IgE (positive), % | – |
| Mixed gramineaes pollens | 26.7 |
| Mixed moulds | 11.6 |
| Mixed weed pollens | 9.3 |
| House dust | 30.2 |
| Dermatophagoides pteronyssinus | 30.2 |
| Japanese cedar pollen | 48.8 |
| Dog dander | 9.9 |
| Cat dander | 8.7 |
| Trichophyton rubrum | 10.5 |
| Sensitization to aeroallergen at least 1 or more, % | 61.0 |
| Number of sensitized allergens | 1.8 ± 2.2 |
| The Leicester Cough Questionnaire, Total scores | 12.9 ± 3.5 |
| FSSG, total scores | 6.5 ± 6.8 |
| Reflux-related symptoms domain | 3.6 ± 3.7 |
| Dysmotility symptoms domain | 2.9 ± 3.6 |
| Cough visual analog scales, mm‡ | 51.5 ± 21.4 |
| Numeric cough scores at daytime‡ | 2.8 ± 0.9 |
| Numeric cough scores at nighttime‡ | 1.5 ± 1.1 |
Data are expressed as means ± SD except for median (range) for serum total IgE levels.
†. Dmin: the cumulative dose of inhaled methacholine at the inflection point at which Rrs began to increase.
‡. n = 54. FSSG, the Frequency Scale for the symptoms of gastroesophageal reflux.
The evaluation of the reliability and validity of the Japanese version of the LCQ
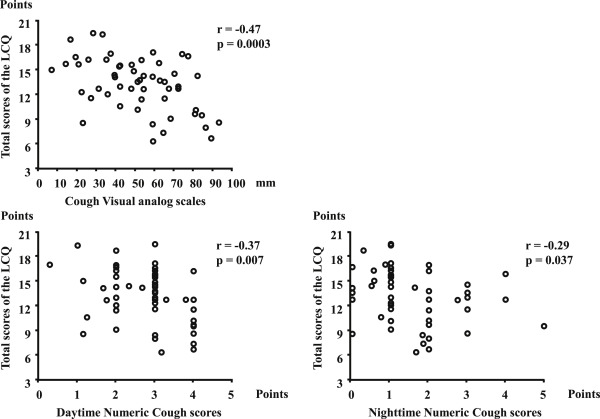
The Cronbachs α coefficient for total scores of the LCQ examined for the whole 172 patients was 0.91 and that for the three domains ranged from 0.79 to 0.85, indicating a good internal consistency for the Japanese version of the LCQ. To assess the construct validity between the Japanese version of the LCQ and other standard instruments utilized to assess cough severity and frequency, correlations of the Japanese version of the LCQ with cough VAS and numeric scores were determined in 54 patients. We found significant correlations between the LCQ scores and cough VAS or numeric cough scores (Fig. 1).
|
|
|
Fig. 1. The association between the LCQ scores and cough VAS or cough numeric scores in patients with cough variant asthma. Associations of cough VAS and cough numeric scores of the LCQ were plotted. |
Measurements of the LCQ and the FSSG
The mean LCQ and FSSG scores are given in Table 1. The average of total, physiological, social and psychological scores of the LCQ were 12.9 (3.5), 4.6 (1.0), 4.1 (1.2) and 4.2 (1.5), respectively. The total score was as low as those reported in previous studies of chronic cough (12.0–14.9),28, 29 and 30 indicating that our patients with CVA had impaired health status due to their coughing.
According to the FSSG scores, acid-reflux symptoms were observed in 57 patients (33%). Meanwhile, 73 patients (42%) complained of dysmotility symptoms.
Relationships between the LCQ and clinical variables
Univariate regression analysis showed that female gender, increased dysmotility symptoms domain and total FSSG scores, sensitization to house dust and Japanese cedar pollen, and increased number of sensitized allergens were associated with lower LCQ scores (Table 2). Lower FEV1 was marginally associated with the impairment of cough-specific QoL. Other variables, such as acid-reflux symptoms, AHR, FeNO50, and sensitization to molds were unrelated to the LCQ scores. Multivariate regression analysis showed that the LCQ scores was independently associated with female gender and higher dysmotility symptoms scores (Table 3). After the adjustment for gender, we found that dysmotility symptoms was solely associated with the impairment of cough-specific QoL (Table 4).
