Highlights
- We review limitations of current diagnostic guidelines for HFPEF.
- We review novel plasma biomarkers applicable to HFPEF.
- The most promising plasma and imaging biomarkers are highlighted.
- We report advanced imaging techniques which may improve diagnosis and risk stratification.
Abstract
Existing diagnostic guidelines for heart failure with preserved ejection fraction (HFPEF) primarily comprise natriuretic peptides and echocardiographic assessment, highlighting the role of diastolic dysfunction. However, recent discoveries of novel plasma markers implicated in pathophysiology of heart failure and technological advances in imaging provide additional biomarkers which are potentially applicable to HFPEF. The evidence base for plasma extra-cellular matrix (ECM) peptides, galectin-3, ST2, GDF-15 and pentraxin-3 is reviewed. Furthermore, the capabilities of novel imaging techniques to assess existing parameters (e.g. left ventricular ejection fraction, systolic & diastolic function, chamber size) and additional derangements of the ECM, myocardial mechanics and ischaemia evaluation are addressed.
Keywords
HFPEF;Biomarkers;Plasma;Imaging;Extra-cellular matrix
1. Introduction
Heart failure with preserved ejection fraction (HFPEF) is the subtype of heart failure (HF) most likely to be encountered in clinical practice in the near future and already accounts for approximately half of all HF cases [1]. Yet importantly, we appear no closer to offering effective treatments [2]. The latest HFPEF diagnostic guidelines [3] were published nearly eight years ago and still remain subject to debate. In the intervening period, technological advances in the fields of plasma biomarkers and imaging have further improved our understanding of this heterogeneous entity, provided insights into potential targets for therapy and improved diagnostic labeling. We review the respective merits of these newer biomarkers and consider their applicability for future use in HFPEF frameworks.
2. Current limitations, potential challenges and the need for biomarker development in HFPEF
A biomarker has been defined as a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic intervention” [4]. The medical condition of interest should: be sufficiently common, significantly impact upon morbidity & mortality, be well defined and with effective treatments available. Likewise, for the biomarker being developed, it should ideally: be a stable product, discriminate between pathology and normal (and between pathologies), enhance clinical care, be acceptable to patients, exhibit a linear relation with change in pathology as well as being reproducible and replicated across multiple studies [5].
Adopting this approach to HFPEF reveals a series of disease- and biomarker-specific factors (see Table 1) that make biomarker development challenging [2]; [3]; [6]; [7]; [8] ; [9]. The primary limiting factor is the marked heterogeneity that characterizes HFPEF populations. To date, various diagnostic criteria (including differing ejection fraction [EF] thresholds) have been employed to define HFPEF. Phenotypic diversity (e.g. obesity, diabetes, atrial fibrillation, right heart failure) coupled with a high prevalence of co-morbidities makes patient identification difficult. Imaging phenocopies such as hypertrophic cardiomyopathy and amyloid are additional confounders. Alternate explanations for pathophysiological mechanisms add to the uncertainty. Furthermore, the discriminatory capabilities of biomarkers (to distinguish HFPEF from heart failure with reduced ejection fraction [HFREF]) are hindered by supportive evidence to suggest the existence of both entities in continuum as part of a single syndrome. While invasive pressure assessments best illustrate the haemodynamic consequences of diastolic dysfunction (DD), they are limited by inherent procedural risks. On the other hand, non-invasive measures of DD are within normal range in up to a third of subjects. These factors highlighted above therefore ensure that existing and newer markers described in this article do not wholly fulfill the aforementioned biomarker criteria [10]; [11]; [12]; [13]; [14]; [15] ; [16].
| Disease specific factors |
|---|
| Population not well defined[2] |
| Variable diagnostic criteria in guidelines and clinical trials [2] |
| Confounders of diagnosis[7] ; [8] |
| Phenotypic variability |
| High prevalence of co-morbidities may alternatively explain clinical features |
| Imaging phenocopies (e.g. hypertrophic cardiomyopathy, amyloid, pericardial constriction) |
| Atrial fibrillation (challenging clinical and imaging assessment) |
| No clear and effective therapies available[2] |
| Evidence for HFPEF as a continuum with HFREF[3]; [6] ; [7] |
| Similar clinical signs and symptoms |
| Unimodal distribution of EF in clinical trials |
| Co-existence of systolic abnormalities and progression over time |
| Eccentric remodeling over time seen in hypertensives |
| Heterogeneity of pathophysiology[6]; [7] ; [9] |
| Diastolic dysfunction — in HFPEF & HFREF, in normal subjects, absent in ≈ 1/3 of HFPEF |
| Alternate abnormalities of: ventricular–arterial coupling, arterial stiffness, systemic & pulmonary vasculature, chronotropic incompetence, endothelial function, LA function volume overloading, LV systolic function |
| Biomarker specific factors |
|---|
| Invasive approach (assessment of diastolic dysfunction or biopsy quantification of fibrosis) |
| Procedural risk |
| Sampling error |
| Non-uniform responses in end-diastolic pressure volume relationship curves |
| Traditional echocardiographic measures for diagnosis[10]; [11]; [12]; [13]; [14]; [15] ; [43] |
| Not the recognized gold standard for EF, LV & LA volumes, LV mass |
| Limitations of methodology and feasibility, less reproducible compared to CMR |
| Markers of diastolic dysfunction: loading dependent |
| Haemodynamic disturbances may not be apparent at rest |
| Plasma natriuretic peptides[16] |
| Lower values in HFPEF versus HFREF |
| Lower values in obesity |
| Higher levels in non-HFPEF conditions but commonly encountered in HFPEF |
Abbreviations: HFPEF = heart failure with preserved ejection fraction; HFREF = heart failure with reduced ejection fraction; EF = ejection fraction; LA = left atrium; LV = left ventricle.
