Summary
Background
Among several minimally invasive adrenalectomy techniques, lateral transperitoneal adrenalectomy (LTA) is the procedure of choice for benign adrenal tumors; however, posterior retroperitoneoscopic adrenalectomy (PRA) is an alternative that is increasing in popularity. This study compared the outcomes of these two approaches.
Methods
Since a single surgeon started adrenalectomy, LTA had been performed exclusively until PRA was adopted and became the standard treatment. Therefore, the consecutive patients were allocated into two groups according to the date of surgery: the first group received LTA and the second group received PRA.
Results
LTA was performed in 29 patients and PRA in 19 patients. There was no difference in sex, age, body mass index, clinical diagnosis, and tumor size between the LTA and the PRA group. The PRA group showed less blood loss (117.0 mL vs. 58.5 mL, p = 0.035) and tended to have a shorter operating time (92.2 minutes vs. 78.1 minutes, p = 0.054) and less pain score on postoperative Day 1 (3.8 vs. 3.0, p = 0.095) and Day 2 (3.2 vs. 2.5, p = 0.051). The mean operation time was significantly shorter for patients in the PRA group undergoing right adrenalectomy (109.2 minutes vs. 80.5 minutes, p = 0.009), but those undergoing left adrenalectomy had a similar operating time to the LTA group (83.2 minutes vs. 74.8 minutes, p = 0.380).
Conclusion
PRA is a good alternative operative technique for an endocrine surgeon who is experienced in the transperitoneal approach.
Keywords
blood loss;lateral transperitoneal adrenalectomy;posterior retroperitoneoscopic adrenalectomy;postoperative outcome;postoperative pain
1. Introduction
Since Gagner and colleagues'1 report of its successful use for the treatment of Cushings disease and pheochromocytoma in 1992, laparoscopic transperitoneal adrenalectomy (LTA) has been the gold standard treatment for benign adrenal tumors. Its benefits over open adrenalectomy were demonstrated in subsequent studies.2; 3 ; 4 However, since its introduction in 1996 (along with improvements in laparoscopic instruments and surgical techniques), posterior retroperitoneal adrenalectomy (PRA) is regarded as a better approach for small benign adrenal tumors.5
LTA is performed within the peritoneal space with the patient in the lateral decubitus position, whereas PRA is performed in the retroperitoneal space with the patient prone. PRA is safe and unaffected by the presence of intra-abdominal adhesions, and can also be performed in obese patients.6; 7 ; 8 Previous studies comparing LTA with PRA report that PRA has advantages in terms of shorter operative time, reduced blood loss, reduced length of hospital stay, less postoperative pain, and a faster return to work5; 9; 10; 11; 12 ; 13; however, variations in the experience of surgeons, their individual preference for a particular technique, and bias in patient selection weaken the impact of many retrospective comparative studies.
In our institution, an endocrine surgeon always removed benign adrenal tumors using LTA prior to 2012; however, he has now exclusively adopted PRA. Therefore, in the present study, patients were allocated into groups according to the date of their surgery rather than the surgeons preference for a particular technique. Thus, the present study is the first to compare the outcomes of LTA and PRA in the absence of selection bias and surgical experience.
2. Materials and methods
2.1. Patient selection
A single surgeon (K.E.L.) performed minimally invasive adrenalectomy using LTA technique exclusively at Seoul National University Hospital from September 2009 until December 2011. Then the surgeon adopted PRA in January 2012, and it has been performed exclusively until September 2012. The medical records of all patients who underwent LTA and PRA performed by the study surgeon at Seoul National University Hospital, Seoul, Korea from September 2009 to September 2012 were reviewed. The inclusion criteria were as follows: patients with a benign adrenal tumor of <5 cm in diameter on the pathology reports in the case of pheochromocytoma, and those with a tumor <7 cm in case of Cushings syndrome, aldosteronism, or for nonfunctioning tumors. The exclusion criteria were bilateral adrenalectomy or conversion to an open procedure. The study was approved by the Institutional Review Board of Seoul National University Hospital.
