Summary
Controversy related to endoscopic or surgical management of pain in patients with chronic pancreatitis remains. Despite improvement in endoscopic treatments, surgery remains the best option for pain management in these patients.
Keywords
chronic pancreatitis;endoscopic;meta-analysis;surgery
1. Introduction
Chronic pancreatitis (CP) is a major cause of morbidity, accounting for 7400 hospital admissions in the United Kingdom during the period 2011/2012. The mainstay of treatment is to avoid precipitating causes, such as alcohol. Pain is the most debilitating symptom and the symptom most resistant to treatment, with patients often becoming reliant on long-term analgesia leading to drug dependency.
The etiology of pain in CP is not completely understood, though it has been studied extensively. Currently its origin is thought to be due to increased pressure within the pancreatic duct (PD) which leads to intraductal and interstitial hypertension. An inflammatory response occurs in the pancreatic tissue mediated by the neuron-specific proteinase-activated receptor 2 (PAR-2), substance P, neurokinin A, and calcitonin-gene related peptide (CGRP), or a combination.1 The resulting fibrosis further contributes to pain as the pancreas parenchyma loses its ability to distend during its excretory function.2 This is exacerbated by the presence of strictures and stones which affect the ability of the gland to drain. Other factors affecting pain have also been advocated. Centrally mediated pancreas-independent pain is a possible contributor and this is due to chronic visceral input leading to plastic changes in the brain, which in turn cause a self-perpetuating and sustained pain.3 ; 4
Treatment has traditionally been medical with analgesics and enzyme supplementation, while surgical management has been reserved for patients with intractable pain or complications.5 The aim of surgical procedures is to decompress the and/or resect the nidus of inflammation. Surgical denervation strategies are ineffective and not appropriate as a first-line treatment.1; 6 ; 7 Recently, combination procedures of drainage and local resection have been increasingly used as an alternative to major resection, with good success in symptom alleviation and low morbidity and mortality.8
Endoscopy has had an increasing role in the treatment of CP. The development of new techniques (PD stenting, stone removal and sphincterotomy) in combination with better patient selection has led to an increasing number of patients being managed without surgery.9; 10 ; 11 However, there are only a few, small studies available that evaluate the outcomes of these interventions. While current studies suggest that surgery is better than endoscopy in the short term, it is not clear whether these benefits are sustained. Endoscopic treatments are becoming more complex, for example, the use of lithotripsy which has not been evaluated in earlier studies. The aim of this study is to provide an update on evidence evaluating the outcomes of surgery versus endoscopy for pain control in CP.
2. Methods
2.1. Study selection
A systematic literature search of the published studies comparing the outcomes of endoscopic or surgical treatment for CP was performed using a PubMed search covering the MEDLINE, EMBASE, Ovid, and Cochrane databases. Publications from January 1994 to October 2014 were reviewed. The following medical subject headings were used for the search: chronic pancreatitis, surgery, endoscopic, stent, resection, and drainage. These terms, and their combinations, were also searched as text words. The “related articles” function was used to broaden the search, and all abstracts, studies, and citations retrieved were reviewed. Relevant references of the articles acquired were also included.
2.2. Inclusion and exclusion criteria
Studies were independently assessed by two authors; conflicts were resolved by a third assessor until consensus was reached. Included studies were: (1) human; (2) reported the indication for surgery; and (3) were written in English.