3. Key pathophysiological substrates that comprise potential biomarkers
Various pathophysiological derangements have been implicated in HFPEF (see Table 1). The central disturbance remains diastolic dysfunction, which in turn is governed by myocardial stiffness [6] ; [7]. Hypertensive heart disease accounts for a significant cohort of HFPEF and is associated with left ventricular hypertrophy (LVH), pressure overload, concentric remodeling and myocardial fibrosis. Structural remodeling results in alterations in both the intra- (e.g. larger cardiomyocytes and predominance of the stiffer isoform of the protein Titin) & extracellular compartments [6]. Stiffness is increased by fibrosis resulting in reduced left ventricular (LV) compliance and elevated LV filling pressures which are the haemodynamic hallmarks of HFPEF. Myocardial stiffness is primarily determined by the turnover rates of the extra-cellular matrix (ECM) and its constituents (predominantly collagen). However, additional factors such as inflammatory processes, endothelial dysfunction, ischaemia, and neurohormonal activation may contribute [6]; [7]; [9]; [16] ; [17]. These pathological changes and consequences may be detectable by either plasma or imaging techniques (see Table 2 and Supplementary online Table 1) and form the basis of subsequent sections.
| LVEF | Contractile function (LV/LA) | Chamber quantification | ECM quantification (fibrosis) | Myocardial mechanics | Haemodynamics | CAD/ischaemia/flow reserve | Molecular imaging | Metabolic imaging | |
|---|---|---|---|---|---|---|---|---|---|
| TTE | ++ | ++ | ++ | + | ++ | +++ | + | n/a | n/a |
| CMR | +++ | +++ | +++ | +++ | +++ | ++ | +++ | + | ++ |
| PET | + | + | + | ++ | n/a | n/a | +++ | ++ | ++ |
| SPECT | + | + | + | + | n/a | n/a | ++ | ++ | ++ |
| CT | + | + | +++ | + | + | n/a | + | + | n/a |
Adapted from Paterson et al. [100] and Jellis et al. [50].
Abbreviations: HFPEF = heart failure with preserved ejection fraction; LVEF = left ventricular ejection fraction; LV = left ventricle; LA = left atrium; ECM = extra-cellular matrix; CAD = coronary artery disease; TTE = trans-thoracic echocardiography; CMR = cardiac magnetic resonance; PET = positron emission tomography; SPECT = single-photon emission computed tomography; CT = computed tomography; n/a = not applicable or not assessed; + = limited evidence but potential future role; ++ = supportive evidence from either at least one large study or registry data; +++ = accepted reference standard or strongly supportive evidence base including meta-analyses or randomized controlled trials.
4. Novel plasma biomarkers
4.1. ECM biomarkers
Matrix metalloproteinases (MMPs) primarily degrade collagen and other ECM components while inhibitors of matrix metalloproteinases (TIMPs) counteract their actions. Generally, in HFPEF, TIMPs are increased and MMPs are decreased such that collagen degradation is reduced and collagen accumulation is increased. Conversely, in HFREF the opposite has been demonstrated [6]; [7] ; [18]. However, the concept of a high TIMP/MMP ratio being synonymous with HFPEF is too rigid since individual MMPs and TIMPs also actively promote fibrosis through alternate (and additional) mechanisms of action [16]. The high levels of MMPs − 1 [19], − 2 [20]; [21] ; [22], − 8 [22], and − 9 [20] ; [21] reported in HFPEF likely reflect this phenomenon. In hypertensive subjects with HFPEF, TIMP-1 moderately predicts the presence of HF with an area under curve (AUC) of 0.71 and higher levels are detected compared to controls [18]. Additionally, TIMP-1 levels correlate with DD and are reportedly more accurate than NT-proBNP for detecting echocardiographic estimates of elevated LV filling pressures using E/E′ [19].
Compared to controls, circulating markers of active collagen turnover i.e. synthesis (e.g. pro-collagen type I carboxy-terminal pro-peptide [PICP] [19] ; [22], collagen III N-terminal pro-peptide [PIIINP] [22]) and degradation (e.g. collagen I telopeptide [CITP]) are elevated in HFPEF[20]; [22] ; [23]. Furthermore, elevated levels appear to correlate with worsening indices of DD [19]; [20] ; [22]. In a study of 446 subjects including healthy controls (n = 241), LVH without HF (n = 144) and LVH with HFPEF (n = 61), a multi-biomarker panel comprising MMP-7 & -9, TIMP-1and PIIINP detected the presence of LVH (AUC = 0.8). A further panel consisting of MMP-2 & -8, TIMP-4 and PIIINP best detected LVH with HFPEF (AUC = 0.79) [22].
4.2. Galectin-3
Galectin-3 is a soluble β-galactoside binding protein secreted by activated macrophages, promoting fibroblast & myo-fibroblast activity and pro-collagen deposition in the ECM. Seminal studies in rat models first highlighted the potential role of Galectin-3 as a pro-fibrotic and pro-inflammatory mediator in HF [24]. While intra-pericardial infusion of galectin-3 induced adverse cardiac remodeling and LV dysfunction, these deleterious effects were counteracted by administration of its inhibitor [25]. Enhanced galectin-3 expression induces fibroblast proliferation, collagen deposition in the ECM and promotes fibrosis [26]. Accordingly, elevated serum levels are associated with an increased risk of HF development [27], worsening grades of DD [28] and adverse outcomes [27] ; [29]. Interestingly, despite similar levels of Galectin-3 measured in both HFPEF and HFREF, increased serum concentrations appear to be a stronger predictor of mortality in HFPEF (n = 114). However, this observation is limited by the small sample size used and a lower EF threshold (> 40%) for defining HFPEF[30].
4.3. Inflammatory processes, endothelial dysfunction and neurohormonal activation
4.3.1. ST2
The ST2 receptor is a member of the Interleukin-1 family existing in trans-membrane and soluble forms. ST2 modulates inflammatory signaling and neurohormonal activation in HF. Soluble ST2 (sST2) acts as a decoy receptor disrupting the binding of interleukin-33 and promotes excess cardiac fibrosis, hypertrophy and LV dysfunction [31]. In both HFPEF and HFREF, ST2 secretion by vascular endothelium is enhanced in response to elevated left ventricular end-diastolic pressure (LVEDP) measured by cardiac catheterization. Furthermore, the rise in ST2 levels is proportional to increasing values of LVEDP [32].
In acute HF, increased ST2 levels correlate with worsening HF symptoms, mortality at one-year and confer incremental prognostic value compared to NT-pro-BNP [33]. As a diagnostic aid in chronic, stable hypertensive patients (n = 107) recruited from the out-patient setting, sST2 performed better than NT-pro BNP (AUC = 0.80 versus AUC = 0.70) [34] for the detection of HFPEF (n = 68). In a further prospective study assessing risk (n = 447), ST2 levels while comparatively lower in HFPEF than HFREF, remained an independent predictor of mortality [35].
4.3.2. Growth differentiating factor-15 (GDF-15)
GDF-15 is a member of the transforming growth factor-β cytokine superfamily and is also expressed by activated macrophages. Although not normally expressed in myocardium, it can be induced in response to metabolic stress such as cardiac ischaemia, pressure overload or inflammation [36]. In apparently healthy, elderly individuals from the community (n = 1004, age > 70), GDF-15 independently predicted HF development and rising levels correlated with higher LV mass and concentric hypertrophy [37]. In a small population study (n = 151), the discriminatory capacity of GDF-15 (AUC = 0.936) was similar to NT-proBNP (AUC = 0.934) for HFPEF versus controls. Increasing levels of both biomarkers correlated with worsening diastolic indices. A ratio of NT-pro BNP: GDF-15 best distinguished HFPEF from HFREF (AUC = 0.709) [38].