| Variables | Estimates | 95% C.I. | p value |
|---|---|---|---|
| Gender, females | −0.78 | −1.34; −0.22 | 0.006 |
| Log disease duration, months | −0.35 | −1.02; 0.32 | 0.31 |
| Age, years old | 0.02 | −0.01; 0.05 | 0.29 |
| Body mass index, kg/m2 | −0.02 | −0.17; 0.13 | 0.79 |
| Smoking, ex-smoking | 0.64 | −0.04; 1.32 | 0.066 |
| Log FEV1, L | 3.15 | −0.55; 6.86 | 0.095 |
| %FEV1, % pred | 0.01 | −0.01; 0.04 | 0.31 |
| Log FEV1/FVC, % | −2.90 | −14.9; 9.07 | 0.63 |
| Log fractional exhaled nitric oxide at 50 ml/s, ppb | 0.94 | −1.28; 3.16 | 0.41 |
| Log Dmin, unit | 0.14 | −0.99; 1.26 | 0.81 |
| Blood eosinophil, μL | 0.001 | −0.002; 0.005 | 0.40 |
| Atopic disposition | −0.68 | −1.75; 0.38 | 0.21 |
| Log total IgE, IU/ml | −0.63 | −1.39; 0.12 | 0.10 |
| Antigen specific IgE, positive | – | – | – |
| Mixed gramineaes pollens | −0.61 | −1.78; 0.56 | 0.31 |
| Mixed moulds | 0.18 | −1.81; 1.44 | 0.82 |
| Mixed weed pollens | −1.49 | −3.27; 0.29 | 0.10 |
| House dust | −1.17 | −2.29; −0.05 | 0.041 |
| Dermatophagoides pteronyssinus | −1.07 | −2.20; 0.05 | 0.061 |
| Japanese cedar pollen | −1.11 | −2.14; −0.08 | 0.034 |
| Dog dander | −0.61 | −2.35; 1.14 | 0.49 |
| Cat dander | −1.58 | −3.41; 0.25 | 0.091 |
| Trichophyton rubrum | −0.48 | −2.18; 1.22 | 0.58 |
| Number of sensitized allergen | −0.26 | −0.49; −0.02 | 0.032 |
| FSSG, total scores | −0.08 | −0.15; 0.00 | 0.050 |
| Reflux-related symptoms domain | −0.09 | −0.23; 0.05 | 0.23 |
| Dysmotility symptoms domain | −0.18 | −0.32; −0.04 | 0.014 |
FSSG, the Frequency Scale for the symptoms of gastroesophageal reflux.
| Variables | Estimates | 95% C.I. | p value |
|---|---|---|---|
| Gender, females | −0.82 | −1.54; −0.11 | 0.024 |
| Log FEV1, L | 2.29 | −2.54; 7.12 | 0.35 |
| Log total IgE, IU/ml | −0.16 | −1.51; 0.58 | 0.38 |
| Antigen specific IgE, positive | – | – | – |
| Mixed weed pollens | −2.00 | −4.70; 0.69 | 0.14 |
| House dust | −3.17 | −7.92; 1.58 | 0.19 |
| Dermatophagoides pteronyssinus | 0.98 | −3.80; 5.76 | 0.69 |
| Japanese cedar pollen | −1.29 | −2.82; 0.24 | 0.097 |
| Cat dander | −0.84 | −3.11; 1.43 | 0.46 |
| Number of sensitized allergen | 0.47 | −0.34; 1.28 | 0.26 |
| Dysmotility symptoms domain | −0.23 | −0.30; −0.02 | 0.026 |
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Estimates | 95% C.I. | p value | Estimates | 95% C.I. | p value | |
| Log disease duration, months | −0.39 | −1.05; 0.27 | 0.31 | |||
| Age, years old | 0.02 | −0.004; 0.05 | 0.094 | −0.001 | −0.03; 0.03 | 0.91 |
| Body mass index, kg/m2 | −0.04 | −0.19; 0.11 | 0.62 | |||
| Smoking, ex-smoking | 0.41 | −0.29; 1.11 | 0.25 | |||
| Log FEV1, L | −0.13 | −4.89; 4.63 | 0.96 | |||
| %FEV1, % pred | 0.01 | −0.01; 0.04 | 0.31 | |||
| Log FEV1/FVC, % | −3.8 | −15.6; 7.96 | 0.52 | |||
| Log fractional exhaled nitric oxide at 50 ml/s, ppb | 0.12 | −2.14; 2.38 | 0.91 | |||
| Log Dmin, unit | 0.02 | −1.04; 1.09 | 0.96 | |||
| Blood eosinophil, μL | 0.001 | −0.002; 0.004 | 0.64 | |||
| Atopic disposition | −0.98 | −2.03; 0.08 | 0.069 | |||
| Log total IgE, IU/ml | −0.76 | −1.50; −0.01 | 0.047 | −0.45 | −1.49; 0.59 | 0.39 |
| Antigen specific IgE, positive | – | – | – | |||
| Mixed gramineaes pollens | −0.77 | −1.93; 0.38 | 0.19 | |||
