Abstract
Protease stimulation in cultured normal human epidermal keratinocytes (NHEK) due to sulfur mustard (SM) exposure is well documented. However, the specific protease(s) stimulated by SM and the protease substrates remain to be determined. In this study, we observed that SM stimulates several proteases and the epidermal-dermal attachment protein laminin-5 is one of the substrates. We propose that following SM exposure of the skin, laminin-5 degradation causes the detachment of the epidermis from the dermis and, therefore, vesication. We utilized gelatin zymography, Western blotting, immuno-fluorescence staining, and real-time polymerase chain reaction (RT-PCR) analyses to study the SM-stimulated proteases and laminin-5 degradation in NHEK. Two major protease bands (64 kDa and 72 kDa) were observed by zymography in SM-exposed cells. Addition of serine protease inhibitor (aprotinin, 100 μM), or the metalloprotease inhibitor (amastatin, 100 μM) to NHEK cultures prior to SM exposure decreased the SM-stimulated protease bands seen by zymography. These inhibitors completely or partially prevented SM-induced laminin-5 γ2 degradation as seen by Western blotting as well as immuno-fluorescence staining. Our results from Western blotting and RT-PCR studies also indicated that the membrane-type matrix metalloproteinase-1 (MT-MM-1) may be involved in SM-induced skin blistering.
To summarize, our results in the NHEK model indicate the following: (a) SM stimulates multiple proteases including serine protease(s), and metalloproteases; (b) SM decreases the level of laminin-5 γ2, which is prevented by either a serine protease inhibitor or a metalloprotease inhibitor and (c) MT-MMP-1 maybe one of the proteases that is involved in skin blistering due to SM exposure.
Keywords
Sulfur mustard ; Serine protease ; Metalloprotease ; Protease inhibiter ; Zymography ; Laminin-5 γ2
1. Introduction
Protease stimulation in epidermal keratinocytes and at the epidermal-dermal junction is one of the mechanisms of SM-induced vesication [1] SM-stimulated proteases cause the separation of the epidermis from the dermis by degrading attachment proteins such as laminin-5 [2] . The use of protease inhibitors is one of the several pharmacological approaches currently under consideration as vesicant medical countermeasure. In this context, the experiments done at the USAMRICD led to two critical observations that (a) in the mini pig skin, which is more akin to the human skin, only one protein in the lamina lucida area i.e., laminin is affected by sulfur mustard [1] , and (b) in human skin explants, laminin-5 immunoreactivity is decreased by SM [1] . A defect in laminin-5 subunit composition, especially β3 and γ2, has been implicated in a human blistering disease at the level of lamina lucida [3] .
The role of proteolysis in SM vesication has been indicated by the results from some experimental studies including those using the skin in the SM-exposed mouse ear model [4] and [5] . Both in vitro and in vivo studies by Cowan et al. [6] indicated that serine protease inhibitors could protect against vesication caused by the blistering agent, Chakrabarti et al. [7] reported from studies using cultured normal human epidermal keratinocytes (NHEK) model that both the amount of membrane bound protease and its proteolytic activity were stimulated following exposure to SM. These SM effects were inhibited by a Ca(2+) chelator, either 2 mM EGTA (ethylene glycol-bis(amino ethyl ether)N ,N ,N ′,N ′ tetraacetic acid) or 50 microM BAPTA-AM (1,2-bis (2-aminophenoxy)ethane-N ,N ,N ′,N ′-tetraacetic acid tetrakis acetoxymethyl ester). A protein purification study by Ray et al. [8] using cultured NHEK model and gel exclusion/hydrophobic chromatography showed that a 70–80 kDa protease was stimulated by SM; this SM-stmulated protease had an amino acid sequence homologous with a mammalian-type bacterial serine endopeptidase. However, there was no direct monitoring and matching of the proteases stimulated by SM. Based on these observations, identifying the protease(s) stimulated by SM in NHEK and further determining their inhibitors may provide important information to evaluate prospective antivesicant drugs.
Zymography analysis of culture medium conditioned by guinea pig tracheal epithelial cells demonstrated that these cells produced 92 kDa gelatinase on exposure to SM [9] . However, Mol et al. reported that the secretion of matrix metalloprotease-9 (MMP-9) in NHEK and skin was decreased following SM exposure. On the contrary, the release of MMP-2 from skin pieces and the release of MMP-3 from cultured NHEK were increased following exposure to moderate concentrations of SM, but suppressed following exposure to higher concentrations of SM [10] . Previously, we purified and partially characterized a single protease that hydrolyzes laminin in vitro [7] . These findings strongly suggest that some specific protease(s) may be responsible for SM-induced vesication involving laminin-5 degradation.
This concept of a specific protease being involved in SM pathology is important because the use of generalized protease inhibitors in preventing SM toxicity may be contraindicated due to a systemic toxicity concern. However, no systematic study has been carried out so far to study and to characterize the types of proteases stimulated by SM and their inhibitors. Here, we utilize gelatin zymography, Western blotting, immunofluorescence staining technique, and RT-PCR to explore and to characterize the SM-stimulated proteases and also laminin-5 degradation in cultured NHEK exposed to SM. The purpose of this study was to establish new technologies and to obtain new knowledge required to identify the specific SM-stimulated protease(s), the logical functions, and the inhibitors.