2.2. Operative techniques
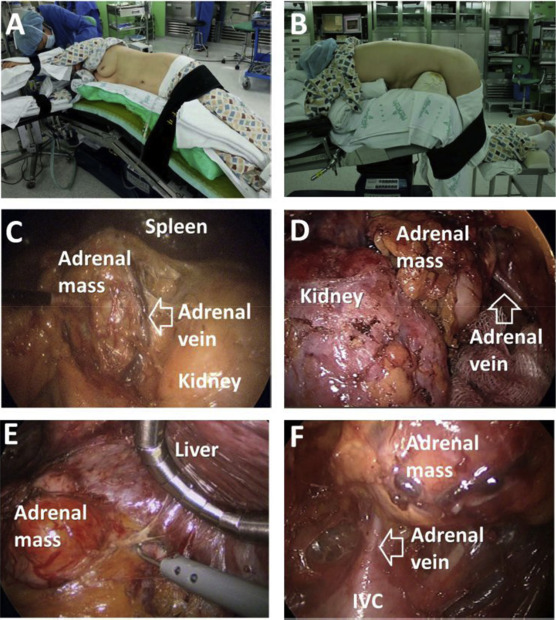
For LTA, the patient was placed in the lateral decubitus position with the affected side facing upward and the operative bed flexed just above the level of the iliac crest. The laparoscopic procedure was performed through a transperitoneal approach as described by Duh et al,14 except that three ports were used on the left side and four on the right (Figure 1).
|
|
|
Figure 1. (A) Position for left lateral transperitoneal adrenalectomy. (B) Position for posterior retroperitoneoscopic adrenalectomy. (C) Intraoperative view of left lateral transperitoneal adrenalectomy. (D) Intraoperative view of left posterior retroperitoneoscopic adrenalectomy. (E) Intraoperative view of right lateral transperitoneal adrenalectomy. (F) Intraoperative view of right posterior retroperitoneoscopic adrenalectomy. |
For PRA, the patient was intubated after anesthesia in a patient bed and then turned over from the bed onto the operating table. PRA was performed with the patient in the prone jackknife position with a moderately bent hip joint. Routinely, three holes were made along the lower tip of the 12th rib, and the operation was performed as described by Walz et al.15
2.3. Outcome evaluation
The operation time was calculated from the time of the first incision to the closing of the final stitch. Operation time and blood loss were obtained from the surgeons operation record or from the anesthesia record. An arterial line was inserted, and blood pressure and heart rate were monitored and recorded automatically during the operation. An intraoperative hypertensive event was defined as systolic blood pressure (SBP) ≥ 170 mmHg and an intraoperative hypotensive event as SBP ≤ 90 mmHg or diastolic blood pressure (DBP) ≤ 50 mmHg; the number of these events was recorded. The tumor size was obtained from pathology reports.
Pain was evaluated using a visual analog scale (VAS). The VAS ranges from 0 (no pain) to 10 (worst pain). The VAS was checked three times a day and expressed as the daily mean value. Acetaminophen (650 mg) was used routinely for postoperative pain management. Analgesic injections (intramuscular ketorolac tromethamine, 30 mg) were prescribed upon patient demand. Sips of water were allowed 6 hours postoperatively, and an oral semi-blended or regular diet was initiated from postoperative Day 1, regardless of whether or not the patient had passed gas. Patients who used patient-controlled analgesia (PCA) were excluded when evaluating the VAS and analgesic requirements.
2.4. Statistical analysis
All statistical analyses were performed using SPSS version 19 (SPSS Inc., Chicago, IL, USA). Numerical data are presented as the mean or median and standard deviation. Comparisons were performed using the Student t test, the Mann–Whitney U test, the Chi-square test, or Fishers exact test as appropriate. A p value <0.05 was considered statistically significant.
