Summary
Background
A pulmonary tumor is occasionally detected on a chest computed tomography (CT) scan before cardiovascular surgery.
Purpose
In this study, we examined clinical courses of patients who had undergone the simultaneous resection of a pulmonary tumor following cardiovascular surgery.
Methods
From 2008 to 2013, 18 patients (13 men and 5 women) with a median age of 69.8 years underwent the wedge pulmonary resection for a lung tumor through a median thoracotomy following cardiovascular surgery in our hospital. Cardiovascular surgeries consisted of off-pump coronary artery bypass grafting (CABG) in six patients, aortic valve replacement and/or mitral valve plasty in 10 patients, total arch replacement in 10 patients and descending aorta replacement in 10 patients.
Results
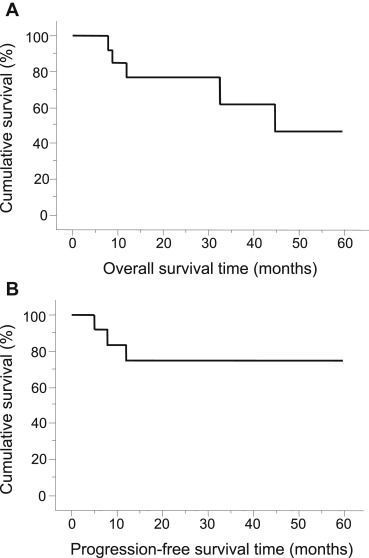
No complications associated with pulmonary resections were observed. Pathological examination revealed that 15 patients (83.3%) were diagnosed with lung cancers including 13 adenocarcinomas and two squamous cell carcinomas, with the clinical stages of 1A in 13 patients, 2A in one patient and 2B in one patient. Among them, five patients received the radical pulmonary resection subsequently, whereas 10 patients were unable to receive it due to their poor cardiopulmonary function. Kaplan-Meier analysis of patients with lung cancer revealed that the 5-year survival rate and progression-free survival (PFS) rate after 3 years from the surgery were 46.2% and 73.8%, respectively.
Conclusion
The simultaneous resection of pulmonary tumor following cardiovascular surgery is safely performed, and is useful for the pathological diagnosis of the tumor. Further studies are warranted, however, this procedure may contribute to controlling the progression of lung cancer in patients with cardiovascular disease with comorbidities.
Keywords
cardiovascular surgery;lung cancer;simultaneous surgery;thoracic surgery
1. Introduction
Lung cancer is the leading cause of cancer-related death in males in the Asia-Pacific region and globally,1 ; 2 and it accounted for 13% (1.6 million) of the total cancer cases and 18% (1.4 million) of deaths in 2008.1 In Japan, the total number of general thoracic surgeries in 2012 was 72,899, and > 49% of those cases underwent surgery for lung cancer.3 The number of patients diagnosed with lung cancer is steadily increasing, leading to an increase in the number of patients undergoing thoracic surgery. Furthermore, the number of cardiovascular surgeries for adults was reported to be > 50,000 in Japan in 2012.3 An unhealthy lifestyle seems to be a major factor for the increase in thoracic and cardiovascular surgeries, and the number of these surgeries continues to increase.
Cases of concomitant lung cancer with cardiovascular disease are also increasing. Cases of incidental pulmonary tumor, which is detected by chest computed tomography (CT) before cardiovascular surgery is performed, present the greatest challenge for surgeons. Johnson and colleagues reviewed 3364 consecutive patients who had undergone coronary artery bypass grafting (CABG) and reported that 191 patients (5.7%) had pulmonary nodules.4 As opposed to cases of pulmonary nodules with calcification, for pulmonary nodules suspected to be lung cancer in patients with cardiovascular disease, guidelines for the diagnosis and treatment of pulmonary nodules are yet to be established.