2. Materials and methods
2.1. Materials
Normal human epidermal keratinocytes (NHEK), human keratinocyte growth supplement (Insulin, BPE, Transferrin, EGF, Hydrocortisone, PSA), and basal media for epithelial cells—Epilife with calcium, kit for splitting cells were purchased from Cascade Biologics (Portland, OR). Sulfur mustard (>98% pure) was from the Edgewood Chemical and Biological Center (ECBC), Aberdeen Proving Ground, Maryland. Protein molecular weight markers, zymogram gel, zymogram renaturing and developing buffers, precast SDS-PAGE gels and buffers were from Invitrogen (Carlsbad, CA). Anti-laminin-5 γ2 polyclonal antibody and horseradish peroxidase-conjugated secondary goat antibody were from Santa Cruz (Santa Cruz, CA). Enhanced chemiluminescence (ECL) detection reagent was from Amersham (Piscataway, CA). Protease inhibitor cocktail, chromozym TRY (serine specific substrate), and aprotinin were from Roche (Basel, Switzerland). Amastatin was purchased from Axxora, LLC (San Diego, CA). Dimethyl sulfoxide (DMSO), coomassie blue R-250, and E-64 were from Sigma (St. Louis, MO). Anti-membrane type matrix metalloptotease-1 antibody (MT-MMP-1), anti-matrix metalloprotease-2 antibody (MMP-2), and anti-matrix metalloprotease-9 (MMP-9) antibody were from Research Diagnostics, Inc. (Flanders, NJ).
2.2. NHEK culture and exposure to SM
NHEK cultures were initiated in basal media from frozen stock (passage 2, P2) using 0.2 × 106 cells per 75 cm2 plastic tissue culture flasks. Cells were grown at 37 °C in a humidified atmosphere of 95% air/5% CO2 according to the method described by Rhoads et al. [11] . When cells became approximately 80% confluent in the flasks, the cells were sub cultured to passage 3 (P3) to be used in experiments. Treatment of cells with 200 μM sulfur mustard was carried out according to the method described by Broomfield and Gross [12]. Cells were exposed to desired SM concentrations using a formulation originally described by Broomfield and Gross [12] and by a method as described by Ray et al. [13] . The stock SM formulation consisted of a frozen binary mixture of 5 μl undiluted SM (oil) and 10 ml aqueous culture medium. The frozen SM Stock was kept on ice until cell exposure. Just prior to cell exposure, the stock was thawed and immediately vortexed hard at room temperature for 1 min to make a SM solution in the medium. Appropriate dilutions were made as quickly as possible for exposure of the cells by adding aliquots of stock SM solution to cells. Cells were exposed to this SM concentration because this was considered to be the in vitro equivalent to an in vivo vesicating concentration of SM. Protease inhibitors were dissolved in DMSO reagent, and then further diluted in NHEK culture medium. Protease inhibitor studies were conducted using about 80% confluent NHEK cultures and the inhibitor was added to cell cultures 30 min prior to cell exposure to 200 μM SM. Each experiment was repeated at least three times or more times to test the statistical significance of the data obtained.
2.3. Zymography
Gelatin zymography was conducted by electrophoresis as described by Heussen and Dowdle [14] . Cell lysates were prepared 16–18 h after SM exposure in Mammalian Protein Extraction Reagent (PIERCE, Rockford, IL). Protein concentration was determined with the BCA Protein Assay Kit (PIERCE, Rockford, IL). Cell lysates were normalized to equal protein concentration. A 50 μg protein equivalent of each sample was lyophilized and mixed with 20 μl Novex Tris-Glycine SDS sample buffer (2×) in the absence of reducing agent, and incubated for 10 min at room temperature. The samples were electrophoresed on zymogram gel (10% polyacrylamide gels co-polymerized with 1 mg/ml gelatin). After electrophoresis, the gels were incubated in zymogram renaturing buffer containing 2.5% Triton-100 with gentle agitation for 30 min at room temperature and then incubated in zymogram developing buffer for 30 min followed by changing to fresh zymogram developing buffer and incubation at 37 °C overnight. After incubation, the gels were stained with 0.5% Coomassie Brilliant Blue R-250 and de-stained with de-staining solution (40% methanol, 10% acetic acid in distilled water). Protease activities were detected as clear bands against a dark blue background of Coomassie-Blue R-250 stained gelatin. For inhibition studies, 100 μM amastatin [15] and [16] , and 100 μM aprotinin [17] and [18] were added to flasks followed by 200 μM SM exposure. The inhibitory activity of each compound was ascertained by comparing the thickness of the gelatinolytic bands in gels developed in the presence or absence of inhibitors.
2.4. Protein elution from gels with Bio-Rad Model 422 electroeluter
Clear protease bands were cut from zymogram gels into 3 mm2 pieces and each piece was separately placed in electroeluter glass tubes. Each tube was filled not past the half-way point. 1 l of the elution buffer (25 mM tris base, 192 mM glycine, 0.1% SDS) was added in the tubes and the elution was carried out at 10 mA/tube for 4–5 h using the Bio-Rad Model 422 electroeluter. After elution, the upper (elution) buffer was removed from each glass tube down to the level of the frit. The adapter and cap were removed and the eluate was carefully recovered by aspiration. The cap was rinsed with 200 μl of fresh elution buffer and the solution was added to the eluate.
2.5. Chromozym TRY assay
Synthetic substrate was used to determine the substrate specificity of the purified enzyme. Protease activity in eluate from electroeluter was assayed according to method by Cowan et al. [19] using 50 μl Chromozym TRY as substrate. The total volume of the reaction mixture was 100 μl in which 50 μl of eluate was used as the enzyme source. The reaction was carried out for 2 h at 37 °C and stopped by incubating on ice for 10 min. Enzyme activity was measured by reading the reaction mixture absorbance at 405 nm. Trypsin standard was used instead of the purified enzyme as positive control. The assay can detect protease at a ng/ml level, for example, 50 μl of 100 μg/ml of trypsin assayed with 50 μl of 2.5 mM Chromozym TRY substrate for 2 h yielded an absorbance of 0.602 ± 0.036 at 405 nm.