3. Results
A total of 29 LTA and 19 PRA patients were eligible for the study. Patient demographics and clinical data are shown in Table 1. There was no significant difference between the two study groups in terms of gender, age, body mass index, antihypertensive medication history, preoperative blood pressure, preoperative heart rate, history of abdominal surgery, clinical diagnosis, tumor location, or tumor size.
| LTA (n = 29) | PRA (n = 19) | p | |
|---|---|---|---|
| Sex | 0.835 | ||
| Male | 10 (34.5) | 6 (65.5) | |
| Female | 19 (65.5) | 13 (68.4) | |
| Age (y) | 48.6 ± 15.3 | 45.8 ± 14.4 | 0.543 |
| Body mass index (kg/m2) | 23.8 ± 2.7 | 23.5 ± 3.3 | 0.712 |
| Anti-hypertensive medication | 22 (75.9) | 14 (77.8) | 1.0 |
| Systolic blood pressure (mmHg) | 133.3 ± 19.9 | 128.3 ± 13.6 | 0.336 |
| Diastolic blood pressure (mmHg) | 83.6 ± 18.2 | 80.8 ± 13.1 | 0.566 |
| Heart rate | 79.8 ± 17.3 | 75.3 ± 10.6 | 0.272 |
| History of abdominal surgery | 12 (41.4) | 7 (38.9) | 0.866 |
| Clinical diagnosis | 0.701 | ||
| Aldosteronism | 14 (48.3) | 7 (36.8) | |
| Cushings syndrome | 7 (24.1) | 4 (21.1) | |
| Pheochromocytoma | 5 (17.2) | 6 (31.6) | |
| Nonfunctioning tumor | 3 (10.3) | 2 (10.5) | |
| Location of tumor | 0.110 | ||
| Right | 10 (34.5) | 11 (57.9) | |
| Left | 19 (65.5) | 8 (42.1) | |
| Mean tumor size (cm) | 2.7 ± 1.4 | 2.9 ± 1.4 | 0.696 |
Data are expressed as the n (%) or mean ± SD.
LTA = lateral transperitoneal adrenalectomy; PRA = posterior retroperitoneal adrenalectomy.
The operative outcomes and intraoperative hemodynamic parameters are shown in Table 2. The mean operation time for the PRA group was less than that for the LTA group (92.2 ± 27.0 minutes vs. 78.1 ± 18.9 minutes, p = 0.054), although the difference was not statistically significant. Blood loss was significantly lower in the PRA group than in the LTA group (117.0 ± 83.4 mL vs. 58.5 ± 58.1 mL, p = 0.035). The peak SBP was lower in the PRA group (151.8 ± 26.4 mmHg vs. 138.0 ± 14.2 mmHg, p = 0.041), but there were no significant differences in the lowest SBP, lowest DBP, highest/lowest heart rates, hypertensive/hypotensive events during the operation, or intraoperative use of inotropic or antihypertensive drugs. Intraoperative hypertensive events occurred in four LTA patients. One event occurred once in each of the four patients and was controlled within 5 minutes either with or without antihypertensive medication. The highest end tidal CO2 was significantly greater in the PRA group than in the LTA group (36.0 ± 2.9 mmHg vs. 40.8 ± 4.7 mmHg, p < 0.001). Differences in operation time were more prominent for those undergoing right adrenalectomy ( Table 3): the operation time was significantly shorter for those in the PRA group (109.2 minutes vs. 80.5 minutes, p = 0.009), whereas the operation time for those undergoing left adrenalectomy was similar to that for the LTA group (83.2 minutes vs. 74.8 minutes, p = 0.785).