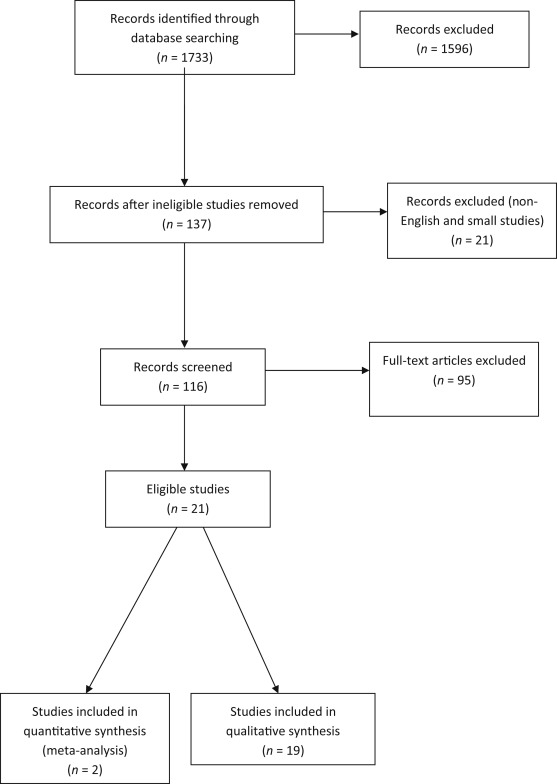
Excluded studies had failed to: (1) describe their methods for treatment options; (2) clearly report the outcomes and variables of interest; (3) data extraction was impossible or not quantifiable; or (4) there was overlap between authors and centres. Figure 1 shows the flow chart of publication selection.
|
|
|
Figure 1. Study selection flow-chart. |
2.3. Variables and outcomes of interest
These include indication for surgery for CP, surgical methods, result of treatment measured by pain control, postoperative morbidity and mortality, and recurrent symptoms. The main focus during the selection process was the presence of data on pain relief and adequate follow up.
2.4. Statistical analysis
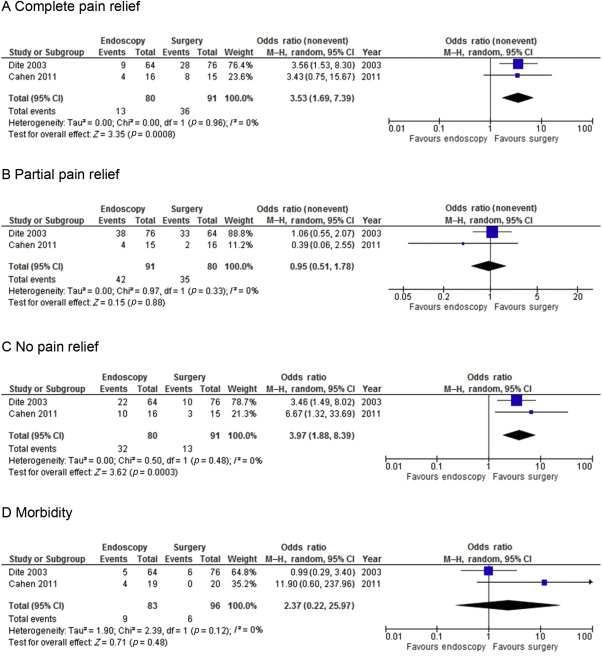
The meta-analysis was performed in line with recommendations from the Cochrane Collaboration.12 Statistical analysis of dichotomous variables was performed using the odds ratio (OR) as the summary statistic. Postprocedural morbidity and mortality after surgery and endoscopy were analyzed, and the results were compared. Both were reported with 95% confidence intervals (CIs). An OR of <1 favored the particular type of procedure. The point estimate of the OR was considered statistically significant at p < 0.05 if the 95% CI did not include 1. In the tabulation of the results ( Figure 2), squares indicate the point estimates of the treatment effect (OR or weighted mean difference), with 95% CIs indicated by horizontal bars. The diamond represents the summary estimate from the pooled studies with 95% CIs. The Mantel–Haenszel method was used to combine the OR for the outcomes of interest using a “random-effect” meta-analytical technique. This is a more conservative method than a fixed effects model and takes into account clinical heterogeneity. Statistical heterogeneity, by inspection of the I2 statistic, reveals no observed heterogeneity across the studies when the value is close to 0%. Heterogeneity was also assessed by graphical exploration with funnel plots to evaluate publication bias. Statistical analysis was performed using Review Manager for Windows, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, England, UK) and SPSS for Windows, version 15.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were analyzed using the Fischers exact test.
|
|
|
Figure 2. Meta-analysis of two RCTs. (A) Complete pain relief. (B) Partial pain relief. (C) No pain relief. (D) Morbidity. M–H = Mantel–Haenszel method. |
3. Results
3.1. Description of studies
The systematic literature search initially identified 1733 potential articles. After irrelevant citations were excluded the remaining 137 articles were retrieved in full and reviewed for further assessment (Figure 1). The titles and abstracts were first reviewed eliminating non-English ones (7 articles), as well as case reports and small volume trials or series, as defined by patient numbers of <15 (14 articles). A further 95 articles were excluded because of insufficient data on pain relief, including those with follow up of <6 months.