4.3.3. Pentraxin-3
As part of a superfamily of proteins including C-reactive protein (CRP) and serum amyloid protein (SAP), Pentraxin-3 has become recognized as an inflammatory marker in HF. When exposed to pressure overload, pentraxin-3 expression is increased in wild type mice. Conversely, cardiac hypertrophy, fibrosis and LV dysfunction are attenuated in pentraxin-3 knockout mice [39]. In a study inclusive of both subtypes of HF (total n = 196), elevated plasma pentraxin-3 correlated with advancing NHYA grade and was a strong, independent predictor of adverse cardiac events in multivariate analysis also incorporating BNP (AUC = 0.8047 versus 0.7107) [40]. In a further study, pentraxin-3 levels were significantly higher in HFPEF (n = 82) compared to controls and independently correlated with the presence of DD (as measured by E/E′) in both groups [41].
While all the aforementioned candidate biomarkers have shown promise in studying HFPEF, ST2 and Galectin-3 appear closest to routine clinical application at present. These markers are the most extensively evidence based, provide prognostic data and better identify those at risk of developing incident HF. However, prospective studies in HFPEF evaluating plasma biomarker guided management and treatments are currently lacking with all [42].
5. Novel imaging biomarkers
5.1. Quantification of the ECM (surrogate measures of DD)
5.1.1. Echocardiography
Traditional echocardiographic parameters used to diagnose HFPEF (e.g. E/E′, E/A ratio, LA volume, LV mass) are reliant on assessing the resting functional and structural consequences of fibrosis (see Table 1). Beyond ischaemia evaluation, diastolic stress echocardiography may further unmask patients with HFPEF in whom resting filling pressures do not meet current diagnostic criteria but rise with exercise (E/E′ > 15) [43]. However, indirect assessment of fibrosis is also possible via echocardiography. Collagen content governs the elasticity of myocardial tissue thereby altering its acoustic impedence, density and reflectivity in response to ultrasound signals used in echocardiography [44]. Using the pericardium as an internal control, these integrated signals scatter back (IBS) to the imaging probe permitting estimates of regional fibrotic burden.
Myocardial reflectivity is enhanced in fibrosis and conditions associated with the phenotype of HFPEF (e.g. hypertension, chronic renal failure [45] and obesity [46]). Furthermore, indices of IBS have been validated against biopsy measured collagen content in non-fibrotic myocardium [44] and interstitial fibrosis in hypertensive subjects [47]. Significant correlations have been shown with plasma ECM biomarkers [48], Doppler echocardiographic measures [45] ; [46] and increasing severity of DD [49]. Currently, fibrosis assessment by echocardiography remains subject to limitations of limited acoustic windows, low signal-to-noise, operator skill and the reproducibility is unknown.
5.1.2. Cardiac magnetic resonance (CMR)
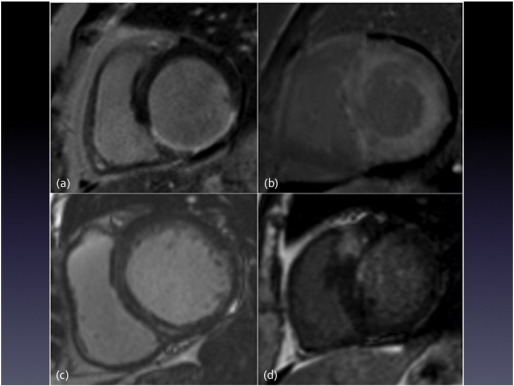
Focal myocardial fibrosis is detectable by echocardiography, computed tomography (CT), single-photon emission computed tomography (SPECT) and CMR with reasonable agreement in all [50] ; [51]. CMR late gadolinium enhancement (LGE) imaging was initially developed upon an understanding that infarcted (scarred) myocardium is associated with regional increases in collagen content, extra-cellular volume (ECV) expansion and a slower washout of extra-cellular contrast agents (e.g. gadolinium) from such areas. Due to the accumulation of gadolinium based contrast agents in these areas T1 times (relaxation properties of tissue) are reduced such that fibrotic regions appear as areas of high signal intensity compared to ‘nulled’ (black) normal myocardium using inversion recovery CMR sequences [13]. CMR LGE is the accepted gold standard due to its superior spatial resolution and high contrast-to-noise ratio enabling the detection of very small infarcts. [52]. Furthermore, focal non-ischaemic scarring is also seen in a range of other conditions such as dilated cardiomyopathy, aortic stenosis and sarcoidosis. The pattern of LGE (see Fig. 1) allows discrimination between aetiologies (e.g. ischaemic versus non-ischaemic and HFPEF ‘phenocopies’ such as hypertrophic cardiomyopathy [HCM], amyloid, pericardial constriction), provides prognostic information and identifies vulnerable myocardium amenable to targeted therapies [8]; [13] ; [53].
|
|
|
Fig. 1. Cardiac magnetic resonance imaging examples of late gadolinium enhancement patterns seen in differing aetiologies of heart failure. (a) Sub-endocardial pattern in myocardial infarction; (b) global sub-endocardial pattern with mid-myocardial extension in amyloidosis; (c) mid-wall pattern typical of non-ischaemic dilated cardiomyopathy; (d) marked focal “scar” in the region of maximal left ventricular hypertrophy and the superior right ventricular insertion point seen in hypertrophic cardiomyopathy. |
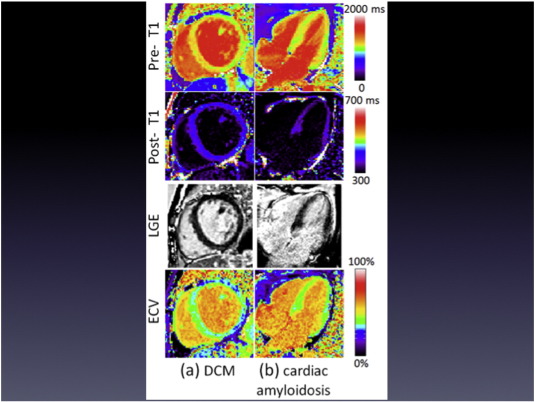
In HFPEF however, the pattern of fibrosis, at least in the early stages, is typically diffuse and the signal differences between diseased and normal myocardium are less distinct, rendering the LGE technique insensitive. T1 mapping and ECV quantification techniques are promising recent developments in CMR addressing this issue (see Fig. 2). Native T1 values (non-contrast) are a reflection of myocardial tissue properties (such as fat and water content) and may be altered in diseased states. Estimates of T1 values encoded within pixel intensity of images enable both focal and diffuse myocardium to be studied. T1 values can discriminate pathology from normal (e.g. high T1 in diffuse fibrosis and amyloid, low T1 in iron overload) and may detect pre-clinical disease. ECV quantification (reliant on measurement of hematocrit, contrast administration and pre- and post-contrast T1 values) permits the myocardium to be further dichotomized into both intra- and extra-cellular compartments. Differing ECV techniques have been validated against collagen volume fraction measured at histology [54] and also tested across a range of pathologies (HFREF, aortic stenosis [AS], HCM, amyloid) whereby derived values discriminated between healthy controls and disease [55]. Recently in small studies, post-contrast T1 times (n = 61) have shown association with adverse outcomes (hospitalization or death) [56] and ECV values (n = 62) appear to correlate with CMR measures of DD in HFPEF[57].