| LTA (n = 29) | PRA (n = 19) | p | |
|---|---|---|---|
| Operation time (min) | 92.2 ± 27.0 | 78.1 ± 18.9 | 0.054 |
| Blood loss (mL) | 117.0 ± 83.4 | 58.5 ± 58.1 | 0.035 |
| Intraoperative highest SBP (mmHg) | 151.8 ± 26.4 | 138.0 ± 14.2 | 0.041 |
| Intraoperative lowest SBP (mmHg) | 93.1 ± 14.0 | 91.0 ± 12.3 | 0.580 |
| Intraoperative lowest DBP (mmHg) | 57.1 ± 10.7 | 55.7 ± 10.5 | 0.672 |
| Intraoperative highest HR (beats/min) | 82.9 ± 16.2 | 84.3 ± 19.9 | 0.787 |
| Intraoperative lowest HR (beats/min) | 57.3 ± 9.2 | 60.6 ± 13.6 | 0.316 |
| Intraoperative highest ETCO2 (mmHg) | 36.0 ± 2.9 | 40.8 ± 4.7 | <0.001 |
| Intraoperative hypertensive events (SBP ≥ 170 mmHg) | 4 (13.8) | 0 (0) | 0.142 |
| Intraoperative hypotension events (SBP ≤ 90 mmHg or DBP ≤ 50 mmHg) | 19 (65.5) | 10 (53.6) | 0.372 |
| Intraoperative antihypertensive drug use | 6 (20.7) | 2 (10.5) | 0.451 |
| Intraoperative inotropic drug use | 3 (10.3) | 3 (15.8) | 0.669 |
Data are expressed as n (%) or as mean ± SD.
DBP = diastolic blood pressure; ETCO2 = end tidal CO2; HR = heart rate; SBP = systolic blood pressure.
| Right | p | Left | p | |||
|---|---|---|---|---|---|---|
| LTA | PRA | LTA | PRA | |||
| Number of patients | 10 | 11 | 19 | 8 | ||
| Operation time (min), mean | 109.2 | 80.5 | 0.009 | 83.2 | 74.8 | 0.380 |
| Body mass index (kg/m2), mean | 23.9 | 22.9 | 0.344 | 23.7 | 24.3 | 0.685 |
| Tumor size (cm), mean | 2.6 | 2.8 | 0.774 | 2.8 | 3.0 | 0.785 |
LTA = lateral transperitoneal adrenalectomy; PRA = posterior retroperitoneal adrenalectomy.
The pain scores for the 24 patients who did not use patient-controlled analgesia are shown in Table 4. The pain score on the day of the operation was similar between the two groups; however, the pain score on the 1st day, 2nd day, and 3rd day postoperation tended to be lower in the PRA group than in the LTA group, although the difference was not statistically significant (3.8 ± 1.4 vs. 3.0 ± 0.8, p = 0.095; 3.2 ± 1.1 vs. 2.5 ± 0.7, p = 0.051; and 2.8 ± 0.8 vs. 2.0 ± 0.6, p = 0.091, respectively). The analgesic requirements of both groups were similar (PRA, 1.0 ± 0.9 units vs. LTA, 1.2 ± 0.7 units; p = 0.492). There was no significant difference in the length of postoperative hospital stay (PRA, 2.7 ± 0.9 days vs. LTA, 2.9 ± 0.7 days, p = 0.558).
| LTA (n = 11) | PRA (n = 13) | p | |
|---|---|---|---|
| VAS on Day 0 | 5.6 ± 1.3 | 6.2 ± 1.9 | 0.365 |
| VAS on Day 1 | 3.8 ± 1.4 | 3.0 ± 0.8 | 0.095 |
| VAS on Day 2 | 3.2 ± 1.1 | 2.5 ± 0.7 | 0.051 |
| VAS on Day 3 | 2.8 ± 0.8 (n = 3) | 2.0 ± 0.6 (n = 7) | 0.091 |
| Postoperative analgesic requirements | 1.0 ± 0.9 | 1.2 ± 0.7 | 0.492 |
Data are expressed as mean ± SD.
LTA = lateral transperitoneal adrenalectomy; PRA = posterior retroperitoneal adrenalectomy.