| Mixed moulds | −0.46 | −2.07; 1.14 | 0.57 | |||
| Mixed weed pollens | −1.88 | −3.64; −0.13 | 0.036 | −1.63 | −4.23; 0.96 | 0.22 |
| House dust | −1.78 | −2.91; −0.64 | 0.002 | −2.56 | −7.30; 2.18 | 0.29 |
| Dermatophagoides pteronyssinus | −1.67 | −2.81; −0.54 | 0.004 | 0.80 | −3.98; 5.58 | 0.74 |
| Japanese cedar pollen | −1.42 | −2.44; −0.41 | 0.006 | −1.21 | −2.75; 0.32 | 0.12 |
| Dog dander | −1.04 | −2.78; 0.69 | 0.24 | |||
| Cat dander | −1.64 | −3.44; 0.15 | 0.072 | −0.73 | −3.01; 1.55 | 0.53 |
| Trichophyton rubrum | −0.64 | −2.32; 1.03 | 0.45 | |||
| Number of sensitized allergen | −0.36 | −0.60; −0.13 | 0.003 | 0.27 | −0.43; 0.98 | 0.44 |
| FSSG, total scores | −0.07 | −0.14; 0.004 | 0.063 | |||
| Reflux-related symptoms domain | −0.08 | −0.22; 0.06 | 0.27 | |||
| Dysmotility symptoms domain | −0.17 | −0.31; −0.03 | 0.017 | −0.16 | −0.30; −0.02 | 0.026 |
FSSG, the Frequency Scale for the symptoms of gastroesophageal reflux.
Among the whole 172 patients, 24 patients were treated with proton pump inhibitors and/or prokinetic therapy, and 10 patients reported a reduction in cough, verified by review of case notes. When these 10 patients who responded to GERD therapy in addition to CVA were removed from analysis, dysmotility symptoms still remained a significant predictor of the impairment of cough-specific QoL together with female gender (Table 5).
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Estimates | 95% C.I. | p value | Estimates | 95% C.I. | p value | |
| Gender, females | −0.85 | −1.42; −0.27 | 0.004 | −1.04 | −1.62; −0.46 | 0.0005 |
| Log disease duration, months | −0.27 | −0.96; 0.42 | 0.44 | |||
| Age, years old | 0.02 | −0.007; 0.05 | 0.13 | |||
| Body mass index, kg/m2 | 0.01 | −0.15; 0.18 | 0.87 | |||
| Smoking, ex-smoking | 0.64 | −0.05; 1.34 | 0.069 | |||
| Log FEV1, L | 2.99 | −0.79; 6.78 | 0.12 | |||
| %FEV1, % pred | 0.02 | −0.005; 0.05 | 0.11 | |||
| Log FEV1/FVC, % | −4.05 | −16.1; 8.03 | 0.51 | |||
| Log fractional exhaled nitric oxide at 50 ml/s, ppb | 1.08 | −1.20; 3.35 | 0.35 | |||
| Log Dmin, unit | 0.17 | −1.03; 1.38 | 0.78 | |||
| Blood eosinophil, μL | 0.002 | −0.002; 0.005 | 0.33 | |||
| Atopic disposition | −0.78 | −1.89; 0.33 | 0.17 | |||
| Log total IgE, IU/ml | −0.63 | −1.42; −0.15 | 0.11 | |||
| Antigen specific IgE, positive | – | – | – | |||
| Mixed gramineaes pollens | −0.55 | −1.76; 0.64 | 0.36 | |||
| Mixed moulds | −0.04 | −1.75; 1.68 | 0.97 | |||
| Mixed weed pollens | −1.35 | −3.20; 0.50 | 0.15 | |||
| House dust | −1.18 | −2.33; −0.03 | 0.045 | −2.33 | −6.97; 2.30 | 0.32 |
| Dermatophagoides pteronyssinus | −1.08 | −2.23; 0.08 | 0.067 | 1.13 | −3.57; 5.84 | 0.63 |
| Japanese cedar pollen | −1.16 | −2.23; −0.10 | 0.032 | −1.10 | −2.58; 0.39 | 0.15 |
| Dog dander | −0.48 | −2.34; 1.37 | 0.61 | |||
| Cat dander | −1.62 | −3.47; 0.22 | 0.084 | −0.85 | −3.16; 1.47 | 0.50 |
| Trichophyton rubrum | −0.49 | −2.35; 1.37 | 0.61 | |||
| Number of sensitized allergen | −0.26 | −0.51; −0.02 | 0.037 | 0.08 | −0.46; 0.62 | 0.777 |
| FSSG, total scores | −0.07 | −0.15; 0.01 | 0.095 | |||
| Reflux-related symptoms domain | −0.07 | −0.22; 0.08 | 0.33 | |||
| Dysmotility symptoms domain | −0.16 | −0.31; −0.01 | 0.034 | −0.15 | −0.29; −0.007 | 0.040 |