|
|
|
Fig. 2. Cardiac magnetic resonance imaging examples of “normal” appearing late gadolinium enhancement but with diffuse abnormalities in myocardial extra-cellular volume. Pre-contrast (top row) and post-contrast (2nd row) T1 maps, late gadolinium enhancement (3rd row) and extra-cellular volume maps (bottom row) in (a) non-ischaemic dilated cardiomyopathy (DCM); (b) amyloidosis. Adapted from Kellman et al. [101] with permission from the publisher. |
Before the aforementioned techniques enter routine clinical practice however, significant limitations need to be addressed including: a lack of consensus on scanning parameters and ECV techniques, the absence of normative reference ranges across sex and age, potential confounders of T1 values such as heart rate, respiratory motion, magnet strength and the lack of large scale multi-centre studies [58]. The reproducibility of T1 mapping ECV is excellent but there is a large overlap between ECV measurements in most disease states and age-matched controls which is likely to render this technique unsuitable for guiding diagnosis or therapy in an individual patient [60] ; [58].
5.1.2.1. Positron emission tomography (PET) and CT
PET (using labeled H215O and C15O) measured perfusable tissue index (PTI) reflects the fraction of myocardium that is perfusable by water. Fibrosis prevents rapid exchange of water and there is moderate correlation of PTI with LGE CMR in phenotypically similar HCM patients with preserved EF [61]. Recently, using a technique analogous to equilibrium contrast CMR [54], CT derived quantification of diffuse myocardial fibrosis has also been validated. CT based techniques appear to correlate with histology and CMR albeit with relatively wide Bland–Altman limits of agreement [62].
5.2. Myocardial mechanics
Remodeling of the ECM compartment alters myocardial tissue mechanics resulting in abnormalities of both diastole and systole in HFPEF[63]. Longitudinal function is typically depressed in HFPEF and can be measured with echocardiography (tissue Doppler) and CMR (velocity-encoded or tissue phase mapping) [50] ; [63]. A more detailed assessment of LV performance can now be made using strain (or deformation) analysis. Simplistically, strain imaging assesses myocardial tissue lengthening, shortening or thickening in orthogonal planes.
Significant correlations between early diastolic strain rates, regional stiffness and the extent of myocardial fibrosis were initially described in animal studies [64]. Subsequently, regional strain disturbances have demonstrated a strong relation with LV catheter derived relaxation abnormalities and LVEDP in HCM [65]. Furthermore, the ratio of mitral E wave velocity: global strain rate correctly predicts LVEDP and is more accurate than E/E′ ratios in patients with preserved EF and regional dysfunction [66]. In an exercise echocardiographic study of 56 patients with HFPEF, both resting and exertional reductions in longitudinal & radial strain as well as apical rotation were observed [67]. As prognostic biomarkers, strain parameters (global longitudinal peak strain and longitudinal early diastolic strain) are important predictors of adverse outcomes (one-year follow up) in HFPEF[68].
Whereas strain measurements with echocardiography (tissue Doppler, speckle tracking) and CMR techniques (tagging) are well established [15]; [50] ; [69], recent developments in CT [70] also show promise. With CMR, feature tracking has recently emerged as a promising alternative to tagging. In comparison, feature tracking does not necessitate prolonged breath-holding for image acquisition, has been recently studied in HFPEF[69] with good feasibility, has shorter analysis times and shows good reproducibility at both 1.5- and 3-Tesla magnet strengths [71].
5.3. Haemodynamic consequences of ECM remodeling
A dilated left atrium (LA) is the haemodynamic consequence of chronically elevated LVEDP and provides supportive evidence for HFPEF diagnosis [3]. CMR has already superseded echocardiography for LA volumetric measurement with superior reproducibility and feasibility [8] ; [12]. Beyond structural assessment, disturbances in atrial myocardial mechanics have also been observed in HFPEF. LA dysfunction reportedly discriminates between HFPEF, hypertensive and healthy control groups [72] ; [73]. Using similar techniques as for LV assessment, LA strain parameters can be measured with echocardiography [15], CMR [74] and CT [75]. Abnormal measures of LA strain have been noted in the HFPEF antecedent conditions of hypertension and diabetes despite normal LA dimensions, highlighting the potential for early disease profiling [76] as a marker of DD in HF, speckle tracking echocardiography performed better than E/E′ (AUC = 0.93 versus 0.69) and correlated strongly with LV filling pressures [77]. Recently, global peak longitudinal atrial strain measured by CMR (feature tracking) had excellent feasibility (> 86%), reproducibility (intra-class correlation co-efficients > 0.92) and was an independent predictor of HF development (n = 112, including n = 39 HFPEF) [74].
5.4. Detection of coronary artery disease (CAD), ischaemia and myocardial blood flow assessment
At present, the role of CAD and ischaemia in the natural history of HFPEF is incompletely defined. Not only is CAD associated with an increased risk of developing HFPEF but worsens prognosis in this setting [17]. Epidemiological studies have reported lower prevalence of CAD in HFPEF compared to HFREF. However, pooled analysis of prospective studies suggests that CAD is present in nearly half of all HFPEF cases [78]. Unfortunately, the majority of these studies failed to systematically look for CAD and were further hindered by the lack of a universal definition and incomplete documentation in many.