2.6. Western blotting analysis
NHEK lysates were normalized to 20 μg protein and mixed with 4× SDS sample buffer and loaded onto 4–12% NuPAGE Bis-Tris gels, and then subjected to electrophoresis at constant voltage of 125 V for about 90 min. After electrophoresis, the proteins were transferred to a polyvinylidence difluoride (PVDF) membrane at a constant voltage of 30 V for about 90 min. Blots were blocked in PBS blocking buffer with 5% non-fat milk overnight at 4 °C, and then incubated with primary antibodies for 1 h at room temperature followed by washing 5 min each three times with washing buffer (0.05% Tween-20 in PBS solution). Then the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody for 1 h followed by washing 5 min each three times with washing buffer (0.05% Tween-20 in PBS solution). The signal detection of Western blotting was performed by using enhanced chemiluminescence (ECL) kit. Image analysis was performed by using Bio-Rad Quantity One software.
2.7. Immunofluorescence staining
Laminin-5 distribution and degradation, and the effect of protease inhibitors on laminin-5 γ2 degradation were determined by immunofluorescence staining using laminin-5 γ2 primary antibody. Briefly, NHEK were cultured in chamber slides for 3–4 days, fixed in 3.7% para-formaldehyde solution, washed with phosphate buffered saline (PBS), and blocked with 5% normal goat serum for 1 h at room temperature, followed by 1 h incubation with a primary antibody. After washing 5 min each 3 times, the Rhodamine-conjugated secondary antibody against goat IgG was added for 1 h at room temperature and then the slides were washed with PBS 3 times for 5 min each. The slides were mounted with mounting solution (SIGMA). The cells were viewed with a Bio-Rad laser confocal system attached to an Olympus microscope.
2.8. Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was prepared from NHEK at the end of the experiments. Briefly, the cells were washed once with ice-cold PBS, collected by trypsinization and stored in RNAlater® (Ambion, Austin, TX) at −20 °C until use. Upon thawing of the frozen cells, total RNA was purified using the RNAqueous® kit (Ambion). First-strand cDNA was synthesized from 5 μg total RNA using the High Capacity cDNA Archive kit® (Applied Biosystems, Foster City, CA). Of each 100 μl cDNA reaction, 9 μl was used in 20 μl reactions for human cDNA-specific TaqMan Gene Expression assays for MT-MMP-1 and β-actin (Applied Biosystems). All assays were carried out in a 96-well format. Real-time fluorescent detection of PCR products was performed using an Applied Biosystems Prism 7500 System using the following thermocycling conditions: 1 cycle of 50 °C for 2 min and 95 °C for 10 min; 50 cycles of 95 °C for 15 s and 60 °C for 1 min β-actin was used as a control for endogenous gene expression. All samples were run in duplicate, and amplification data were analyzed using Applied Biosystem’s Fast System Sequence Detection Software, version 1.3. Relative quantification was calculated according to the ΔΔCt method (Applied Biosystems) using a statistical confidence of 99.9%.
2.9. Statistical analysis
Comparisons to detect significant differences were made using student’s t -test with p < 0.05.
3. Results
3.1. Zymogram analysis for proteases
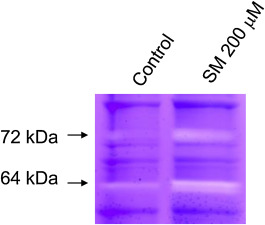
Gelatin zymogram of the untreated (no SM) control NHEK showed that small amounts of proteases, mainly as 72-kDa and 64 kDa. Increased intensities of both 72 kDa and 64 kDa protease bands were observed in SM-exposed NHEK (Fig. 1 ).
|
|
|
Fig. 1. Zymography of untreated (no SM) control NHEK and 200 mM SM-exposed NHEK extracts. Cell extracts were prepared as described under Methods at 16–18 h after SM exposure. Enhanced protease bands at both 72 kDa and 64 kDa protein molecular weight regions on the gel indicated SM-induced protease stimulation in the NHEK model. |
3.2. Prevention of SM-induced protease stimulation by inhibitors of both serine protease and metalloprotease
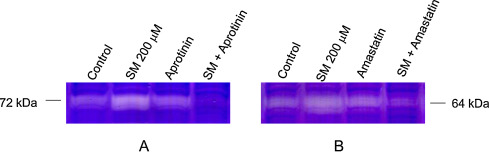
Addition of a serine protease inhibitor, aprotinin (100 μM) to NHEK 30 min prior to 200 μM SM exposure abolished the SM stimulated 72 kDa band (Fig. 2 A). Addition of a metalloprotease inhibitor, amastatin (100 μM) 30 min prior to 200 μM SM exposure decreased the 64 kDa protease bands (Fig. 2 B). However, a cysteine protease inhibitor, N -[N -(l -3-trans -carboxyoxyrane-2-carbonyl)-l -leucyl]-agmatine (E-64, 100 μM), did not decrease 72 kDa or 64 kDa band stimulated by SM (data not shown). These results indicated the roles of both serine protease and metalloprotease in protease stimulation due to SM in NHEK.