To obtain the diagnosis for an incidental pulmonary tumor detected before cardiovascular surgery, patients undergo conventional radiological and serological examinations, which are in part useful for the diagnosis. However, physicians appear reluctant to perform examinations to obtain the pathological diagnosis of the tumor before cardiovascular surgery because of the poor cardiovascular function of the patients. Thus, some physicians may consider performing a bronchoscopy after cardiovascular surgery; however, there is an increased risk of bleeding following a transbronchial lung biopsy (TBLB) due to the administration of anticoagulant drugs after cardiovascular surgery.5
To resolve such a dilemma, we performed simultaneous resection of pulmonary tumor during cardiovascular surgery. In previous papers on this surgical procedure, patients who had undergone a radical surgery, such as a lobectomy or a pneumonectomy, for lung cancer and cardiovascular surgery simultaneously were included.6; 7; 8 ; 9 The pulmonary vascular bed is decreased by a lobectomy or a pneumonectomy, causing a serious strain on cardiac function. Thus, although it has been reported that this surgical procedure is safe, postoperative complications may increase because of the radical lung surgery.
With this in mind, during cardiovascular surgery, we decided to perform simultaneous wedge resection of incidental pulmonary tumor which had been detected prior to cardiovascular surgery. We previously reported early to mid-term results of this procedure, demonstrating that there was neither operative mortality nor any major cardiac complication associated with it and that it had a mean progression-free survival (PFS) time of 17 months.10
In the present report that included more patients than the previous report, the safety and effectiveness of this procedure were evaluated retrospectively. We herein report that this procedure can be safely performed and is useful to obtain the pathological diagnosis of incidental pulmonary tumors. In addition, this procedure may be useful for controlling the progression of lung cancer in patients with severe cardiovascular disease.
2. Patients and methods
2.1. Patients
From February 2008 to April 2013, incidental pulmonary tumors were detected using preoperative chest CT in 18 patients who had been scheduled for cardiovascular surgery in our hospital (Table 1). The median age was 69.8 years, and the patients consisted of 13 men and five women, none of whom reported any symptoms associated with the pulmonary tumor. Cardiovascular surgeries that had been planned were as follows: off-pump CABG in six patients, aortic valve replacement and/or mitral valve plasty in nine patients, aortic valve replacement and total arch replacement in one patient, total arch replacement in one patient, and descending aorta replacement in one patient. The preoperative CT scan revealed that pulmonary tumors were located in the right lung in 11 patients (upper, 7; middle, 2; lower, 2) and in the left lung in seven patients (upper, 2; lower, 5). In all cases, through the findings of preoperative chest CT, the pulmonary tumors were suspected to be lung cancers, with neither hilar adenopathy nor mediastinal masses observed. Common preoperative examinations for cardiovascular surgery, such as brain CT and pulmonary function tests were performed. To obtain the pathological diagnosis of the pulmonary tumor, cytological examination of the sputum was performed, and neither TBLB nor CT-guided needle biopsy were preoperatively planned.
| Age (median) | 50∼86 (69.8) |
|---|---|
| Gender | |
| Male (%) | 13 (72) |
| Female (%) | 5 (28) |
| Cardiovascular surgery | |
| OPCAB (%) | 6 (33) |
| AVR and/or MVP (%) | 9 (50) |
| AVR and TAR (%) | 1 (5.6) |
| TAR (%) | 1 (5.6) |
| DAR (%) | 1 (5.6) |
| Location of pulmonary tumor | |
| Right lung (%) | 11 (61) |
| Upper | 7 |
| Middle | 2 |
| Lower | 2 |
| Left lung (%) | 7 (39) |
| Upper | 2 |
| Lower | 5 |
AVR = aortic valve replacement; DAR = descending aorta replacement; MVP = mitral valve plasty; OPCAB = off-pump coronary artery bypass grafting; TAR = total arch replacement.
2.2. Surgical procedure
Informed consent to perform simultaneous resection of the pulmonary tumor was obtained from all patients prior to surgery. During surgery, a double lumen endotracheal tube was introduced in the patient. A median sternotomy was performed in 17 patients and a left posterolateral thoracotomy in one patient. After completion of cardiovascular surgery and administration of protamine, wedge resection of the pulmonary tumor was performed using an automatic suture instrument via median sternotomy or left posterolateral thoracotomy; consequently, an additional incision was not required for the pulmonary resection. Hilar and mediastinal lymph nodes were not sampled.
2.3. Statistical analysis
The overall survival (OS) and PFS after simultaneous pulmonary resection were estimated by the Kaplan-Meier method. A log-rank test was used to compare these survival times in different groups of patients.