FSSG, the Frequency Scale for the symptoms of gastroesophageal reflux.
Discussion
The present study demonstrated that cough-specific QoL is severely impaired in patients with CVA. We also report for the first time to our knowledge, that GERD symptoms, particularly dysmotility symptoms, are associated with impaired cough-specific QoL measured by the LCQ. Meanwhile, there were no relationships between the LCQ scores and spirometry, AHR, or FeNO.
Relationships between chronic cough and GERD have been well known.31 Róka et al. showed that 18% of patients with GERD had chronic respiratory complications, of whom 75% complained of chronic cough. 32 Two mechanisms are assumed for the chronic cough caused by GERD: microaspiration of gastric contents into the proximal airways, and vagally-mediated esophageal-tracheobronchial reflex from distal esophageal exposure to gastric contents.33 The latter mechanism is thought to be evoked by an increased frequency of transient lower esophageal sphincter relaxation caused by gastric distension.34 Patterson et al. demonstrated higher sputum levels of tachykinins in asthmatic or chronic cough patients with acid reflux as demonstrated by 24 h esophageal pH monitoring than in those without acid reflux, 35 which suggests that acid perfusion into the distal esophagus promotes release of tachykinins into the airway through the afferent nerve which may evoke cough.35 Indeed, we previously found an association between GERD symptoms and cough sensitivity to capsaicin inhalation in patients with subacute or chronic cough15 and further demonstrated an association between GERD symptoms and cough-specific QoL in patients with CVA in the present study.
Several lines of evidence show the importance of gastroesophageal dysmotility in GERD-associated cough or chronic cough itself. In a study of patients with GERD-associated cough, 38% of patients had esophageal dysmotility as determined by esophageal manometry.36 Among 42 patients with GERD-related cough who responded to proton-pump inhibitors, 24 patients responded to monotherapy with proton-pump inhibitor, while 18 patients required additional prokinetic agents (metoclopramide or cisapride) for the resolution of cough.11 Furthermore, a cross-sectional survey of the prevalence of chronic cough in the community in the United Kingdom revealed that irritable bowel syndrome and regurgitation, but not heartburn, were strong predictors for chronic cough, in addition to smoking and lower social class.12 According to a new disease concept of chronic cough termed “cough hypersensitivity syndrome”, upregulation of intrinsic cough reflex triggered by common causes of chronic cough was fundamental feature of the pathophysiology of chronic cough.37 Morice et al. advocated that gaseous non-acid reflex that was undetectable by currently available technique may play fundamental role in the pathophysiology of “cough hypersensitivity syndrome”. 38 Current finding, the impairment of cough-specific QoL in patients with CVA by the presence of gastroesophageal dysmotility, may support Morices hypothesis. However, the effect of gastroesophageal dysmotility on the pathophysiology of “cough hypersensitivity syndrome” in patients with CVA remains to be further clarified.