|
|
|
Fig. 2. Reduction of SM-induced protease stimulation by pretreatment with protease inhibitors prior to SM exposure in NHEK. Either the serine protease inhibitor, Aprotinin (100 mM; panel A) or the metalloprotease inhibitor, Amastatin (200 mM; panel B) was added to NHEK cultures 30 min prior to 200 mM SM exposure. Zymographic analyses of protease stimulation were conducted using cell extracts prepared at 16–18 h after SM exposure. Both Aprotinin and Amastatin prevented SM-induced protease stimulation at 72 kDa and 64 kDa zones, respectively in gelatin zymograms. |
3.3. Prevention of SM-induced laminin-5 degradation in NHEK by protease inhibitors as shown by Western blotting
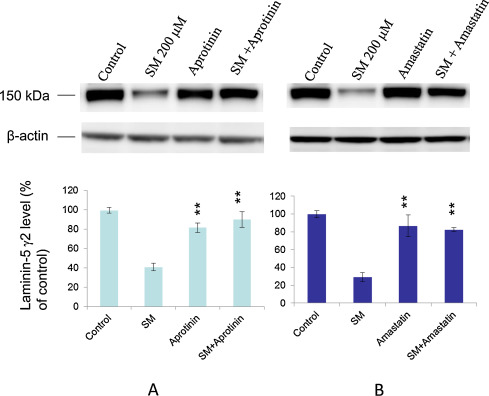
Laminin-5 degradation was measured at 16 h after 200 μM SM exposure in NHEK. Pretreatment of NHEK cultures with either the serine protease inhibitor, aprotinin (100 μM, Fig. 3 A) or the metalloprotease inhibitor, amastatin (100 μM, Fig. 3 B) prevented laminin-5 γ2 degradation. However, the cysteine protease inhibitor, E-64 (100 μM) had no effect (data not shown).
|
|
|
Fig. 3. Prevention of SM-induced laminin-5 γ2 degradation in NHEK by protease inhibitors as shown by Western blotting. Cells were treated with respective protease inhibitors 30 min prior to 200 μM SM exposure. Prevention of laminin-5 g2 degradation was noticed with 100 μM of aprotinin (A) or 100 mM amastatin (B). Both the immune blotting (top panels) and corresponding densitometric measurements (bottom panels) are shown here. Prevention of laminin-5 g2 degradation by aprotinin or amastatin after SM exposure were statistically significant (** means P < 0.05) compared to SM alone. The average laminin-2 γ2 levels (% of control) and error bars are from three repeated separate experiments done at three different times. |
3.4. Prevention of SM-induced laminin-5 degradation in NHEK by protease inhibitors as shown by immunofluorescence staining
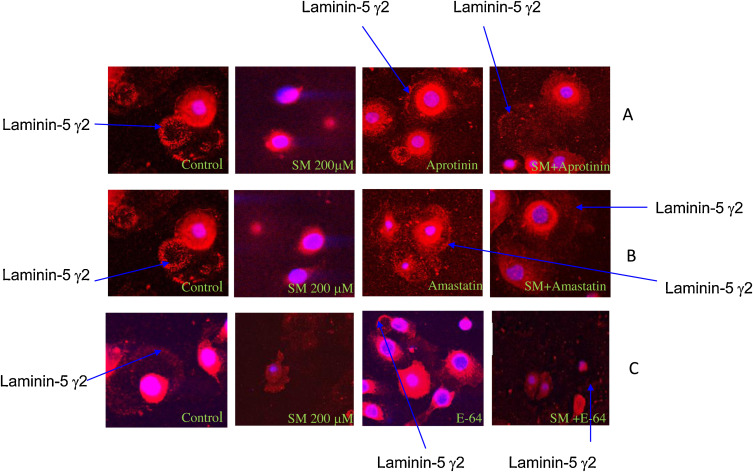
Immunofluorescence staining of NHEK with anti-laminin-5 γ2 antibody showed SM (200 μM)-induced laminin-5 γ2 degradation at 16 h after SM exposure; this was prevented by pretreatment of NHEK cultures with either the serine protease inhibitor, aprotinin (100 μM, Fig. 4 A) or the metalloprotease inhibitor, amastatin (100 μM, Fig. 4 B). However, the cysteine protease inhibitor E-64 (100 μM) had no effect (Fig. 4 C).
|
|
|
Fig. 4. Prevention of laminin-5 γ2 degradation in NHEK by protease inhibitors as shown by immunofluorescence staining. Immunofluorescence staining with anti laminin–5 γ2 antibody revealed that degradation of laminin-5 γ2 seen at 16-h after 200 mM SM exposure was prevented by pretreatment of NHEK cultures 30 min prior to SM exposure with either the serine protease inhibitor, aprotinin (100 μM) (A), or the metalloprotease inhibitor, amstatin (100 μM) (B), the cysteine protease inhibitor E-64 (100 μM) (C). The images were representatives of 10 viewed fields. |
3.5. Analysis of 72 kDa protease band by chromozym TRY
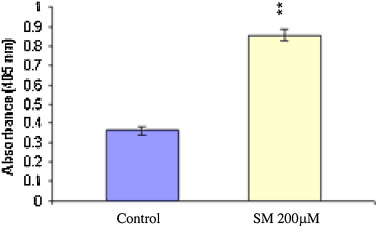
The 72 kDa bands from SM-exposed and unexposed NHEK extracts were cut, electro-eluted and determined for protease activity by the chromozym TRY (substrate for serine proteases) method. It was found that 72 kDa serine protease activity was increased by 2.5 fold in the SM treated group compared to the untreated group (Fig. 5 ). Increased numbers in A405 means that protease activity was increased with SM-exposure in electro-eluted NHEK samples.