Ischaemia in HFPEF may result from macrovascular (CAD) or microvascular disease (MVD). Ischaemia reduces LV chamber compliance, increases LVEDP, causes DD and accentuates adverse ECM remodeling. In conjunction with pressure overload typical of HFPEF, LV wall stress is further increased, blunting sub-endocardial perfusion and coronary reserve [9] ; [17]. Non-invasive imaging can identify haemodynamically significant CAD with good sensitivity and specificity in: stress echocardiography (dipyridamole 85% and 89%, dobutamine 86% and 89%) [79], adenosine SPECT (90% and 75%) [80], PET (89% and 89%), CT (87% and 96%) and MRI (84% and 86%) [81]. CMR best detects infarction (which may be silent) and alternatively explain symptoms (angina equivalent), provides prognostic information and enables effective primary and secondary prevention therapies [53] ; [82].
Invasive (angiography) or non-invasive (TTE, CMR or PET) detection of diminished coronary flow reserve (CFR) and MVD confer adverse prognosis in the presence or absence of CAD [83]. Furthermore, these imaging biomarkers appear to be overrepresented in populations typical of HFPEF: increasing age, female, obese, diabetic, hypertensive and in similar pressure overloaded conditions e.g. aortic stenosis (AS), HCM [83]. Indeed MVD, ECM remodeling and microvascular endothelial inflammation appear intimately linked and have recently been proposed as a novel paradigm for HFPEF[84]. In HCM patients with preserved EF at baseline, MVD (assessed by PET) predicted transition to HFREF and development of symptoms [85]. Diminished myocardial perfusion reserve (MPR) as measured by CMR may further detect pre-clinical disease. In a recent study of severe AS patients [86], MPR independently predicted exercise capacity and was determined by the degree of fibrosis and LV mass (remodeling).
5.5. Metabolic imaging
Existing nuclear and MRI techniques permit the detection of metabolic derangements of energetic status and substrate utilization (e.g. free fatty acids) implicated in HF [87]. Irrespective of HF etiology, reductions in energy levels (by measuring phosphocreatine) of approximately 70% have already been shown in human and animal studies [88]. Using magnetic resonance spectroscopy (MRS), the association between reduced myocardial phosphocreatine: adenosine tri-phosphate ratio (PCr:ATP) (phosphocreatine: adenosine tri-phosphate ratio) and DD has been shown in hypertensive patients [89] and in HFPEF during exercise [90]. Furthermore, diminished ATP flux through creatine kinase (CK) may distinguish those patients with LVH who transition to HF [91]. Recently, CMR hyperpolarized imaging (artificially increasing molecular alignment within a magnetic field) has emerged as an exciting new methodology allowing cardiac metabolism to be studied with dramatic increases in signal-to-noise and early studies in HF are keenly awaited [92].
Reduced substrate uptake and oxidation may also limit cardiac performance. Published literature provides conflicting data from studies of cardiac metabolism: both fatty acid and glucose utilization appear enhanced in early stages but diminishes with advancing HF [87]. Several PET radionuclide tracers that reflect utilization and oxidative metabolism (e.g. analogs of fatty acid and glucose) may be of potential benefit in HFPEF[93]. Increasing evidence implicates the role of excessive myocardial triglyceride accumulation (steatosis) in conditions highly prevalent in HFPEF: obesity, diabetes and pressure overload [94]. Steatosis, as quantified by MRS is independently associated with echocardiographic measures of DD [95], strain parameters derived from CMR tagging and correlates with histology [94].
5.6. Molecular imaging
Molecular targeting of the key markers implicated in ECM turnover has recently shown good capabilities, albeit almost exclusively in animal models. Potential targets studied include MMPs, ECM proteins, the renin-angiotensin axis and myofibroblasts. Post-infarct studies have already demonstrated the feasibility of assessing collagen deposition [96] and increased probe activity closely approximates with histological findings [97]. Although most studies have employed nuclear techniques (limited signal from poor tissue penetration), hybrid imaging with PET, SPECT, CT or CMR may further improve spatial resolution, which is the current major limitation. Cost and limited radiotracer availability are additional factors [98].
6. Summary
This review highlights some of the potential biomarkers in HFPEF, each offering alternative pathways for diagnosis and potential prognostic information. Furthermore, they provide unique mechanistic insights into a condition that is already characterized by marked pathophysiological diversity. Targeting biomarkers implicated across the disease spectrum, from a molecular level to macroscopy may enable earlier detection, better phenotyping & sub-classification, monitoring of treatment response and ultimately provide targeted treatment opportunities [93] ; [99]. Before incorporation into routine clinical practice however, these novel plasma and imaging tests must undergo rigorous methodological and clinical validation followed by testing in large scale, prospective studies [4]; [5]; [58] ; [100].
Author contributions
PK performed the literature review and drafted the manuscript. PK, IBS, LLNG and GPM conceived the structure/design and reviewed/edited the article. In particular, IBS provided expertise in heart failure, LLNG provided expertise in plasma biomarkers and GPM provided expertise in imaging aspects relevant to the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
The following are the supplementary data related to this article.
Supplementary online Table 1.
. Summary of major plasma biomarker studies showing promise in HFPEF.
Acknowledgments
This work has been supported by funding from the National Institute for Health Research (NIHR) Cardiovascular Biomedical Research Unit and Department of Cardiovascular Sciences, University Hospitals of Leicester.