Previous studies showed that levels of FEV1, AHR, and FeNO correlated with wheezing39, 40, 41 and 42 and dyspnea39, 43 and 44 in patients with asthma. Meanwhile, there were no association of FEV1,7 and 39 AHR,7, 40 and 41 or FeNO7 with cough frequency, as well as those with cough-specific QoL8 in patients with asthma. In the current study, patients with CVA showed no relationships between these 3 indices and cough-specific QoL. We previously found an association between plasma levels of substance P and methacholine AHR in patients with asthmatic cough.45 Since airway tachykinins may play potential roles in asthma or chronic cough associated with reflux,35 and upregulation of neuronal mechanisms involving transient receptor potential (TRP) nociceptors such as TRPV-1 is considered a key feature of cough hypersensitivity syndrome,37 the relationship between cough in CVA or asthma and neurogenic airway inflammation should be further examined in future studies.
It is well known that chronic cough is more prevalent in females than in males.46 In fact, in patients with chronic cough30 and 47 and healthy volunteers,48 females showed increased cough sensitivity to capsaicin and citric acid than males. However, there are conflicting findings about gender difference on cough-specific QoL.29 and 30 Birring et al. also demonstrated a significant association between LCQ scores and female gender, 29 whereas Kelsall et al. failed to show a gender difference in LCQ scores. 30 Kelsall et al. found that females coughed more frequently during the nighttime (p < 0.01) and for 24 h (p = 0.01), but not during the daytime (p = 0.06), than males. 30 This might explain the lack of sex difference in cough-specific QoL examined by the LCQ in Kellsalls study.29 The present study confirmed the Birrings study that cough-specific QoL examined by the LCQ were impaired more in female patients with CVA than in male patients.
This study involves several limitations. First, cough frequency was not objectively measured using a cough monitor device. However, earlier reports have already confirmed moderate correlations between cough frequency and the LCQ scores (r = −0.547 and −0.628). Second, the symptoms of GERD were merely evaluated by the FSSG questionnaire without the use of objective methods such as using 24-h intraesophageal pH monitoring, manometry, or impedance monitoring. Associations between LCQ scores and these objective measures of GERD could not be addressed in this study. However, current guideline clearly has noted that these maneuver are considered when empiric treatment for GERD fails.49 In addition, the GERD questionnaires, including the FSSG have been used in several studies to evaluate the symptoms of GERD in respiratory research field.15, 16, 17 and 18 Third, the LCQ questionnaire that was translated into Japanese by forward and backward translation method according to the standard protocol24 was used instead of original version of the LCQ. We thus evaluated the reliability and validity of the Japanese version of the LCQ. Japanese version of the LCQ showed high internal consistency and significant correlation with other instruments utilized to assess cough severity and frequency. Indeed, values of Cronbach α coefficient and correlation coefficients between the LCQ and cough VAS/numeric scores obtained from current study were similar to those from the original report.23 Therefore, as was the original LCQ,23 the Japanese version of the LCQ can be considered valid as an instrument to assess cough-specific QoL. However, we have not checked retest repeatability for the Japanese version of the LCQ. Forth, although allergic rhinitis/chronic rhinosinusitis are also known as most common cause of chronic cough and is involved in the pathophysiology of cough hypersensitivity syndrome together with asthma and GERD, we did not evaluate an association between sinonasal symptoms and cough-specific QoL because of the lack of validated questionnaires about sinonasal symptoms. Recently, sinonasal outcome test-22, a validated questionnaire about rhinitis and sinusitis, is developed to assess symptoms of rhinitis and sinusitis and is used in clinical and research fields of respiratory medicine.50 Further study is required whether sinonasal symptoms assessed by validated tools contribute to the impairment of cough-specific QoL in patients with CVA.
In conclusion, the present findings suggest that gastroesophageal dysmotility independently affects the impairment of cough-specific QoL in patients with CVA as measured by Japanese version of the LCQ, which showed very high reliability compatible to the original one. Dysmotility symptoms might potentially be a therapeutic target in patients with CVA whose cough is intractable to anti-asthma treatment.51 Further prospective studies are needed to determine whether specific treatment of gut dysmotility affects cough or cough-specific QoL of CVA patients to further clarify the involvement of gut dysmotility in the pathophysiology of CVA.
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government (23591117).
Conflict of interest
AN received honorarium from Astellas, AstraZeneca, Kyorin, GSK, Boehringer Ingelheim, and received research funding from Astellas. The rest of the authors have no conflict of interest.