|
|
|
Fig. 5. Chromozym TRY assay of protein eluates for SM-exposed and unexposed control NHEK. The 72 kDa bands in gelatin zymograms using SM-exposed and unexposed control cell extracts were cut, electro-eluted and assayed for protease activity by the Chromozym TRY. Protease activity was increased by 2.5 fold at 16 h after SM (200 μM) exposure compared with unexposed control cells. The protease activity after SM exposure was statistically significant (** means P < 0.05) compared to control. |
3.6. Characterization of matrix metalloprotease(s) by Western blotting analysis
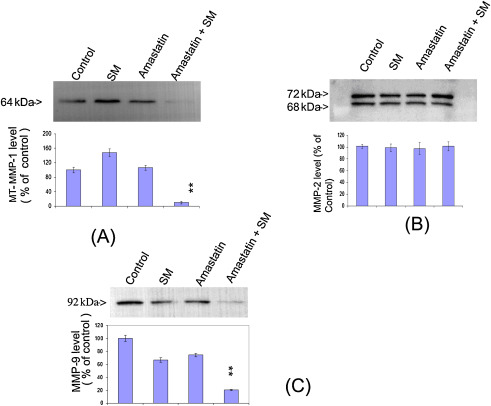
Western blotting technique was used to characterize and analyze matrix metalloproteases. Cell lysates were normalized to 20 μg protein and were subjected to Western blotting analyses. An increase in MT-MMP-1 was detected; however, no change in MMP-2 and a decrease in MMP-9 were observed (Fig. 6 ). MT-MMP-1 appeared as 64 kDa protein band, and matched with our zymogram result. Moreover, this 64 kDa band was blocked by the matrix metalloprotease inhibitor, amastatin further confirming that this was a metalloprotease.
|
|
|
Fig. 6. Detection of MT-MMP-1 after SM exposure in NHEK by Western blotting analysis. Increase of MT-MMP-1 was detected after 200 mM SM exposure compared to unexposed control NHEK, and MT-MMP-1 band was inhibited by adding 100 μM amastatin (metalloprotease protease inhibitor) 30 min prior to SM exposure (A). There was no change in MMP-2 level (B), but a decrease in MMP-9 level (C) at 16 h after SM exposure in NHEK. The changes in MT-MMP-1 and MMP-9 after SM exposure with amastatin pretreatment were statistically significant (** means P < 0.05) compared to either SM or amastatin alone. The average of each protease level (% of control) and error bars were from three or more separate experiments. |
3.7. MT-MMP-1 mRNA expression
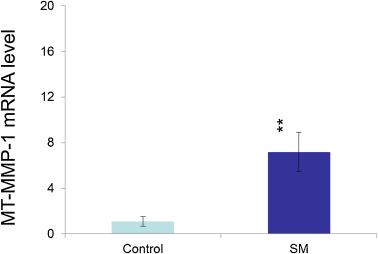
To determine whether the change in the expression level of MT-MMP-1 shown in Fig. 6 A was due to a change in the MT-MMP-1 mRNA level, MT-MMP-1 mRNA was measured by real-time RT-PCR in NHEK at 16 h after SM exposure. MT-MMP-1 mRNA expression in SM-exposed NHEK was increased by about seven-fold over the unexposed control cells (Fig. 7 ). These data suggested that the increase in MT-MMT-1 protein expression with SM-exposure in NHEK was mainly due to transcriptional regulation.
|
|
|
Fig. 7. RT-PCR analysis of MT-MMP-1 mRNA level. MT-MMP-1 mRNA expression increased by about seven-fold in SM (200 μM)-exposed NHEK over that in unexposed control cells at 16 h after SM exposure. These data suggested that the increase in MT-MMP-1 protein expression with SM-exposure in NHEK was mainly due to transcriptional regulation. The change in MT-MMP-1 after SM exposure was statistically significant (** means P < 0.05) compared to untreated control. The average MT-MMP-1 mRNA levels (% of control) and standard error bars are from determinations using 8 separate samples. |
4. Discussion
Enhanced protease activity following SM exposure in NHEK demonstrated that the alkylating agent, SM can cause protease stimulation in cells. Cultured NHEK have been used as an in vitro experimental model of cell death and vesication due to SM [20] . In the present study, zymographic analyses showed that at least two different types of proteases were elevated in lysates of cells treated with SM and these increases were blocked by protease inhibitors such as a metalloprotease inhibitor, amastatin and a serine protease inhibitor, aprotinin. Western blotting showed that SM (200 μM)-exposed NHEK had a decreased level of laminin-5 content and this decrease was prevented by pretreatment of NHEK cultures with a protease inhibitor, amastatin or aprotinin 30 min prior to SM exposure. Immunofluorescence staining indicated that the degradation of laminin-5 γ2 due to SM exposure in NHEK was also blocked by protease inhibitor treatment 30 min prior to SM exposure. These results implied that proteases like serine protease(s) and metalloprotease(s) are induced or activated by SM exposure in NHEK. These proteases may cause the degradation of laminin-5 in SM-exposed NHEK and consequently epidermal-dermal separation and, therefore, vesication.
Previous reports in the literature had shown SM-induced stimulation of protease in cultured NHEK and had proposed its role in skin blistering due to SM, but which type of protease is involved in blistering remained controversial. In contrast to the previous studies [9] and [21] , the findings from the current study suggested that serine protease(s) alone or metalloprotease(s) alone might not be able to completely degrade the epidermal-dermal matrix components, thereby leading to skin blistering. Our results indicated that both types of proteases, serine protease and metalloprotease, may play a role in blistering due to SM. This contributes to the hypothesis of the role of proteolysis in SM vesication, and thus extends the physiological implications for an antivesicant drug development approach.