References
- [1] T.E. Owan, D.O. Hodge, R.M. Herges, S.J. Jacobsen, V.L. Roger, M.M. Redfield; Trends in prevalence and outcome of heart failure with preserved ejection fraction; N. Engl. J. Med., 355 (2006), pp. 251–259
- [2] W.J. Paulus, J.J. van Ballegoij; Treatment of heart failure with normal ejection fraction: an inconvenient truth!; J. Am. Coll. Cardiol., 55 (2010), pp. 526–537
- [3] W.J. Paulus, C. Tschope, J.E. Sanderson, et al.; How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the european society of cardiology; Eur. Heart J., 28 (2007), pp. 2539–2550
- [4] Biomarkers Definitions Working G; Biomarkers and surrogate endpoints: preferred definitions and conceptual framework; Clin. Pharmacol. Ther., 69 (2001), pp. 89–95
- [5] R.S. Vasan; Biomarkers of cardiovascular disease: molecular basis and practical considerations; Circulation, 113 (2006), pp. 2335–2362
- [6] B.A. Borlaug, W.J. Paulus; Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment; Eur. Heart J., 32 (2011), pp. 670–679
- [7] M. Komajda, C.S. Lam; Heart failure with preserved ejection fraction: a clinical dilemma; Eur. Heart J., 35 (2014), pp. 1022–1032
- [8] D.P. Leong, C.G. De Pasquale, J.B. Selvanayagam; Heart failure with normal ejection fraction: the complementary roles of echocardiography and cmr imaging; JACC Cardiovasc. Imaging, 3 (2010), pp. 409–420
- [9] T.T. Phan, G.N. Shivu, K. Abozguia, J.E. Sanderson, M. Frenneaux; The pathophysiology of heart failure with preserved ejection fraction: from molecular mechanisms to exercise haemodynamics; Int. J. Cardiol., 158 (2012), pp. 337–343
- [10] N.G. Bellenger, M.I. Burgess, S.G. Ray, et al.; Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable?; Eur. Heart J., 21 (2000), pp. 1387–1396
- [11] J.L. Hare, J.K. Brown, T.H. Marwick; Performance of conventional echocardiographic parameters and myocardial measurements in the sequential evaluation of left ventricular function; Am. J. Cardiol., 101 (2008), pp. 706–711
- [12] L.E. Hudsmith, A.S. Cheng, D.J. Tyler, et al.; Assessment of left atrial volumes at 1.5 Tesla and 3 Tesla using flash and ssfp cine imaging; J. Cardiovasc. Magn. Reson., 9 (2007), pp. 673–679
- [13] T.D. Karamitsos, J.M. Francis, S. Myerson, J.B. Selvanayagam, S. Neubauer; The role of cardiovascular magnetic resonance imaging in heart failure; J. Am. Coll. Cardiol., 54 (2009), pp. 1407–1424
- [14] D.H. Maciver, M. Townsend; A novel mechanism of heart failure with normal ejection fraction; Heart, 94 (2008), pp. 446–449
- [15] S.F. Nagueh, C.P. Appleton, T.C. Gillebert, et al.; Recommendations for the evaluation of left ventricular diastolic function by echocardiography; Eur. J. Echocardiogr., 10 (2009), pp. 165–193
- [16] M.R. Zile, C.F. Baicu; Biomarkers of diastolic dysfunction and myocardial fibrosis: application to heart failure with a preserved ejection fraction; J. Cardiovasc. Transl. Res., 6 (2013), pp. 501–515
- [17] S.J. Shah; Evolving approaches to the management of heart failure with preserved ejection fraction in patients with coronary artery disease; Curr. Treat. Options Cardiovasc. Med., 12 (2010), pp. 58–75
- [18] S.H. Ahmed, L.L. Clark, W.R. Pennington, et al.; Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease; Circulation, 113 (2006), pp. 2089–2096
- [19] A. Gonzalez, B. Lopez, R. Querejeta, E. Zubillaga, T. Echeverria, J. Diez; Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction; Hypertension, 55 (2010), pp. 1418–1424
- [20] R. Martos, J. Baugh, M. Ledwidge, et al.; Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction; Circulation, 115 (2007), pp. 888–895
- [21] R. Martos, J. Baugh, M. Ledwidge, et al.; Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover; Eur. J. Heart Fail., 11 (2009), pp. 191–197
- [22] M.R. Zile, S.M. Desantis, C.F. Baicu, et al.; Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure; Circ. Heart Fail., 4 (2011), pp. 246–256
- [23] R. Querejeta, B. Lopez, A. Gonzalez, et al.; Increased collagen type i synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis; Circulation, 110 (2004), pp. 1263–1268
- [24] U.C. Sharma, S. Pokharel, T.J. van Brakel, et al.; Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction; Circulation, 110 (2004), pp. 3121–3128
- [25] Y.H. Liu, M. D'Ambrosio, T.D. Liao, et al.; N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin; Am. J. Physiol. Heart Circ. Physiol., 296 (2009), pp. H404–H412
- [26] P.A. McCullough, A. Olobatoke, T.E. Vanhecke; Galectin-3: a novel blood test for the evaluation and management of patients with heart failure; Rev. Cardiovasc. Med., 12 (2011), pp. 200–210
- [27] J.E. Ho, C. Liu, A. Lyass, et al.; Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community; J. Am. Coll. Cardiol., 60 (2012), pp. 1249–1256
- [28] R.V. Shah, A.A. Chen-Tournoux, M.H. Picard, R.R. van Kimmenade, J.L. Januzzi; Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure; Eur. J. Heart Fail., 12 (2010), pp. 826–832
- [29] D.J. Lok, P. Van Der Meer, P.W. de la Porte, et al.; Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the deal-hf study; Clin. Res. Cardiol., 99 (2010), pp. 323–328
- [30] R.A. de Boer, D.J. Lok, T. Jaarsma, et al.; Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction; Ann. Med., 43 (2011), pp. 60–68
- [31] J.L. Januzzi Jr.; St2 as a cardiovascular risk biomarker: from the bench to the bedside; J. Cardiovasc. Transl. Res., 6 (2013), pp. 493–500
- [32] J. Bartunek, L. Delrue, F. Van Durme, et al.; Nonmyocardial production of st2 protein in human hypertrophy and failure is related to diastolic load; J. Am. Coll. Cardiol., 52 (2008), pp. 2166–2174
- [33] J.L. Januzzi Jr., W.F. Peacock, A.S. Maisel, et al.; Measurement of the interleukin family member st2 in patients with acute dyspnea: results from the pride (pro-brain natriuretic peptide investigation of dyspnea in the emergency department) study; J. Am. Coll. Cardiol., 50 (2007), pp. 607–613
- [34] Y.C. Wang, C.C. Yu, F.C. Chiu, et al.; Soluble st2 as a biomarker for detecting stable heart failure with a normal ejection fraction in hypertensive patients; J. Card. Fail., 19 (2013), pp. 163–168