Authors' contributions
YK: contributed to the performance of pulmonary function tests and FeNO measurements, the collection of data, the acquisition and interpretation of data, and drafting the manuscript. AN: contributed to the recruitment of patients, disease diagnosis and management, the conception of the whole study, the design, acquisition, and interpretation of data, and revision of the manuscript. HM: contributed to the recruitment of patients, disease diagnosis and management, and revision of the manuscript. TI: contributed to the performance of pulmonary function tests, the collection of data, and management of patients. II: contributed to disease diagnosis and management of patients. TO, TT: contributed to the performance of pulmonary function tests and methacholine challenge tests. HI, TN, YI, GP: contributed to the performance of pulmonary function tests, and FeNO measurements. SSB: contributed to the development of the Leicester Cough Questionnaire (LCQ) and the Japanese version of the LCQ. MM: contributed to supervision of the study.
Acknowledgments
The authors would like to thank Dr Haruhiko Ogawa, Division of Pulmonary Medicine, Ishikawa-ken Saiseikai Kanazawa Hospital, for the co-translation of the Leicester Cough Questionnaire.
References
- 1 C.L. French, R.S. Irwin, F.J. Curley, C.J. Krikorian; Impact of chronic cough on quality of life; Arch Intern Med, 158 (1998), pp. 1657–1661
- 2 W.M. Corrao, S.S. Braman, R.S. Irwin; Chronic cough as the sole presenting manifestation of bronchial asthma; N Engl J Med, 300 (1979), pp. 633–637
- 3 S. Kohno, T. Ishida, Y. Uchida, H. Kishimoto, H. Sasaki, T. Shioya, et al.; The Japanese Respiratory Society guidelines for management of cough; Respirology, 11 (Suppl. 4) (2006), pp. S135–S186
- 4 R.S. Irwin, M.H. Baumann, D.C. Bolser, L.P. Boulet, S.S. Braman, C.E. Brightling, et al.; Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines; Chest, 129 (2006), pp. 1S–23
- 5 A.H. Morice, L. McGarvey, I. Pavord, B.T.S.C.G. Group; Recommendations for the management of cough in adults; Thorax, 61 (Suppl. 1) (2006), pp. i1–24
- 6 A. Niimi; Geography and cough aetiology; Pulm Pharmacol Ther, 20 (2007), pp. 383–387
- 7 P.A. Marsden, J.A. Smith, A.A. Kelsall, E. Owen, J.R. Naylor, D. Webster, et al.; A comparison of objective and subjective measures of cough in asthma; J Allergy Clin Immunol, 122 (2008), pp. 903–907
- 8 M. Purokivi, H. Koskela, K. Kontra; Determinants of asthma control and quality of life in stable asthma: evaluation of two new cough provocation tests; Clin Respir J, 7 (2013), pp. 253–260
- 9 A.H. Morice, G.A. Fontana, A.R. Sovijarvi, M. Pistolesi, K.F. Chung, J. Widdicombe, et al.; The diagnosis and management of chronic cough; Eur Respir J, 24 (2004), pp. 481–492
- 10 R.S. Irwin, J.M. Madison; The persistently troublesome cough; Am J Respir Crit Care Med, 165 (2002), pp. 1469–1474
- 11 R.H. Poe, M.C. Kallay; Chronic cough and gastroesophageal reflux disease: experience with specific therapy for diagnosis and treatment; Chest, 123 (2003), pp. 679–684
- 12 A.C. Ford, D. Forman, P. Moayyedi, A.H. Morice; Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms; Thorax, 61 (2006), pp. 975–979
- 13 M. Kusano, Y. Shimoyama, S. Sugimoto, O. Kawamura, M. Maeda, K. Minashi, et al.; Development and evaluation of FSSG: frequency scale for the symptoms of GERD; J Gastroenterol, 39 (2004), pp. 888–891
- 14 S. Yasaka, K. Murakami, T. Abe, J. Anan, K. Mizukami, J. Tanahashi, et al.; Evaluation of esophageal function in patients with gastroesophageal reflux disease using transnasal endoscopy; J Gastroenterol Hepatol, 24 (2009), pp. 1677–1682