Only three of the 21 studies eventually selected were randomized control trials (RCTs).13; 14 ; 15 One of these was a report of longer-term data from the same group.15 The latest paper from this group was included in the meta-analysis to avoid duplication of results.
A total of 2754 patients were included in the 21 studies finally selected (Table 1). Of these, 1602 underwent surgery, including resection, drainage, and combination procedures (i.e., Whipples resection, distal resection, Freys procedure). The remaining 1152 patients were treated endoscopically.
| Study | Patient age (y), mean | Patients treated (n = 2754) | |
|---|---|---|---|
| Surgery (n = 1602) | Endoscopy (n = 1152) | ||
| Dite 200313 | 41.7 | 76 | 64 |
| Cahen 201115 | 41.7 | 20 | 19 |
| Adams 199416 | 43.6 | 62 | 0 |
| Strate 200517 | N/A | 51 | 0 |
| Berney 200018 | 44 | 67 | 0 |
| Dumonceau 200719 | 49 | 0 | 24 |
| Farkas 200320 | 44 | 30 | 0 |
| Frey 199421 | 47 | 47 | 0 |
| Izbicki 199822 | 43.8 | 61 | 0 |
| Mobius 200723 | N/A | 51 | 0 |
| Nealon 200124 | 43 | 185 | 0 |
| Rosch 200225 | 50 | 0 | 1018 |
| Sakorafas 200126 | 47 | 31 | 0 |
| Schlosser 200227 | 44.7 | 219 | 0 |
| Schlosser 200528 | 45.8 | 55 | 0 |
| Schnelldorfer 200729 | 46 | 368 | 0 |
| Stapleton 199630 | 42.2 | 52 | 0 |
| Lucas 199931 | 34 | 124 | 0 |
| Byrne 199732 | 47 | 51 | 0 |
| Vasile 201333 | 51 | 17 | 0 |
| Hong 201134 | 52 | 35 | 27 |
N/A = not available.
Analysis of the two RCTs strongly supports the superiority of surgical treatment when complete pain relief is the outcome under consideration. Figure 2 depicts the forest-plot of patients who had complete resolution of symptoms (OR, 3.53; p = 0.0008). When partial pain relief is considered, there was no statistical significance between surgery and endoscopy (OR, 0.95; p = 0.88). However, when comparing the outcome of no pain relief, there is again a significant difference in favor of surgery (OR, 3.97; p = 0.0003). Similarly, there were no major differences in morbidity (p = 0.48) or mortality (p = 0.47) between the two groups. One death was observed in Cahens original study 14 in the endoscopic group, attributed to a perforated duodenal ulcer 4 days post extracorporeal shockwave lithotripsy (ESWL). During their 5-year follow-up period a further six patients died of unrelated causes, two in the endoscopy group and four in the surgical group. These were excluded from the meta-analysis for pain outcomes due to lack of data for these patients. There were a further four morbidities in the endoscopy group, two ruptured PDs and two pancreatic stent occlusions. Table 2 summarizes the complications in the two RCTs.