- [35] S. Manzano-Fernandez, T. Mueller, D. Pascual-Figal, Q.A. Truong, J.L. Januzzi; Usefulness of soluble concentrations of interleukin family member st2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction; Am. J. Cardiol., 107 (2011), pp. 259–267
- [36] D. Frank, C. Kuhn, B. Brors, et al.; Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program; Hypertension, 51 (2008), pp. 309–318
- [37] L. Lind, L. Wallentin, T. Kempf, et al.; Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the prospective investigation of the vasculature in Uppsala seniors (pivus) study; Eur. Heart J., 30 (2009), pp. 2346–2353
- [38] R. Santhanakrishnan, J.P. Chong, T.P. Ng, et al.; Growth differentiation factor 15, st2, high-sensitivity troponin t, and n-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction; Eur. J. Heart Fail., 14 (2012), pp. 1338–1347
- [39] S. Suzuki, T. Shishido, A. Funayama, et al.; Long pentraxin ptx3 exacerbates pressure overload-induced left ventricular dysfunction; PLoS ONE, 8 (2013), p. e53133
- [40] S. Suzuki, Y. Takeishi, T. Niizeki, et al.; Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure; Am. Heart J., 155 (2008), pp. 75–81
- [41] J. Matsubara, S. Sugiyama, T. Nozaki, et al.; Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction; J. Am. Coll. Cardiol., 57 (2011), pp. 861–869
- [42] R.A. de Boer, L.B. Daniels, A.S. Maisel, J.L. Januzzi Jr.; State of the art: Newer biomarkers in heart failure; Eur. J. Heart Fail. (2015)
- [43] E. Picano, P.A. Pellikka; Stress echo applications beyond coronary artery disease; Eur. Heart J., 35 (2014), pp. 1033–1040
- [44] D.A. Lythall, J. Bishop, R.A. Greenbaum, et al.; Relationship between myocardial collagen and echo amplitude in non-fibrotic hearts; Eur. Heart J., 14 (1993), pp. 344–350
- [45] M. Salvetti, M.L. Muiesan, A. Paini, et al.; Myocardial ultrasound tissue characterization in patients with chronic renal failure; J. Am. Soc. Nephrol., 18 (2007), pp. 1953–1958
- [46] C.Y. Wong, T. O'Moore-Sullivan, R. Leano, N. Byrne, E. Beller, T.H. Marwick; Alterations of left ventricular myocardial characteristics associated with obesity; Circulation, 110 (2004), pp. 3081–3087
- [47] M. Ciulla, R. Paliotti, D.B. Hess, et al.; Echocardiographic patterns of myocardial fibrosis in hypertensive patients: endomyocardial biopsy versus ultrasonic tissue characterization; J. Am. Soc. Echocardiogr., 10 (1997), pp. 657–664
- [48] A.M. Maceira, J. Barba, N. Varo, O. Beloqui, J. Diez; Ultrasonic backscatter and serum marker of cardiac fibrosis in hypertensives; Hypertension, 39 (2002), pp. 923–928
- [49] M.A. Losi, B. Memoli, C. Contaldi, et al.; Myocardial fibrosis and diastolic dysfunction in patients on chronic haemodialysis; Nephrol. Dial. Transplant., 25 (2010), pp. 1950–1954
- [50] C. Jellis, J. Martin, J. Narula, T.H. Marwick; Assessment of nonischemic myocardial fibrosis; J. Am. Coll. Cardiol., 56 (2010), pp. 89–97
- [51] T.H. Marwick, M. Schwaiger; The future of cardiovascular imaging in the diagnosis and management of heart failure, part 1: tasks and tools; Circ. Cardiovasc. Imaging., 1 (2008), pp. 58–69
- [52] E. Wu, R.M. Judd, J.D. Vargas, F.J. Klocke, R.O. Bonow, R.J. Kim; Visualisation of presence, location, and transmural extent of healed q-wave and non-q-wave myocardial infarction; Lancet, 357 (2001), pp. 21–28
- [53] R.J. Kim, E. Wu, A. Rafael, et al.; The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction; N. Engl. J. Med., 343 (2000), pp. 1445–1453
- [54] A.S. Flett, M.P. Hayward, M.T. Ashworth, et al.; Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans; Circulation, 122 (2010), pp. 138–144
- [55] D.M. Sado, A.S. Flett, S.M. Banypersad, et al.; Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease; Heart, 98 (2012), pp. 1436–1441
- [56] J. Mascherbauer, B.A. Marzluf, C. Tufaro, et al.; Cardiac magnetic resonance postcontrast t1 time is associated with outcome in patients with heart failure and preserved ejection fraction; Circ. Cardiovasc. Imaging., 6 (2013), pp. 1056–1065
- [57] M.Y. Su, L.Y. Lin, Y.H. Tseng, et al.; Cmr-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFPEF; JACC Cardiovasc. Imaging (2014)
- [58] J.C. Moon, D.R. Messroghli, P. Kellman, et al.; Myocardial t1 mapping and extracellular volume quantification: a society for cardiovascular magnetic resonance (scmr) and cmr working group of the european society of cardiology consensus statement; J. Cardiovasc. Magn. Reson., 15 (2013), p. 92
- [59] A. Singh, M.A. Horsfield, S. Bekele, J. Khan, A. Greiser, G.P. McCann; Myocardial t1 and extracellular volume fraction measurement in asymptomatic patients with aortic stenosis: reproducibility and comparison with age-matched controls; Eur. Heart J. Cardiovasc. Imaging (2015)
- [60] S.K. White, D.M. Sado, A.S. Flett, J.C. Moon; Characterising the myocardial interstitial space: the clinical relevance of non-invasive imaging; Heart, 98 (2012), pp. 773–779
- [61] P. Knaapen, W.G. van Dockum, O. Bondarenko, et al.; Delayed contrast enhancement and perfusable tissue index in hypertrophic cardiomyopathy: comparison between cardiac mri and pet; J. Nucl. Med., 46 (2005), pp. 923–929
- [62] S. Bandula, S.K. White, A.S. Flett, et al.; Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced ct: validation against histologic findings; Radiology, 269 (2013), pp. 396–403
- [63] G. Yip, M. Wang, Y. Zhang, J.W. Fung, P.Y. Ho, J.E. Sanderson; Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition?; Heart, 87 (2002), pp. 121–125
- [64] T.H. Park, S.F. Nagueh, D.S. Khoury, et al.; Impact of myocardial structure and function postinfarction on diastolic strain measurements: implications for assessment of myocardial viability; Am. J. Physiol. Heart Circ. Physiol., 290 (2006), pp. H724–H731
- [65] T. Kato, A. Noda, H. Izawa, et al.; Myocardial velocity gradient as a noninvasively determined index of left ventricular diastolic dysfunction in patients with hypertrophic cardiomyopathy; J. Am. Coll. Cardiol., 42 (2003), pp. 278–285
- [66] J. Wang, D.S. Khoury, V. Thohan, G. Torre-Amione, S.F. Nagueh; Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures; Circulation, 115 (2007), pp. 1376–1383
- [67] Y.T. Tan, F. Wenzelburger, E. Lee, et al.; The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion; J. Am. Coll. Cardiol., 54 (2009), pp. 36–46