3. Results
3.1. Operative mortality and surgical complications
No operative mortalities or hospital deaths were observed in patients who had undergone simultaneous resection of the pulmonary tumor during cardiovascular surgery. Regarding surgical complications, we were mostly concerned about thoracic hemorrhage due to wedge resection of the pulmonary tumor; however, this did not occur in any patient, and therefore, no patients required a re-thoracotomy. We were also concerned about an increased risk of air leakage and pneumonia after the simultaneous pulmonary resection; however, this did not occur in any patient. In addition, other cardiovascular surgical complications, such as perioperative or postoperative myocardial infarction, cardiac tamponade, and stroke, were not increased by the simultaneous pulmonary resection.
3.2. Pathological diagnoses of pulmonary tumors
Among the 18 patients, three patients were diagnosed with benign tumors, including granuloma (1 patient), inflammatory tumor (1 patient), and teratoma (1 patient) (Table 2). The other 15 patients were pathologically diagnosed with non-small cell lung cancer (NSCLC), consisting of nine adenocarcinomas, four bronchioloalveolar cell carcinomas, and two squamous cell carcinomas. Pathological findings also demonstrated that malignant tumor cells were not observed in the resected lung margins in any patient. The clinical stages of NSCLC cases were determined to be 1A in 13 patients, 2A in one patient, and 2B in 1 patient.
| Case | Sex | Age (y) | Cardiovascular surgery | Tumor location | Clinical stage | Radical surgery | Pathology | Prognosis (time from initial surgery) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 73 | OPCAB + Y-graft | Rt. upper | IA | − | BAC | Alive (60 mo) |
| 2 | M | 54 | TAR | Lt. lower | IA | Lob + ND1b | Adeno | Alive (59 mo) |
| 3 | M | 77 | AVR + MVP + maze | Rt. lower | IA | − | SqCC | Alive (53 mo) |
| 4 | M | 70 | OPCAB | Rt. lower | IIA | Lob + ND1b | SqCC | Dead from AMI (45 mo) |
| 5 | M | 86 | OPCAB | Rt. upper | IA | − | Adeno | Follow-up loss (30 mo) |
| 6 | F | 78 | AVR | Lt. lower | IA | − | Adeno | Follow-up loss (2 mo) |
| 7 | M | 50 | OPCAB | Lt. lower | IA | Seg + ND1b | BAC | Dead from colon cancer (8 mo) |
| 8 | M | 78 | MVP + Bentall + maze | Lt. lower | IA | − | Adeno | Dead from lung cancer (9 mo) |
| 9 | M | 83 | DAR | Rt. upper | IA | − | Adeno | Dead from unknown disease (33 mo) |
| 10 | M | 77 | AVR | Rt. upper | IA | − | Adeno | Alive (28 mo) |
| 11 | M | 67 | OPCAB | Lt. upper | IIB | Lob + ND1b | Adeno | Dead from IP (12 mo) |
| 12 | F | 75 | AVR + TAR | Rt. middle | IA | − | Adeno | Alive (14 mo) |
| 13 | F | 60 | AVR + CABG | Lt. lower | IA | Seg + ND1b | BAC | Alive (19 mo) |
| 14 | M | 63 | OPCAB + PVI | Rt. upper | IA | − | Adeno | Alive (19 mo) |
| 15 | F | 78 | MVP | Rt. upper | IA | − | BAC | Follow-up loss (6 mo) |
AAR = ascending aorta replacement; Adeno = adenocarcinoma; AVR = aortic valve replacement; BAC = bronchioloalveolar cell carcinoma; CABG = coronary artery bypass grafting; DAR = descending aorta replacement; Lob = lobectomy; IP = interstitial pneumonitis; MVP = mitral valve plasty; ND = nodal resection; OPCAB = off-pump coronary artery bypass grafting; PVI = pulmonary vein isolation; Seg = segmentectomy; SqCC = squamous cell carcinoma; TAR = total arch replacement.