| Complications | Dite et al13, 2003 | Cahen et al15, 2011 | ||
|---|---|---|---|---|
| Surgery | Endoscopy | Surgery | Endoscopy | |
| Morbidity | 6 | 5 | 7 (4) | 18 (6) |
| Acute pancreatitis | 2 | 2 | 0 | 4 |
| Bleeding | 0 | 2 | 2 | 0 |
| Cholecystitis | 0 | 0 | 0 | 1 |
| Pancreatic abscess | 0 | 1 | 0 | 0 |
| Fistula | 2 | 0 | 0 | 0 |
| Anastomotic leak | 1 | 0 | 1 | n/a |
| Ileus | 1 | 0 | 0 | n/a |
| Wound infection | 0 | 0 | 3 | 1 |
| Cardiopulmonary | 0 | 0 | 1 | 0 |
| Stent related | 0 | 0 | n/a | 5 (2) |
| Ruptured pancreatic duct | 0 | 0 | 0 | (2) |
| Other minor complications | 0 | 0 | 0 | 7 |
| Mortality | 0 | 0 | 0 (4) | 1 (2) |
Numbers in brackets show additional complications at 5-year follow up.
n/a = not available.
The remaining 19 articles reviewed were systematically analyzed16; 17; 18; 19; 20; 21; 22; 23; 24; 25; 26; 27; 28; 29; 30; 31; 32; 33 ; 34 Specifically, surgical treatment was effective in alleviating pain symptoms completely in 58.4% of patients, partially effective in 22.0%, and completely failed in 19.4% of cases. Endoscopic treatment was effective in 64.0%, partially effective in 8.5%, and failed in 27.5%. The ability for a treatment to give any pain relief was significantly better after surgery (80.4%) compared with endoscopy (72.6%; p < 0.0001).
Morbidity was higher in the surgical group (12.7%) compared with the endoscopy group (10.1%). Mortality was also higher at 0.6% (n = 13), with no deaths occurring following endoscopy.
4. Discussion
The progressive nature of CP leads to at least 50% of patients developing intractable symptoms (i.e., endocrine and/or exocrine insufficiency and pain). During its natural course, traditional medical treatment is usually the first to fail in controlling the symptoms leaving endoscopic and surgical management as the only alternatives.35 ; 36
Endoscopy for CP usually involves a combination of pancreatic sphincterotomy, ESWL, and PD stenting. This, however, involves multiple procedures due to stent occlusion or displacement and carries a fourfold increased risk of associated acute pancreatitis. Radiological changes in ductal and parenchymal morphology that are indistinguishable from those in CP after PD stenting have been reported in up to 50% of patients, which supports a possible causative association between the two.37; 38 ; 39 A further downside of endoscopy is the need for compliance in patients to sustain repeated procedures, which may be another obstacle to its success.40
Surgical treatment for CP traditionally consisted of pancreatic head resection (e.g., Whipples resection) or drainage procedures (e.g., Partington–Rochelle).41 ; 42 A shift towards more conservative, less aggressive treatment however has led to the development of pylorus preserving pancreaticoduodenectomy and duodenum preserving pancreatic head resection procedures as well as combination procedures (e.g., Freys procedure) which have been increasingly performed successfully with minimal morbidity and mortality.43; 44; 45 ; 46 The results of the reviewed publications show a significantly better response to surgical treatment, with good long-term results.
However, a major limitation in this review is the lack of randomized studies which include a relatively small number of patients. Furthermore, in the two RCTs identified there are some important differences to note. Firstly, in Dite et als13 study only 72 of 140 patients agreed to be randomised, with the majority of the remaining patients opting for surgery. The data in the randomised group was analyzed individually as well as for the entire patient group, with no significant differences. Dite et als13 study included a higher number of participants (72 vs. 39), more surgical resections than drainage procedures (80% vs. 10%), as well as a lower number of patients with intraductal stones (23% vs. 95%) when compared with Cahen et als14 ; 15 study. Moreover, lithotripsy was not available, unlike in Cahens series were 88% of patients in the endoscopic group underwent ESWL. ESWL is an important component of endoscopic treatment, and the lack of it in the endoscopy arm may reduce its efficacy when comparing it to surgery.