- 15 H. Matsumoto, R.P. Tabuena, A. Niimi, H. Inoue, I. Ito, M. Yamaguchi, et al.; Cough triggers and their pathophysiology in patients with prolonged or chronic cough; Allergol Int, 61 (2012), pp. 123–132
- 16 K. Terada, S. Muro, S. Sato, T. Ohara, A. Haruna, S. Marumo, et al.; Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation; Thorax, 63 (2008), pp. 951–955
- 17 M. Jinnai, A. Niimi, M. Takemura, H. Matsumoto, Y. Konda, M. Mishima; Gastroesophageal reflux-associated chronic cough in an adolescent and the diagnostic implications: a case report; Cough, 4 (2008), p. 5
- 18 T. Shirai, M. Mikamo, T. Tsuchiya, Y. Shishido, T. Akita, S. Morita, et al.; Real-world effect of gastroesophageal reflux disease on cough-related quality of life and disease status in asthma and COPD; Allergol Int, 64 (2015), pp. 79–83
- 19 R.S. Irwin, C.T. French, N.A. Smyrnios, F.J. Curley; Interpretation of positive results of a methacholine inhalation challenge and 1 week of inhaled bronchodilator use in diagnosing and treating cough-variant asthma; Arch Intern Med, 157 (1997), pp. 1981–1987
- 20 M.R. Miller, R. Crapo, J. Hankinson, V. Brusasco, F. Burgos, R. Casaburi, et al.; General considerations for lung function testing; Eur Respir J, 26 (2005), pp. 153–161
- 21 A. Niimi, H. Matsumoto, M. Takemura, T. Ueda, K. Chin, M. Mishima; Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma; Am J Respir Crit Care Med, 168 (2003), pp. 983–988
- 22 A.T. Society, E.R. Society; ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005; Am J Respir Crit Care Med, 171 (2005), pp. 912–930
- 23 S.S. Birring, B. Prudon, A.J. Carr, S.J. Singh, M.D. Morgan, I.D. Pavord; Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ); Thorax, 58 (2003), pp. 339–343
- 24 S. Fukuhara, J.E. Ware, M. Kosinski, S. Wada, B. Gandek; Psychometric and clinical tests of validity of the Japanese SF-36 health survey; J Clin Epidemiol, 51 (1998), pp. 1045–1053
- 25 A. Kelsall, L.A. Houghton, H. Jones, S. Decalmer, K. McGuinness, J.A. Smith; A novel approach to studying the relationship between subjective and objective measures of cough; Chest, 139 (2011), pp. 569–575
- 26 J.Y. Hsu, R.A. Stone, R.B. Logan-Sinclair, M. Worsdell, C.M. Busst, K.F. Chung; Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder; Eur Respir J, 7 (1994), pp. 1246–1253
- 27 J.R.A. Santos; Cronbachs alpha: a tool for assessing the reliability of scales; J Ext, 37 (1999), pp. 1–5
- 28 S.S. Birring, S. Matos, R.B. Patel, B. Prudon, D.H. Evans, I.D. Pavord; Cough frequency, cough sensitivity and health status in patients with chronic cough; Respir Med, 100 (2006), pp. 1105–1109
- 29 S.S. Birring, I.D. Pavord; Assessment of gender differences in health status with the Leicester Cough Questionnaire (LCQ); Thorax, 64 (2009), pp. 1008–1009
- 30 A. Kelsall, S. Decalmer, K. McGuinness, A. Woodcock, J.A. Smith; Sex differences and predictors of objective cough frequency in chronic cough; Thorax, 64 (2009), pp. 393–398
- 31 H. Matsumoto, A. Niimi, M. Takemura, T. Ueda, M. Yamaguchi, H. Matsuoka, et al.; Prevalence and clinical manifestations of gastro-oesophageal reflux-associated chronic cough in the Japanese population; Cough, 3 (2007), p. 1
- 32 R. Róka, A. Rosztóczy, F. Izbéki, Z. Taybani, I. Kiss, J. Lonovics, et al.; Prevalence of respiratory symptoms and diseases associated with gastroesophageal reflux disease; Digestion, 71 (2005), pp. 92–96
- 33 D.O. Castell, P.F. Schnatz; Gastroesophageal reflux disease and asthma. Reflux or reflex?; Chest, 108 (1995), pp. 1186–1187