- [68] T. Ohtani, S.F. Mohammed, K. Yamamoto, et al.; Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction; Eur. Heart J., 33 (2012), pp. 1742–1749
- [69] W.E. Moody, R.J. Taylor, N.C. Edwards, et al.; Comparison of magnetic resonance feature tracking for systolic and diastolic strain and strain rate calculation with spatial modulation of magnetization imaging analysis; J. Magn. Reson. Imaging (2014)
- [70] S.J. Buss, F. Schulz, D. Mereles, et al.; Quantitative analysis of left ventricular strain using cardiac computed tomography; Eur. J. Radiol., 83 (2014), pp. e123–e130
- [71] A. Singh, C.D. Steadman, J.N. Khan, et al.; Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 tesla: a comparison of feature-tracking and tagging in patients with aortic stenosis; J. Magn. Reson. Imaging, 41 (2015), pp. 1129–1137
- [72] V. Melenovsky, B.A. Borlaug, B. Rosen, et al.; Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban baltimore community: the role of atrial remodeling/dysfunction; J. Am. Coll. Cardiol., 49 (2007), pp. 198–207
- [73] Y.T. Tan, F. Wenzelburger, E. Lee, et al.; Reduced left atrial function on exercise in patients with heart failure and normal ejection fraction; Heart, 96 (2010), pp. 1017–1023
- [74] M. Habibi, H. Chahal, A. Opdahl, et al.; Association of cmr-measured la function with heart failure development: results from the mesa study; JACC Cardiovasc. Imaging., 7 (2014), pp. 570–579
- [75] G. Bastarrika, B. Zudaire, M. Ferreira, M. Arraiza, R. Saiz-Mendiguren, G. Rabago; Assessment of left atrial volumes and function in orthotopic heart transplant recipients by dual-source CT: comparison with MRI; Investig. Radiol., 45 (2010), pp. 72–76
- [76] S. Mondillo, M. Cameli, M.L. Caputo, et al.; Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size; J. Am. Soc. Echocardiogr., 24 (2011), pp. 898–908
- [77] M. Cameli, M. Lisi, S. Mondillo, et al.; Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure; Cardiovasc. Ultrasound, 8 (2010), p. 14
- [78] J.B. Somaratne, C. Berry, J.J. McMurray, K.K. Poppe, R.N. Doughty, G.A. Whalley; The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis; Eur. J. Heart Fail., 11 (2009), pp. 855–862
- [79] E. Picano, S. Molinaro, E. Pasanisi; The diagnostic accuracy of pharmacological stress echocardiography for the assessment of coronary artery disease: a meta-analysis; Cardiovasc. Ultrasound, 6 (2008), p. 30
- [80] C. Kim, Y.S. Kwok, P. Heagerty, R. Redberg; Pharmacologic stress testing for coronary disease diagnosis: a meta-analysis; Am. Heart J., 142 (2001), pp. 934–944
- [81] R.S. Beanlands, B.J. Chow, A. Dick, et al.; Ccs/car/canm/cncs/canscmr joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease–executive summary; Can. J. Cardiol., 23 (2007), pp. 107–119
- [82] C. Jaarsma, S. Schalla, E.C. Cheriex, et al.; Incremental value of cardiovascular magnetic resonance over echocardiography in the detection of acute and chronic myocardial infarction; J. Cardiovasc. Magn. Reson., 15 (2013), p. 5
- [83] F. Crea, P.G. Camici, C.N. Bairey Merz; Coronary microvascular dysfunction: an update; Eur. Heart J., 35 (2014), pp. 1101–1111
- [84] W.J. Paulus, C. Tschope; A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation; J. Am. Coll. Cardiol., 62 (2013), pp. 263–271
- [85] I. Olivotto, F. Cecchi, R. Gistri, et al.; Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy; J. Am. Coll. Cardiol., 47 (2006), pp. 1043–1048
- [86] C.D. Steadman, M. Jerosch-Herold, B. Grundy, et al.; Determinants and functional significance of myocardial perfusion reserve in severe aortic stenosis; JACC Cardiovasc. Imaging., 5 (2012), pp. 182–189
- [87] S. Neubauer; The failing heart–an engine out of fuel; N. Engl. J. Med., 356 (2007), pp. 1140–1151
- [88] J.S. Ingwall, R.G. Weiss; Is the failing heart energy starved? On using chemical energy to support cardiac function; Circ. Res., 95 (2004), pp. 135–145
- [89] H.J. Lamb, H.P. Beyerbacht, A. van der Laarse, et al.; Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism; Circulation, 99 (1999), pp. 2261–2267
- [90] T.T. Phan, K. Abozguia, G. Nallur Shivu, et al.; Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency; J. Am. Coll. Cardiol., 54 (2009), pp. 402–409
- [91] C.S. Smith, P.A. Bottomley, S.P. Schulman, G. Gerstenblith, R.G. Weiss; Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium; Circulation, 114 (2006), pp. 1151–1158
- [92] O.J. Rider, D.J. Tyler; Clinical implications of cardiac hyperpolarized magnetic resonance imaging; J. Cardiovasc. Magn. Reson., 15 (2013), p. 93
- [93] B. McArdle, T.F. Dowsley, M.S. Cocker, et al.; Cardiac pet: metabolic and functional imaging of the myocardium; Semin. Nucl. Med., 43 (2013), pp. 434–448
- [94] M. Mahmod, S. Bull, J.J. Suttie, et al.; Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis; Circ. Cardiovasc. Imaging, 6 (2013), pp. 808–816
- [95] L.J. Rijzewijk, R.W. van der Meer, J.W. Smit, et al.; Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus; J. Am. Coll. Cardiol., 52 (2008), pp. 1793–1799
- [96] Z.H. Sahul, R. Mukherjee, J. Song, et al.; Targeted imaging of the spatial and temporal variation of matrix metalloproteinase activity in a porcine model of postinfarct remodeling: relationship to myocardial dysfunction; Circ. Cardiovasc. Imaging, 4 (2011), pp. 381–391
- [97] S.W. van den Borne, S. Isobe, H.R. Zandbergen, et al.; Molecular imaging for efficacy of pharmacologic intervention in myocardial remodeling; JACC Cardiovasc. Imaging, 2 (2009), pp. 187–198
- [98] H.J. de Haas, E. Arbustini, V. Fuster, C.M. Kramer, J. Narula; Molecular imaging of the cardiac extracellular matrix; Circ. Res., 114 (2014), pp. 903–915
- [99] O.R. Coelho-Filho, R.V. Shah, T.G. Neilan, et al.; Cardiac magnetic resonance assessment of interstitial myocardial fibrosis and cardiomyocyte hypertrophy in hypertensive mice treated with spironolactone; J. Am. Heart Assoc., 3 (2014)
- [100] I. Paterson, L.M. Mielniczuk, E. O'Meara, A. So, J.A. White; Imaging heart failure: current and future applications; Can. J. Cardiol., 29 (2013), pp. 317–328
- [101] P. Kellman, J.R. Wilson, H. Xue, et al.; Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience; J. Cardiovasc. Magn. Reson., 14 (2012), p. 64
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?