There was one case each of morbidity and mortality. One patient in the PRA group who had a history of chronic kidney disease suffered acute renal failure for 9 days postoperation, but recovered after conservative treatment. One patient in the PRA group who had a history of coronary vessel disease died from acute myocardial infarction on postoperative Day 8. No patient experienced nerve root pain.
Biochemical markers were tested postoperatively and a biochemical cure was achieved in all patients with a functioning tumor. No patient suffered recurrence.
4. Discussion
Minimally invasive adrenalectomy has been the gold standard treatment for benign adrenal tumors for the past two decades.16 Among the different minimally invasive adrenalectomy techniques, LTA is the most widely practiced.17 This approach offers a familiar anatomical view and a large working space in which to manipulate the intra-abdominal organs. For this reason, LTA was the procedure used by the study surgeon since he began performing adrenalectomies in September 2009. In recent years, however, many institutes have adopted the PRA technique and favorable results have been reported.10; 11; 12; 13; 16; 17 ; 18 The advantages of PRA include direct exposure of the adrenal gland without the need for adjacent organ displacement, and improved visualization and access to the adrenal vein.7 Inspired by the potential advantages of PRA, the study surgeon began performing PRA in January 2012.
For the patient selection, PRA has limitations. Walz et al15 recommended a size limit of 7 cm for tumors associated with aldosteronism and Cushings syndrome, and for other nonfunctioning tumors. In addition, patients with extremely obesity or concomitant intra-abdominal pathologies used to be discouraged for PRA.6 On the contrary, PRA can be performed safely in patients with a history of abdominal surgery because the peritoneum does not need to be breached.16 As studies have reported, the safety of PRA and the surgeons' experience is increasing and the indication has been more liberal.19 Furthermore, PRA using single port or robotic systems has been successfully performed.20; 21 ; 22 In this study, a more conservative size limit of 5 cm was applied to pheochromocytomas because of the risk of an intraoperative hypertensive crisis, even though preoperative α-blockade was used in every patient. PRA was performed safely in all patients who met these size requirements. Because the tumor size limit for LTA patients is more liberal, we excluded patients with tumors outside the criteria set for PRA to allow a more robust comparison of surgical outcomes. During the study period, open adrenalectomy was performed in 20 patients due to tumors being outside the size criteria or due to suspected malignancy.
Open conversion was necessary in two cases. One LTA patient was converted to an open procedure because of uncontrollable bleeding caused by a tear to the left renal vein. One PRA patient, who had a history of total pancreatectomy and subtotal gastrectomy with jejunal transposition due to multiple pancreatic endocrine tumors, required open conversion because of severe adhesions around the adjacent liver and inferior vena cava.
There have been concerns that the high CO2 insufflation required for PRA could stimulate catecholamine secretion and cause hypertension.23 ; 24 We set the insufflation pressure at 25–30 mmHg, which is reported to be safe for PRA.6 The high pressure is mandatory to create enough retroperitoneal space to manipulate the kidney and adrenal gland; however, the inevitable consequence is significant hypercarbia, as evidenced by the higher end tidal CO2 levels in PRA patients (36.0 ± 2.9 mmHg vs. 40.8 ± 4.7 mmHg, p < 0.001). However, there were no complications associated with hypercarbia because it was controlled by appropriate manipulation of the ventilator settings. We also evaluated the incidence of intraoperative hypertensive events to assess whether hypercarbia influenced hemodynamics. A strict definition of an intraoperative hypertensive event (SBP ≥ 170 mmHg) was applied as per the report of Tonaito. 25 Interestingly, intraoperative hypertensive events occurred only in the LTA group. The nonincidence of intraoperative hypertensive events in PRA patients is consistent with previous studies, 9; 26 ; 27 and is partially due to the fact that the surgeon can avoid direct manipulation of the adrenal gland when treating pheochromocytoma. Instead, the adrenal gland is manipulated gently with a cotton ball or the backside of a clamp.