It was proposed that serine protease inhibiters could delay and protect skin from vesication caused by SM [22] . Non-reducing 4–12% SDS gel electrophoresis of cell extract from SM-exposed NHEK revealed a mustard-stimulated protein band at about 70–80 kDa that was associated with protease activity; this was inhibited by adding EGTA, BAPTA, DFP or cycloheximide in NHEK culture prior to SM exposure [23] . These results implicated that the SM-stimulated protease could be a calcium-dependent serine protease. Another group reported a complete inhibition of SM increased proteolysis in NHEK by using protease inhibitors such as antipain, leupeptin, and 4-(2-aminoethyl)-benzenesulfonylfonyl fluoride) [24] . These data also suggested that serine protease was induced by SM treatment. In the present study, we used multiple techniques like zymography, Western blotting, chromozym TRY assay and immuno-fluorescence staining to confirm that serine protease is one of the major SM-induced proteases that may be responsible for vescication. In our chromozym TRY substrate assay, the increase in serine protease activity due to SM was 4 fold higher compared to untreated control cell. The chromozym TRY assay can identify substrate specificity, e.g., serine-like proteolysis, and can be used to indicate the general class of proteases. However, this assay cannot identify a specific protease unless more selective reagent is used to neutralize protease activity.
Papirmeister et al. [25] proposed a hypothesis of the biochemical mechanism of skin blistering due to SM via protease stimulation. This hypothesis was as follows. SM being a strong bifunctional alkylating compound induces DNA double strand breaks in cells exposed to SM. This DNA damage causes the activation of the DNA repair enzyme poly (ADP ribose) polymerase (PARP), which utilizes NAD+ (nicotinamide adenine dinucleotide) as its substrate and as such cellular NAD is depleted. Since NAD is also required as a cofactor for cellular energy metabolism, its depletion causes hexose monophosphate shunt metabolic pathway stimulation and consequently protease activation and release. Another possible mechanism of SM-induced protease stimulation has been proposed to be related to an increase in intracellular free calcium ion concentration due to SM [13] , which may activate calcium-dependent enzymes like phospholipases and/or proteases [26] . The major target of SM in the skin are the replicating basal epidermal keratinocytes, which remain attached to the sub epithelial layer (dermis) via some attachment proteins e.g., laminin-5, integrin etc. Protease activation and release at this epidermal-dermal junction degrades these attachment proteins and as a consequence the epithelial layer detaches from the substratum and eventually micro blister formation [27] . The above discussion suggests that SM-induced protease activation may contribute to the pathology of blister formation; therefore, inhibitors of SM-stimulated proteases may serve as prospective therapeutics for intervention for sulfur mustard toxicity.
Matrix metalloproteases (MMPs) are a family of zinc-dependent enzymes that are primarily responsible for the degradation of extracellular matrix proteins [28] and [29] . These enzymes are secreted as zymogens and cleaved to their active forms by other enzymes in the extracellular space. Gelatinolytic activity of MMP-9 was up-regulated by cytokine (for example, TNF-α) in both soluble and immobilized forms. However, MMP-2 was secreted in pro-active form and only activated by interaction with membrane-type MT-MMPs associated to the cell surface [30] , [31] and [32] . In previous reports on MMPs only a few were implicated in laminin-5 processing. Exogenous addition of matrix metalloprotease 2 (MMP-2) to breast epithelial cells, cleaved the γ2 subunit of rat laminin-5 [33] . A subsequent study suggested that membrane type 1 matrix metalloprotease (MT1-MMP) may play a role in cleaving laminin-5 [31] . One report indicated that matrix metalloprotease-2 and -9 are involved in blistering induced by SM or nitrogen mustard [9] . They also found that there was a greater increase in MMP-9 (92 kDa) than in MMP-2 (72 kDa) induced by SM intoxication [9] . In the present study, the western blot results indicated that among the matalloproteases, the membrane type 1 MMP (MT-MMP-1, 64 kDa) was most likely involved in the vesication processes. MT1-MMP has been described as a major activator of MMP-2 [34] , but MT1-MMP also possesses the ability to degrade extracellular component, including gelatin, fibronectin, collagen, and laminin-5 [35] and [36] . From Western blotting it appeared that MT-MMP-1 was increased and MMP-9 was decreased after SM exposure (Fig. 6 ). MT-MMP-1 and MMP-9 were almost eliminated by pretreatment of the metalloprotease inhibitor, amastatin 30 min prior to SM exposure; this indicated that the MT-MMP-1 and MMP-9 were sensitive proteases for the amastatin’s inhibitory effect. The reason for this drastic reduction of MT-MMP-1 and MMP-9 in cells pretreated with amastatin and then exposed to SM remains to be investigated. These results were consistent with our findings by zymography that the 64 kDa protease was increased and the increase was blocked by metalloprotease inhibitor, amastatin. These results supported the idea that MT- MMP-1 could be one important enzyme induced or activated by SM exposure. It is also known that some serine proteases can release matrix metalloprotease, MMP-2 from human vascular smooth muscle cells and can stimulate the conversion of pro-MMP-2 to MMP-2 [32] . The relationship between serine protease and MMP-2 remains to be clarified.
The basement membrane between the epidermis and the dermis contains unique
structural proteins that maintain the attachment of the two layers one of the key components of the anchoring complex is laminin-5 [3] . It has been postulated that (a) laminin-5 directly binds to type VII collagen, which forms the anchoring fibrils that insert into the papillary dermis [37] ; and (b) laminin-5 forms a covalent complex with laminin-6 or -7 and this laminin 5–6/7 complex interacts with type IV collagen in the basement membrane [38] . It has also been reported that laminin-5 cleavage by MMPs may be a wide spread mechanism that triggers migration of cells contacting basement membranes [39] . Our study demonstrated that laminin-5 was degraded after SM exposure and the degradation of laminin-5 was reduced by metalloprotease inhibitor or serine protease inhibitor. An intact basement membrane at the dermal-epidermal junction is essential to the viability of the skin and in the process of communications between the dermis and the epidermis [39] .