- 34 T. Tomonaga, Z.T. Awad, C.J. Filipi, R.A. Hinder, M. Selima, F. Tercero, et al.; Symptom predictability of reflux-induced respiratory disease; Dig Dis Sci, 47 (2002), pp. 9–14
- 35 R.N. Patterson, B.T. Johnston, J.E. Ardill, L.G. Heaney, L.P. McGarvey; Increased tachykinin levels in induced sputum from asthmatic and cough patients with acid reflux; Thorax, 62 (2007), pp. 491–495
- 36 J.A. Kastelik, A.E. Redington, I. Aziz, G.K. Buckton, C.M. Smith, M. Dakkak, et al.; Abnormal oesophageal motility in patients with chronic cough; Thorax, 58 (2003), pp. 699–702
- 37 A.H. Morice, E. Millqvist, M.G. Belvisi, K. Bieksiene, S.S. Birring, K.F. Chung, et al.; Expert opinion on the cough hypersensitivity syndrome in respiratory medicine; Eur Respir J, 44 (2014), pp. 1132–1148
- 38 A.H. Morice, S. Faruqi, C.E. Wright, R. Thompson, J.M. Bland; Cough hypersensitivity syndrome: a distinct clinical entity; Lung, 189 (2011), pp. 73–79
- 39 J. Sunyer, X. Basagaña, J. Roca, I. Urrutia, A. Jaen, J.M. Antó, et al.; Relations between respiratory symptoms and spirometric values in young adults: the European community respiratory health study; Respir Med, 98 (2004), pp. 1025–1033
- 40 A.L. Parker, F.D. McCool; Pulmonary function characteristics in patients with different patterns of methacholine airway hyperresponsiveness; Chest, 121 (2002), pp. 1818–1823
- 41 H. Matsumoto, A. Niimi, M. Takemura, T. Ueda, M. Yamaguchi, H. Matsuoka, et al.; Features of cough variant asthma and classic asthma during methacholine-induced bronchoconstriction: a cross-sectional study; Cough, 5 (2009), p. 3
- 42 A.C. Olin, A. Rosengren, D.S. Thelle, L. Lissner, K. Torén; Increased fraction of exhaled nitric oxide predicts new-onset wheeze in a general population; Am J Respir Crit Care Med, 181 (2010), pp. 324–327
- 43 Y.I. Koh, I.S. Choi, H. Lim; Airway responsiveness as a direct factor contributing to the dyspnoea perception in asthma; Respir Med, 95 (2001), pp. 464–470
- 44 J.M. Sippel, W.E. Holden, S.A. Tilles, M. O'Hollaren, J. Cook, N. Thukkani, et al.; Exhaled nitric oxide levels correlate with measures of disease control in asthma; J Allergy Clin Immunol, 106 (2000), pp. 645–650
- 45 K. Otsuka, A. Niimi, H. Matsumoto, I. Ito, M. Yamaguchi, H. Matsuoka, et al.; Plasma substance P levels in patients with persistent cough; Respiration, 82 (2011), pp. 431–438
- 46 L.P. McGarvey, A.J. Ing; Idiopathic cough, prevalence and underlying mechanisms; Pulm Pharmacol Ther, 17 (2004), pp. 435–439
- 47 J.A. Kastelik, R.H. Thompson, I. Aziz, J.C. Ojoo, A.E. Redington, A.H. Morice; Sex-related differences in cough reflex sensitivity in patients with chronic cough; Am J Respir Crit Care Med, 166 (2002), pp. 961–964
- 48 M. Fujimura, K. Kasahara, Y. Kamio, M. Naruse, T. Hashimoto, T. Matsuda; Female gender as a determinant of cough threshold to inhaled capsaicin; Eur Respir J, 9 (1996), pp. 1624–1626
- 49 R.S. Irwin; Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines; Chest, 129 (2006), pp. 80S–94S
- 50 A.E. Dixon, M. Castro, R.I. Cohen, L.B. Gerald, J.T. Holbrook, C.G. Irvin, et al.; Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma; J Allergy Clin Immunol, 135 (2015) 701–9.e5
- 51 H. Hijikata, M. Takamura, T. Asamo, H. Ichikawa, A. Niimi; High prevalence and impact of GERD in patients with persistent cough: an experience from a specialist clinic in Japan [abstract]; Am J Respir Crit Care Med, 189 (2014) A1608
Document information
Published on 05/04/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?