Dickson et al9 attributed the shorter operation time for PRA to the directness of the approach to the adrenal glands, which does not require visceral dissection. In the present study, the mean operation time for the PRA group was shorter than that for the LTA group, but the difference did not reach statistical significance (92.2 ± 27.0 minutes vs. 78.1 ± 18.9 minutes, p = 0.054). However, when we compared the mean operation time of the two approaches according to tumor location, we found that the time for PRA was significantly shorter than that for LTA when performed on the right side (109.2 minutes vs. 80.5 minutes, p = 0.009); however, there was no difference for the left side. The difficulty of performing LTA on the right side may be due to three reasons. First, the right adrenal gland drains directly into the inferior vena cava via the short adrenal vein, an anatomic location that makes right LTA more difficult. Second, mobilization of the liver is more time consuming than that of the spleen and colon. Third, an additional port and assistant are needed for adequate handling of the liver during right adrenalectomy, and this necessitates additional time (for example, to direct the assistant during the operation). The difficulty of performing LTA on the right side was demonstrated by the significantly longer operation time compared with that for left-sided LTA (109.2 minutes vs. 83.2 minutes, p = 0.011).
PRA is associated with less pain than LTA,5; 10 ; 11 possibly due to the shorter operation time and the reduced level of dissection required.5 Furthermore, PRA requires only three trocar sites regardless of tumor location, whereas four trocar sites are needed for a right-sided LTA, leading to increased pain. During the study period, the patients were asked if they wanted to use PCA on the previous day of surgery, and prescribed PCA if they wanted. Therefore, we could not compare pain scores for all of the patients because some patients used PCA and they were excluded in evaluating the VAS. However, the pain score for the PRA group after the day of operation tended to be lower than for the LTA group. Considering previous and present studies, PRA results in less pain for the patient, particularly if there is no injury to the subcostal nerve, which can cause severe neuromuscular pain.6 ; 28
Using operation times as a measure, the learning curves for LTA and PRA are estimated to be 30–50 procedures.29; 30 ; 31 The limited number of cases in the present study precludes the determination of the learning curves for both procedures; however, the mean operation time for PRA was comparatively shorter than that for LTA. This suggests that PRA is a viable alternative for surgeons with experience in laparoscopic adrenalectomy, as has been previously reported.11; 16 ; 32
In the retrospective studies including case–control studies, the patient selection for specific operative methods depended on the surgeons preference, meaning that selection bias was inevitable. Specifically, surgeons may preferentially use the newer technique for easier cases. The current study is also a retrospective study and this is a major drawback. However, we could minimize the surgeon selection bias because patient allocation in each group was made by time rather than surgeons preference. Another limitation of this study is the possibility that previous experience of LTA may have reduced the learning curve required for PRA, thereby resulting in improved outcomes. However, our study showed that PRA is at least as effective and safe as LTA in terms of operation time, blood loss, intraoperative hemodynamics, postoperative pain, and recovery when performed by a surgeon with experience in transperitoneal adrenalectomy. Further randomized controlled studies with larger patient populations are necessary to validate the feasibility and/or superiority of PRA.
5. Conclusion
PRA provides a direct approach to the adrenal gland and avoids manipulation of intra-abdominal organs. If a surgeon has had sufficient experience in LTA, PRA can be performed safely, resulting in improved outcomes for patients with benign adrenal tumors.