In conclusion, based on the findings presented here from our experiments using the in vitro NHEK model of SM toxicity, we propose a working hypothesis of the SM-induced skin vesication as follows. SM exposure may stimulate multiple proteases to include serine protease and metalloprotease in the basal epidermal keratinocytes (NHEK). These proteases are released at the basement membrane zone (BMZ) i.e., the epidermal-dermal junction and degrade the epidermal-dermal attachment proteins e.g., laminin-5, which is one of the anchoring filaments responsible for maintaining the BMZ integrity. As a result, the epidermal layer gets detached from the dermis causing a separation of the epidermis from the dermis; this triggers a hydrodynamic effect at the site of the separation and, thus, the formation of a fluid filled blister or vesication. The fact that protease inhibitors like aprotinin or amastatin attenuate protease stimulation and laminin-5 degradation suggest their prospect for application as a medical countermeasure for SM vesication. However, since these concepts are derived from an in vitro model, these need to be validated in vivo in an appropriate animal skin vesication model such as the hairless guinea pig skin and/or the mouse ear.
Transparency document
Transparency Document.
Acknowledgments
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. We acknowledge the expert technical assistance of Ms. Betty J. Benton of the USAMRICD, APG, MD for exposure of cells to SM.
References
- [1] J.P. Petrali, S. Oglesby-Megee; Toxicity of mustard gas skin lesions; Microsc. Res. Tech., 37 (1997), pp. 221–228
- [2] K.J. Smith, W.J. Smith, T. Hamilton, H.G. Skelton, J.S. Graham, C. Okerberg, R. Moeller, B.E. Hackley Jr.; Histopathologic and immunohistochemical features in human skin after exposure to nitrogen and sulfur mustard; Am. J. Dermatopathol., 20 (1998), pp. 22–28
- [3] C. Matsui, C.K. Wang, C.F. Nelson, E.A. Bauer, W.K. Hoeffler; The assembly of laminin-5 subunits; J. Biol. Chem., 270 (1995), pp. 23496–23503
- [4] R.P. Casillas, K.J. Smith, R.B. Lee, L.R. Castrejon, F.W. Stemler; Effect of topically applied drugs against HD-induced cutaneous injury in the mouse ear edema model; Paper Presented at the Proceedings of the 1996 Medical Defense Bioscience Review. U. S. Army Medical Research Institute of Chemical Defense. AD A321841.2.801 (1996)
- [5] F.M. Cowan, C.A. Broomfield; Putative roles of inflammation in the dermatopathology of sulfur mustard; Cell Biol. Toxicol., 9 (1993), pp. 201–213
- [6] F.M. Cowan, C.A. Broomfield, D.E. Lenz, W.J. Smith; Putative role of proteases and other inflammatory-neuronal molecules in the toxicity of nerve and blister chemical warfare agents; Proceedings of the 2000 Medical Defense Bioscience Review. US Army Medical Research Institute of Chemical Defense: APG, Maryland (2000)
- [7] A.K. Chakrabarti, P. Ray, C.A. Broomfield, R. Ray; Purification and characterization of protease activated by sulfur mustard in normal human epidermal keratinocytes; Biochem. Pharmacol., 56 (1998), pp. 467–472
- [8] P. Ray, A.K. Chakrabarti, C.A. Broomfield, R. Ray; Sulfur mustard-stimulated protease: a target for antivesicant drugs?; J. Appl. Toxicol., 22 (2) (2002), pp. 139–140
- [9] J.H. Calvet, E. Planus, P. Rouet, S. Pezet, M. Levame, C. Lafuma, A. Harf, M.P. D'Ortho; Matrix metalloproteinase gelatinases in sulfur mustard-induced acute airway injury in guinea pigs; Am. J. Physiol., 276 (1999), pp. L754–L762
- [10] M.A. Mol, R.M. van den Berg, H.P. Benschop; Involvement of caspases and transmembrane metalloproteases in sulphur mustard-induced microvesication in adult human skin in organ culture: directions for therapy; Toxicology, 258 (1) (2009), pp. 39–46
- [11] L.S. Rhoads, J.R. Cook, L.M. Patrone, R.G. Van Buskirk; A human epidermal model that can be assayed employing a multiple fluorescent endpoint assay and the CytoFluor 2300; J. Toxicol. Cutan. Ocul. Toxicol., 12 (1993), pp. 87–108
- [12] C.A. Broomfield, C.L. Gross; Stability of sulfur mustard in a two-phase storage configuration; Proceedings of the 1989 Medical Defense Bioscience Review, Aberdeen Proving Ground. United States Army Medical Research Institute of Chemical Defense, AD B139550, 1989 (1989), pp. 433–436
- [13] R. Ray, R.H. Legere, B.J. Majerus, J.P. Petrali; Sulfur mustard-induced increase in intracellular free calcium level and arachidonic acid release from cell membrane; Toxicol. Appl. Pharmacol., 131 (1995), pp. 44–52
- [14] C. Heussen, E.B. Dowdle; Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates; Anal. Biochem., 102 (1980), pp. 196–202
- [15] J.R. Baker, T.A. Kylstra, T.D. Bigby; Effects of metalloproteinase inhibitors on leukotriene A4 hydrolase in human airway epithelial cells?; Biochem. Pharmacol., 50 (7) (1995), pp. 905–912