3.3. Staged radical surgery for NSCLC
Of the 15 patients with NSCLC, five underwent staged radical surgery for NSCLC 1–14 months after the initial simultaneous pulmonary resection (Table 2). The pathological stages of them were determined to be 1A in three patients, 2A in one patient, and 2B in one patient. Among them, three patients underwent a lobectomy and two underwent a segmentectomy. Their pulmonary function became worse after cardiovascular surgery; however, we considered that this was acceptable given the benefits of the radical pulmonary resection. The remaining 10 patients were considered not suitable for additional pulmonary surgery due to low cardiopulmonary function, poor performance status, or severe comorbidities.
3.4. Postoperative clinical courses of patients with NSCLC
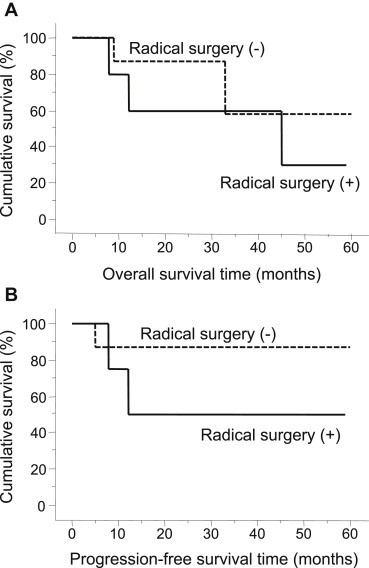
The median follow-up period of patients with NSCLC was 19 months, ranging from 2 to 60 months. Two patients died from NSCLC, and others died from other medical issues including cardiac insufficiency, colon cancer, and unknown reasons. Local recurrences were observed in three patients 5–12 months after the initial simultaneous pulmonary resection. Kaplan-Meier analysis in all patients with NSCLC revealed that the median OS was 45 months (Fig. 1A), and the median PFS was not reached (Fig. 1B). The PFS at 3 years after the initial simultaneous pulmonary resection was 73.8%, and the 5-year survival rate was 46.2%. In patients who underwent staged radical surgery for NSCLC, the median OS after the initial simultaneous pulmonary resection was 45 months, which tended to be shorter than that of patients who did not undergo the radical surgery (median OS is not reached, p = 0.3678) ( Fig. 2A). The median PFS also tended to be shorter in patients who had undergone the staged radical surgery than in those who had not (11 months vs. not reached, p = 0.2914) ( Fig. 2B). The PFS at 3 years and the 5-year survival rate after the initial simultaneous pulmonary resection was not improved by the staged radical surgery for NSCLC (30.0% vs. 58.3%, p = 0.3678; 40.0% vs. 87.5%, p = 0.2914, respectively).
|
|
|
Figure 1. (A) Kaplan-Meier analysis on overall survival time of patients with lung cancer; (B) Kaplan-Meier analysis on disease-free survival time of patients with lung cancer. |
|
|
|
Figure 2. (A) Kaplan-Meier analysis on overall survival time of patients with lung cancer by radical pulmonary surgery; (B) Kaplan-Meier analysis on disease-free survival time of patients with lung cancer by radical pulmonary surgery. |
4. Discussion
In this study, we evaluated the safety and efficacy of simultaneous resection of the pulmonary tumor during cardiovascular surgery, demonstrating that this procedure was safe and useful for the pathological diagnosis of the tumor and may control the progression of lung cancer in patients with a high risk of severe cardiovascular disease.
The incidence of perioperative rebleeding is more frequent in cardiovascular surgeries than other types of surgery due to the administration of anticoagulants. Thus, we suspected that simultaneous resection of the pulmonary tumor during cardiovascular surgery might increase the risk of perioperative thoracic hemorrhage. However, our study revealed that this was not the case. Previous papers have reported that this is quite a rare complication of simultaneous pulmonary resection during cardiovascular surgery,6; 8; 11; 12 ; 13 demonstrating that simultaneous pulmonary resection does not significantly enhance the risk of perioperative thoracic hemorrhage in cardiovascular surgery. In all cases of the present study, the resection of pulmonary tumor was performed after completion of cardiovascular surgery. The surgical procedure for the pulmonary tumor in all cases was the wedge resection, the less complicated surgical technique. Thus, the resection of pulmonary tumor could have been performed before cardiovascular surgery in the present study. However, pulmonary resection before cardiovascular surgery might cause intrapulmonary hemorrhage, leading to respiratory dysfunction during cardiovascular surgery. Thus, the resection of pulmonary tumor was performed after completion of cardiovascular surgery. Recently, there have been improvements in automatic suture instruments for pulmonary resection, and this has contributed to a lower incidence of perioperative thoracic hemorrhage despite administration of anticoagulants.