References
- 1 M. Gagner, A. Lacroix, E. Bolté; Laparoscopic adrenalectomy in Cushings syndrome and pheochromocytoma; N Engl J Med, 327 (1992), p. 1033
- 2 L.M. Brunt, G.M. Doherty, J.A. Norton, N.J. Soper, M.A. Quasebarth, J.F. Moley; Laparoscopic adrenalectomy compared to open adrenalectomy for benign adrenal neoplasms; J Am Coll Surg, 183 (1996), pp. 1–10
- 3 G. Guazzoni, F. Montorsi, A. Bocciardi, et al.; Transperitoneal laparoscopic versus open adrenalectomy for benign hyperfunctioning adrenal tumors: a comparative study; J Urol, 153 (1995), pp. 1597–1600
- 4 S. Naito, J. Uozumi, H. Ichimiya, et al.; Laparoscopic adrenalectomy: comparison with open adrenalectomy; Eur Urol, 26 (1994), pp. 253–257
- 5 V.A. Constantinides, I. Christakis, P. Touska, K. Meeran, F. Palazzo; Retroperitoneoscopic or laparoscopic adrenalectomy? A single–centre UK experience; Surg Endosc, 27 (2013), pp. 4147–4152
- 6 M.K. Walz, P.F. Alesina, F.A. Wenger, et al.; Posterior retroperitoneoscopic adrenalectomy—results of 560 procedures in 520 patients; Surgery, 140 (2006), pp. 943–948 discussion 948–950
- 7 N.D. Perrier, D.L. Kennamer, R. Bao, et al.; Posterior retroperitoneoscopic adrenalectomy: preferred technique for removal of benign tumors and isolated metastases; Ann Surg, 248 (2008), pp. 666–674
- 8 G.G. Callender, D.L. Kennamer, E.G. Grubbs, J.E. Lee, D.B. Evans, N.D. Perrier; Posterior retroperitoneoscopic adrenalectomy; Adv Surg, 43 (2009), pp. 147–157
- 9 P.V. Dickson, G.C. Alex, E.G. Grubbs, et al.; Posterior retroperitoneoscopic adrenalectomy is a safe and effective alternative to transabdominal laparoscopic adrenalectomy for pheochromocytoma; Surgery, 150 (2011), pp. 452–458
- 10 A. Kiriakopoulos, K.P. Economopoulos, E. Poulios, D. Linos; Impact of posterior retroperitoneoscopic adrenalectomy in a tertiary care center: a paradigm shift; Surg Endosc, 25 (2011), pp. 3584–3589
- 11 C.R. Lee, M.K. Walz, S. Park, et al.; A comparative study of the transperitoneal and posterior retroperitoneal approaches for laparoscopic adrenalectomy for adrenal tumors; Ann Surg Oncol, 19 (2012), pp. 2629–2634
- 12 C.P. Lombardi, M. Raffaelli, C. De Crea, et al.; Endoscopic adrenalectomy: is there an optimal operative approach? Results of a single-center case-control study; Surgery, 144 (2008), pp. 1008–1014 discussion 1014–1015
- 13 V.A. Constantinides, I. Christakis, P. Touska, F.F. Palazzo; Systematic review and meta-analysis of retroperitoneoscopic versus laparoscopic adrenalectomy; Br J Surg, 99 (2012), pp. 1639–1648
- 14 Q.Y. Duh, A.E. Siperstein, O.H. Clark, et al.; Laparoscopic adrenalectomy. Comparison of the lateral and posterior approaches; Arch Surg, 131 (1996), pp. 870–875 discussion 875–876
- 15 M.K. Walz, K. Peitgen, R. Hoermann, R.M. Giebler, K. Mann, F.W. Eigler; Posterior retroperitoneoscopy as a new minimally invasive approach for adrenalectomy: results of 30 adrenalectomies in 27 patients; World J Surg, 20 (1996), pp. 769–774
- 16 P.V. Dickson, C. Jimenez, G.B. Chisholm, et al.; Posterior retroperitoneoscopic adrenalectomy: a contemporary American experience; J Am Coll Surg, 212 (2011), pp. 659–665 discussion 665–667