The endoscopy protocol in the Cahen study involves repeated endoscopy as required, whereas Dite reserves repeat only under certain circumstances such as stent occlusion. Despite this, 47% of patients in Cahens group eventually required surgery due to recurrent PD stenosis. In these patients, only two of nine achieved complete pain relief which suggests that delay in surgical treatment may reduce its efficacy. Dite et al13 demonstrated the same initial success rate in both the endoscopic and the surgical groups, in contrast to Cahen et al14 ; 15 who showed an average difference of 24 points on the Izbicki score (0–100) between the two groups, which was consistent during the entire follow-up period. Therefore Dite’s study only favors surgery in the long-term management.
While endoscopy can achieve improvement in the management of pain, the benefit is usually short lived and the need to repeat the procedure is frequent.47; 48 ; 49 Furthermore, an overall mortality rate of 0.6% following 1622 pancreatic operations is quite remarkable and evidence that as surgical techniques evolve, risk is further minimized.50 Again a lack of properly designed, multicenter RCTs is a limiting factor in evaluating current practice and assessing the different modalities for treating CP, and it is important to discuss these limitations with the patient. It may be useful to design studies that classify different anomalies seen at endoscopic retrograde cholangiopancreatography (ERCP) or endoscopic ultrasound and study the effect of treatment accordingly with the aim of tailoring treatment to individuals. Another avenue to evaluate is whether early surgery is more effective rather than leaving this as a last resort. This is being evaluated in the ESCAPE trial by the Dutch Pancreatitis Study Group.51 It may also be useful to define suitable treatments for low and high risk groups. However, at present, analysis of the existing studies can provide evidence that continues to support the superiority of surgical treatment in CP.
References
- 1 D.K. Andersen, C.F. Frey; The evolution of the surgical treatment of chronic pancreatitis; Ann Surg, 251 (2010), pp. 18–32
- 2 K.E. Fasanella, B. Davis, J. Lyons, et al.; Pain in chronic pancreatitis and pancreatic cancer; Gastroenterol Clin North Am, 36 (2007), pp. 335–364
- 3 F. Fregni, A. Pascual-Leone, S.D. Freedman; Pain in chronic pancreatitis: a salutogenic mechanism or a maladaptive brain response?; Pancreatology, 7 (2007), pp. 411–422
- 4 J.G. Lieb 2nd, C.E. Forsmark; Review article: pain and chronic pancreatitis; Aliment Pharmacol Ther, 29 (2009), pp. 706–719
- 5 Q. Liao, Y.P. Zhao, W.W. Wu, et al.; Diagnosis and treatment of chronic pancreatitis; Hepatobiliary Pancreat Dis Int, 2 (2003), pp. 445–448
- 6 G.Y. Wong, G.H. Sakorafas, G.G. Tsiotos, M.G. Sarr; Palliation of pain in chronic pancreatitis. Use of Neural Blocks and Neurotomy; Surg Clin North Am, 79 (1999), pp. 873–893
- 7 H.C. Buscher, J.B. Jansen, R. van Dongen, R.P. Bleichrodt, H. van Goor; Long-term results of bilateral thoracoscopic splanchnicectomy in patients with chronic pancreatitis; Br J Surg, 89 (2002), pp. 158–162
- 8 S.A. Ahmad, C.J. Wray, H.R. Rilo, et al.; Chronic pancreatitis: recent advances and ongoing challenges; Curr Probl Surg, 43 (2006), pp. 135–238
- 9 M. Hirota, T. Asakura, A. Kanno, T. Shimosegawa; Endoscopic treatment for chronic pancreatitis: indications, technique, results; J Hepatobiliary Pancreat Surg, 17 (2010), pp. 770–775