In patients with an incidental pulmonary tumor, TBLB by flexible bronchoscopy is performed for pathological diagnosis. In some cases, TBLB is useful to obtain the pathological diagnosis of pulmonary tumors. However, as seen in the present study, physicians may be reluctant to perform a bronchoscopy before cardiovascular surgery because of the unstable cardiac function. If TBLB is performed after the surgery, the risk of hemorrhage may increase due to the administration of anticoagulants. In addition, bronchoscopy seems to be less suitable for pathological diagnosis compared with the finding of grand glass opacity detected on chest CT. On the basis of these results, we conclude that simultaneous resection of pulmonary tumor can be safely performed during cardiovascular surgery.
It may be possible to perform a single-stage complete resection for lung cancer, such as pneumonectomy, lobectomy, or segmentectomy during cardiovascular surgery via a thoracotomy. In our study, all patients underwent wedge resection for their pulmonary tumors during cardiovascular surgery. Several months later, curative complete resection for lung cancer was only performed on 33.3% of patients who were evaluated to be capable of enduring additional lung surgery. The other patients who did not undergo subsequent complete pulmonary resection had low cardiopulmonary function, poor performance status, and severe comorbidities. Previous papers have reported that simultaneous complete resection for lung cancer during cardiovascular surgery was associated with high perioperative mortality rates.3 Given that high-risk patients were included in these studies, single-stage complete resection for lung cancer during cardiovascular surgery may not have been suitable.
In patients with resectable NSCLC, the recommended surgical procedure should still be a lobectomy or a greater pulmonary resection, with hilar and mediastinal lymph nodes dissection.14 ; 15 Concerning lymph nodes dissection, Darling and colleagues16 reported that as compared with mediastinal lymph nodes sampling, mediastinal lymph nodes dissection did not improve the chance of survival in patients in the early stages of NSCLC, suggesting that dissection or sampling of mediastinal lymph nodes would provide significant value to the staging of lung cancer. In the present study, we assessed whether subsequent complete pulmonary resection after simultaneous wedge resection of pulmonary tumor during cardiovascular surgery would improve the prognosis in patients in the early stages of NSCLC. Unfortunately, the prognosis tended to be relatively worse than that in patients who had not undergone the additional pulmonary resection. Although our study included a limited and small cohort of patients, we could still conclude that complete pulmonary resection did not seem to contribute to the improved prognoses in patients with severe cardiovascular diseases. We speculated on the reasons for the association between additional pulmonary resection and a poorer prognosis. The patients who underwent simultaneous resection of pulmonary tumor during cardiovascular surgery may have had less residual cardiopulmonary function, which impeded the physiological recovery from the additional lung surgery. In addition, those patients had multiple comorbidities such as diabetes, renal failure, and hyperglycemia.
The number of patients in whom an incidental pulmonary tumor is detected before cardiovascular surgery will increase in the future with the advancements in chest CT scanning; however, the standard surgical strategy for patients with such tumors remains unclear. Johnson and colleagues4 reviewed 3364 consecutive patients undergoing CABG and reported that 191 patients (5.7%) had pulmonary nodules. Among those 191 patients, 40 patients had noncalcified pulmonary nodules requiring follow-up. In that study, 18 patients with noncalcified pulmonary nodules underwent simultaneous resection of pulmonary nodules during CABG, and 11 patients (61.1%) were diagnosed with lung cancer. Similar to our report, 15 of 18 patients (83.3%) were diagnosed with lung cancer, demonstrating that patients with lung cancer were frequently those who underwent the concomitant surgery. These findings indicate that careful interpretation of chest CT is necessary to avoid the resection of benign tumors that can be left untreated.