- 17 E. Berber, G. Tellioglu, A. Harvey, J. Mitchell, M. Milas, A. Siperstein; Comparison of laparoscopic transabdominal lateral versus posterior retroperitoneal adrenalectomy; Surgery, 146 (2009), pp. 621–625 discussion 625–626
- 18 Q.Y. Li, F. Li; Laparoscopic adrenalectomy in pheochromocytoma: retroperitoneal approach versus transperitoneal approach; J Endourol, 24 (2010), pp. 1441–1445
- 19 M.K. Walz, S. Petersenn, J.A. Koch, K. Mann, H.P. Neumann, K.W. Schmid; Endoscopic treatment of large primary adrenal tumours; Br J Surg, 92 (2005), pp. 719–723
- 20 M.K. Walz, H. Groeben, P.F. Alesina; Single-access retroperitoneoscopic adrenalectomy (SARA) versus conventional retroperitoneoscopic adrenalectomy (CORA): a case-control study; World J Surg, 34 (2010), pp. 1386–1390
- 21 M.K. Walz, P.F. Alesina; Single access retroperitoneoscopic adrenalectomy (SARA)— one step beyond in endocrine surgery; Langenbecks Arch Surg, 394 (2009), pp. 447–450
- 22 J.H. Park, S.Y. Kim, C.R. Lee, et al.; Robot-assisted posterior retroperitoneoscopic adrenalectomy using single-port access: technical feasibility and preliminary results; Ann Surg Oncol, 20 (2013), pp. 2741–2745
- 23 M. Flávio Rocha, R. Faramarzi-Roques, P. Tauzin-Fin, V. Vallee, P.R. Leitao de Vasconcelos, P. Ballanger; Laparoscopic surgery for pheochromocytoma; Eur Urol, 45 (2004), pp. 226–232
- 24 R.M. Giebler, M.K. Walz, K. Peitgen, R.U. Scherer; Hemodynamic changes after retroperitoneal CO2 insufflation for posterior retroperitoneoscopic adrenalectomy; Anesth Analg, 82 (1996), pp. 827–831
- 25 A. Toniato, I.M. Boschin, G. Opocher, A. Guolo, M. Pelizzo, F. Mantero; Is the laparoscopic adrenalectomy for pheochromocytoma the best treatment?; Surgery, 141 (2007), pp. 723–727
- 26 F.J. Berends, E.V. Harst, G. Giraudo, et al.; Safe retroperitoneal endoscopic resection of pheochromocytomas; World J Surg, 26 (2002), pp. 527–531
- 27 I. Gockel, G. Vetter, A. Heintz, T. Junginger; Endoscopic adrenalectomy for pheochromocytoma: difference between the transperitoneal and retroperitoneal approaches in terms of the operative course; Surg Endosc, 19 (2005), pp. 1086–1092
- 28 A.E. Siperstein, E. Berber, K.L. Engle, Q.Y. Duh, O.H. Clark; Laparoscopic posterior adrenalectomy: technical considerations; Arch Surg, 135 (2000), pp. 967–971
- 29 M. Guerrieri, R. Campagnacci, A. De Sanctis, M. Baldarelli, M. Coletta, S. Perretta; The learning curve in laparoscopic adrenalectomy; J Endocrinol Invest, 31 (2008), pp. 531–536
- 30 J.M. Schreinemakers, G.J. Kiela, G.D. Valk, M.R. Vriens, I.H. Rinkes; Retroperitoneal endoscopic adrenalectomy is safe and effective; Br J Surg, 97 (2010), pp. 1667–1672
- 31 P. Fiszer, S. Toutounchi, R. Pogorzelski, E. Krajewska, W. Ciesla, M. Skorski; Laparoscopic adrenalectomy—assessing the learning curve; Pol Przegl Chir, 84 (2012), pp. 293–297
- 32 M. Barczynski, A. Konturek, F. Golkowski, et al.; Posterior retroperitoneoscopic adrenalectomy: a comparison between the initial experience in the invention phase and introductory phase of the new surgical technique; World J Surg, 31 (2007), pp. 65–71
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?