- 10 L. Heyries, J. Sahel; Endoscopic treatment of chronic pancreatitis; World J Gastroenterol, 13 (2007), pp. 6127–6133
- 11 T. Nguyen-Tang, J.M. Dumonceau; Endoscopic treatment in chronic pancreatitis, timing, duration and type of intervention; Best Pract Res Clin Gastroenterol, 24 (2010), pp. 281–298
- 12 M. Clarke, R. Horton; Bringing it all together: Lancet–Cochrane collaborate on systematic reviews; Lancet, 357 (2001), p. 1728
- 13 P. Dite, M. Ruzicka, V. Zboril, I. Novotny; A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis; Endoscopy, 35 (2003), pp. 553–558
- 14 D.L. Cahen, D.J. Gouma, Y. Nio, et al.; Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis; N Engl J Med, 356 (2007), pp. 676–684
- 15 D.L. Cahen, D.J. Gouma, P. Laramee, et al.; Long-term outcomes of endoscopic vs surgical drainage of the pancreatic duct in patients with chronic pancreatitis; Gastroenterology, 141 (2011), pp. 1690–1695
- 16 D.B. Adams, M.C. Ford, M.C. Anderson; Outcome after lateral pancreaticojejunostomy for chronic pancreatitis; Ann Surg, 219 (1994), pp. 481–487
- 17 T. Strate, Z. Taherpour, C. Bloechle, et al.; Long-term follow-up of a randomized trial comparing the beger and Frey procedures for patients suffering from chronic pancreatitis; Ann Surg, 241 (2005), pp. 591–598
- 18 T. Berney, T. Rudisuhli, J. Oberholzer, et al.; Long-term metabolic results after pancreatic resection for severe chronic pancreatitis; Arch Surg, 135 (2000), pp. 1106–1111
- 19 J.M. Dumonceau; Endoscopic versus surgical treatment for chronic pancreatitis; N Engl J Med, 356 (2007), pp. 2103–2104
- 20 G. Farkas, L. Leindler, M. Daroczi, G. Farkas Jr.; Organ-preserving pancreatic head resection in chronic pancreatitis; Br J Surg, 90 (2003), pp. 29–32
- 21 C.F. Frey, K. Amikura; Local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy in the management of patients with chronic pancreatitis; Ann Surg, 220 (1994), pp. 492–504
- 22 J.R. Izbicki, C. Bloechle, D.C. Broering, et al.; Longitudinal V-shaped excision of the ventral pancreas for small duct disease in severe chronic pancreatitis: prospective evaluation of a new surgical procedure; Ann Surg, 227 (1998), pp. 213–219
- 23 C. Mobius, D. Max, D. Uhlmann, et al.; Five-year follow-up of a prospective non-randomised study comparing duodenum-preserving pancreatic head resection with classic Whipple procedure in the treatment of chronic pancreatitis; Langenbecks Arch Surg, 392 (2007), pp. 359–364
- 24 W.H. Nealon, S. Matin; Analysis of surgical success in preventing recurrent acute exacerbations in chronic pancreatitis; Ann Surg, 233 (2001), pp. 793–800
- 25 T. Rosch, S. Daniel, M. Scholz, et al.; Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up; Endoscopy, 34 (2002), pp. 765–771
- 26 G.H. Sakorafas, M.G. Sarr, C.M. Rowland, M.B. Farnell; Postobstructive chronic pancreatitis: results with distal resection; Arch Surg, 136 (2001), pp. 643–648
- 27 W. Schlosser, B. Poch, H.G. Beger; Duodenum-preserving pancreatic head resection leads to relief of common bile duct stenosis; Am J Surg, 183 (2002), pp. 37–41
- 28 W. Schlosser, A. Schwarz, H.G. Beger; Surgical treatment of chronic pancreatitis with pancreatic main duct dilatation: long term results after head resection and duct drainage; HPB (Oxford), 7 (2005), pp. 114–119
- 29 T. Schnelldorfer, D.N. Lewin, D.B. Adams; Operative management of chronic pancreatitis: longterm results in 372 patients; J Am Coll Surg, 204 (2007), pp. 1039–1045
- 30 G.N. Stapleton, R.C. Williamson; Proximal pancreatoduodenectomy for chronic pancreatitis; Br J Surg, 83 (1996), pp. 1433–1440