- [16] M. Sajid, R.E. Isaac, I.D. Harrow; Purification and properties of a membrane aminopeptidase from Ascaris suum muscle that degrades neuropeptides AF1 and AF2 ; Mol. Biochem. Parasitol., 89 (2) (1997), pp. 225–234
- [17] Anisuzzaman, M.K. Islam, M.A. Alim, T. Miyoshi, T. Hatta, K. Yamaji, Y. Matsumoto, K. Fujisaki, N. Tsuji; Longistatin is an unconventional serine protease and induces protective immunity against tick infestation?; Mol. Biochem. Parasitol., 182 (1–2) (2012), pp. 45–53
- [18] J. Hirata, L.P. Chung, F. Ariese, H. Irth, C. Gooijer; Coupling of size-exclusion chromatography to a continuous assay for subtilisin using a fluorescence resonance energy transfer peptide substrate: testing of two standard inhibitors?; J. Chromatogr. A, 1081 (2) (2005), pp. 140–144
- [19] F.M. Cowan, C.A. Broomfield, W.J. Smith; Effect of sulfur mustard exposure on protease activity in human peripheral blood lymphocytes; Cell Biol. Toxicol., 7 (1991), pp. 239–348
- [20] R. Ray, S. Hauck, R. Kramer, B. Benton; A convenient fluorometric method to study sulfur mustard-induced apoptosis in human epidermal keratinocytes monolayer microplate culture; Drug Chem. Toxicol., 1 (2005), pp. 105–116
- [21] F.M. Cowan, C.A. Broomfield, D.E. Lenz, W.J. Smith; Putative role of proteolysis and inflammatory response in the toxicity of nerve and blister chemical warfare agents: implications for multi-threat medical countermeasures; J. Appl. Toxicol., 23 (2003), pp. 177–186
- [22] W.J. Smith, F.M. Cowan, C.A. Broomfield; Increased proteolytic activity in human epithelial cells following exposure to sulfur mustard; FASEB J., 5 (1991), p. 528
- [23] P. Ray, S.T. Ali; Protease in normal human epidermal keratinocytes; Drug Chem. Toxicol., 21 (1998), pp. 319–327
- [24] F.M. Cowan, C.A. Broomfield, W.J. Smith; Inhibition of sulfur mustard-increased protease activity by niacinamide, N -acetyl-l -cysteine or dexamethasone ; Cell Biol. Toxicol., 8 (1992), pp. 129–138
- [25] B. Papirmeister, C.L. Gross, H.L. Meier, J.P. Petrali, J.B. Johnson; Molecular basis for mustard-induced vesication; Fundam. Appl. Toxicol., 5 (1985), pp. S134–149
- [26] G.E. Kass, S. Orrenius; Calcium signaling and cytotoxicity; Environ. Health Perspect., 107 (Suppl. 1) (1999), pp. 25–35
- [27] X. Jin, R. Ray, Y. Leng, P. Ray; Molecular determination of laminin-a biomarker for mustard gas exposure its mechanism of action?; Exp. Dermatol., 17 (1) (2008), pp. 49–56
- [28] A.M. Romanic, J.A. Madri; Extracellular matrix-degrading proteinases in the nervous system; Brain Pathol., 4 (1994), pp. 145–156
- [29] E.J. Goetzl, M.J. Banda, D. Leppert; Matrix metalloproteinase in immunity; J. Immunol., 156 (1996), pp. 1–4
- [30] G.A. Limb, J.T. Daniels, R. Pleass, D.G. Charteris, P.J. Luthert, P.T. Khaw; Differential expression of matrix metalloproeinases 2 and 9 by glial muller cells. Response to soluble and extracellular matrix-bound tumor necrosis factor-α; Am. J. Pathol., 160 (2002), pp. 1847–1855
- [31] N. Koshikawa, G. Giannelli, V. Cirulli, K. Miyazaki, V. Quaranta; Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5; J. Cell Biol., 148 (2000), pp. 615–624
- [32] N.H. Rauch, E. Bretschneider, M. Braun, K. Schror; Factor X a releases matrix metalloproteinase-2 (MMP-2) from human vascular smooth muscle cells and stimulates the conversion of pro-MMP-2 to MMP-2; Circ. Res., 90 (2002), pp. 1122–1127
- [33] G. Giannelli, J. Falk-Marzillier, O. Schiraldi, W.G. Stetler-Stevenson, V. Quaranta; Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5; Science, 277 (1997), pp. 225–228
- [34] H. Sato, M. Seiki; Membrane-type metalloproteinases (MT-MMPs) in tumor metastasis; J. Biochem., 119 (1996), pp. 209–215
- [35] E. Ohuchi, K. Imai, Y. Fuji, H. Sato, M. Seiki, Y. Okada; Membrane type I matrix metalloprotease digests interstitial collagens and other extracellular matrix macromolecules; J. Biol. Chem., 272 (1997), pp. 2446–2451
- [36] C. Gilles, M. Polette, C. Coraux, J.M. Tournier, G. Meneguzzi, C. Munaut, L. Volders, P. Rousselle, P. Birembaut, J.M. Foidart; Contribution of MT1-MMP and of human laminin-5 γ2 chain degradation to mammary epithelial cell migration; J. Cell Sci., 114 (2001), pp. 2967–2976
- [37] P. Rousselle, D.R. Keene, F. Ruggiero, M.F. Champliaud, M. Rest, R.E. Burgeson; Laminin 5 binds the NC-1 domain of type VII collagen; J. Cell Biol., 138 (1997), pp. 719–728
- [38] M.F. Champliaud, G.P. Lunstrun, P. Rousselle, T. Nishiyama, D.R. Keene, R.E. Burgeson; Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment; J. Cell Biol., 132 (1996), pp. 1189–1198
- [39] T. Nishiyama, S. Amano, M. Tsunenaga, K. Kadoya, A. Takeda, E. Adachi, R.E. Bergeson; The impoetance of laminin 5 in the dermal-epidermal basement membrane; J. Dermatol. Sci., 25 (2000), pp. S51–S59
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?