Concerning the prognosis of patients who underwent simultaneous resection of lung cancer, Zhang and colleagues7 reported that the 5-year survival rate for these patients was 43.6%. In that report, 14 patients underwent wedge resection for lung cancer, and four of them subsequently underwent lobectomy staged as IB or IIA. In our report, which included patients with relatively early stages of lung cancer, the 5-year survival rate was 46.2%, consistent with the previous report. In contrast, Dyszkiewicz and colleagues8 reported a 5-year survival rate of 0% in patients who had undergone simultaneous resection of Stage II and III lung cancers, suggesting that the pathological stage of lung cancer is likely to be associated with the prognosis of patients, as well as in the case of simultaneous pulmonary resection.
In conclusion, simultaneous resection of pulmonary tumor following cardiovascular surgery can be performed safely and is useful for pathological diagnosis. In patients with severe multiple comorbidities, this procedure possesses some possibility to control the progression of lung cancer.
References
- 1 A. Jemal, F. Bray, M.M. Center, J. Ferlay, E. Ward, D. Forman; Global cancer statistics; CA Cancer J Clin, 61 (2011), pp. 69–90
- 2 E. Jamrozik, A.W. Musk; Respiratory health issues in the Asia-Pacific region: an overview; Respirology, 16 (2011), pp. 3–12
- 3 M. Masuda, H. Kuwano, M. Okumura, et al.; Thoracic and cardiovascular surgery in Japan during 2012 : Annual report by The Japanese Association for Thoracic Surgery; Gen Thorac Cardiovasc Surg, 62 (2014), pp. 734–764
- 4 J.A. Johnson, R.J. Landreneau, T.M. Boley, et al.; Should pulmonary lesions be resected at the time of open heart surgery?; Am Surg, 62 (1996), pp. 300–303
- 5 A. Ernst, R. Eberhardt, M. Wahidi, H.D. Becker, F.J. Herth; Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans; Chest, 129 (2006), pp. 734–737
- 6 M.H. Danton, V.A. Anikin, K.G. McManus, J.A. McGuigan, G. Campalani; Simultaneous cardiac surgery with pulmonary resection: presentation of series and review of literature; Eur J Cardiothorac Surg, 13 (1998), pp. 667–672
- 7 R. Zhang, B. Wiegmann, S. Fischer, et al.; Simultaneous cardiac and lung surgery for incidental solitary pulmonary nodule: learning from the past; J Thorac Cardiovasc Surg, 60 (2012), pp. 150–155
- 8 W. Dyszkiewicz, M. Jemielity, C. Piwkowski, et al.; The early and late results of combined off-pump coronary artery bypass grafting and pulmonary resection in patients with concomitant lung cancer and unstable coronary heart disease; Eur J Cardiothorac Surg, 34 (2008), pp. 531–535
- 9 V. Rao, T.R. Todd, R.D. Weisei, et al.; Results of combined pulmonary resection and cardiac operation; Ann Thorac Surg, 62 (1996), pp. 342–347
- 10 S. Hosoba, J. Hanaoka, T. Suzuki, et al.; Early to midterm results of cardiac surgery with concomitant pulmonary resection; Ann Thorac Cardiovasc Surg, 18 (2012), pp. 8–11
- 11 A. Brutel de la Rivière, P. Knaepen, H. Van Swieten, R. Vanderschueren, J. Ernst, J. Van den Bosch; Concomitant open heart surgery and pulmonary resection for lung cancer; Eur J Cardiothorac Surg, 9 (1995), pp. 310–314
- 12 A. Terzi, G. Furlan, G. Magnelli, et al.; Lung resections concomitant to coronary artery bypass grafting; Eur J Cardiothorac Surg, 8 (1994), pp. 580–584
- 13 K.S. Ulicny Jr., V. Schmelzer, J.B. Flege Jr., et al.; Concomitant cardiac and pulmonary operation: the role of cardiopulmonary bypass; Ann Thorac Surg, 54 (1992), pp. 289–295
- 14 R.J. Ginsberg, L.V. Rubinstein; Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group; Ann Thorac Surg, 60 (1995), pp. 615–622
- 15 F. Al-Shahrabani, D. Vallbohmer, S. Angenendt, W.T. Knoefel; Surgical strategies in the therapy of non-small cell lung cancer; World J Clin Oncol, 5 (2014), pp. 595–603
- 16 G.E. Darling, M.S. Allen, P.A. Decker, et al.; Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial; J Thorac Cardiovasc Surg, 141 (2011), pp. 662–670
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?