- 31 C.E. Lucas, B. McIntosh, D. Paley, et al.; Surgical decompression of ductal obstruction in patients with chronic pancreatitis; Surgery, 126 (1999), pp. 790–795
- 32 R.L. Byrne, R.H. Gompertz, C.W. Venables; Surgery for chronic pancreatitis: a review of 12 years' experience; Ann R Coll Surg Engl, 79 (1997), pp. 405–409
- 33 D. Vasile, P. Ilco, A. Belega, et al.; The surgical treatment of chronic pancreatitis: a clinical series of 17 cases; Chirurgia, 108 (2013), pp. 794–799
- 34 J. Hong, J. Wang, A.M. Keleman, et al.; Endoscopic versus surgical treatment of downstream pancreatic duct stones in chronic pancreatitis; Am Surg, 77 (2011), pp. 1531–1538
- 35 P.G. Lankisch, F. Seidensticker, A. Lohr-Happe, et al.; The course of pain is the same in alcohol- and nonalcohol-induced chronic pancreatitis; Pancreas, 10 (1995), pp. 338–341
- 36 R.H. Bell Jr.; Current surgical management of chronic pancreatitis; J Gastrointest Surg, 9 (2005), pp. 144–154
- 37 S. Sherman, R.H. Hawes, T.J. Savides, et al.; Stent-induced pancreatic ductal and parenchymal changes: correlation of endoscopic ultrasound with ERCP; Gastrointest Endosc, 44 (1996), pp. 276–282
- 38 R.A. Kozarek; Pancreatic stents can induce ductal changes consistent with chronic pancreatitis; Gastrointest Endosc, 36 (1990), pp. 93–95
- 39 H. Ramesh; Alterations in pancreatic ductal morphology following polyethylene pancreatic stent therapy; Gastrointest Endosc, 46 (1997), pp. 196–197
- 40 K. Kienhe, U.R. Folsh, R. Nitsche; High complication rate of bile duct stents in patients with chronic alcoholic pancreatitis due to noncompliance; Endoscopy, 32 (2000), pp. 377–380
- 41 H.S. Ho, C.F. Frey; Current approach to the surgical management of chronic pancreatitis; Gastroenterologist, 5 (1997), pp. 128–136
- 42 K. Bachmann, A. Kutup, O. Mann, et al.; Surgical treatment in chronic pancreatitis timing and type of procedure; Best Pract Res Clin Gastroenterol, 24 (2010), pp. 299–310
- 43 T. Keck, U.F. Wellner, H. Riediger, et al.; Long-term outcome after 92 duodenum-preserving pancreatic head resections for chronic pancreatitis: comparison of Beger and Frey procedures; J Gastrointest Surg, 14 (2010), pp. 549–556
- 44 S. Isaji; Has the Partington procedure for chronic pancreatitis become a thing of the past? A review of the evidence; J Hepatobiliary Pancreat Surg, 17 (2009), pp. 763–769
- 45 D.W. Crist, J.V. Sitzmann, J.L. Cameron; Improved hospital morbidity, mortality, and survival after the Whipple procedure; Ann Surg, 206 (1987), pp. 358–365
- 46 P.A. Grace, H.A. Pitt, R.K. Tompkins, et al.; Decreased morbidity and mortality after pancreatoduodenectomy; Am J Surg, 151 (1986), pp. 141–149
- 47 S.H. Moon, M.H. Kim, D.H. Park, et al.; Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration; Gastrointest Endosc, 72 (2010), pp. 86–91
- 48 A.S. Ross, R.A. Kozarek; Therapeutic pancreatic endoscopy; Dig Liver Dis, 42 (2010), pp. 749–756
- 49 S. Chauhan, C.E. Forsmark; Pain management in chronic pancreatitis: a treatment algorithm; Best Pract Res Clin Gastroenterol, 24 (2010), pp. 323–335
- 50 A. Chaudhary, S.S. Negi, S. Masood, M. Thombare; Complications after Freys procedure for chronic pancreatitis; Am J Surg, 188 (2004), pp. 277–281
- 51 U. Ahmed Ali, Y. Issa, M.J. Bruno, et al.; Early surgery versus optimal current step-up practice for chronic pancreatitis (ESCAPE): design and rationale of a randomized trial; BMC Gastroenterol, 13 (2013 Mar 18), p. 49 https://doi.org/10.1186/1471-230X-13-49
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?