Abstract
Background
Subjective health status is an increasingly important parameter to assess the effect of percutaneous coronary intervention (PCI) in clinical practice.
Aim of this study was to determine medical and psychosocial predictors of poor subjective health status over a 10 years' post-PCI period.
Methods
We included a series of consecutive PCI patients (n = 573) as part of the RESEARCH registry, a Dutch single-center retrospective cohort study.
Results
These patients completed the 36-item Short-Form Health Survey (SF-36) at baseline and 10 years post-PCI. We found 6 predictors of poor subjective health status 10 years post-PCI: SF-36 at baseline, age, previous PCI, obesity, acute myocardial infarction as indication for PCI, and diabetes mellitus (arranged from most to least numbers of sub domains).
Conclusions
SF-36 scores at baseline, age, and previous PCI were significant predictors of subjective health status 10 years post-PCI. Specifically, the SF-36 score at baseline was an important predictor. Thus assessment of subjective health status at baseline is useful as an indicator to predict long-term subjective health status. Subjective health status becomes better by optimal medical treatment, cardiac rehabilitation and psychosocial support. This is the first study determining predictors of subjective health status 10 years post-PCI.
Keywords
Percutaneous coronary intervention;Subjective health status;Health surveys;Risk factors
1. Introduction
Percutaneous coronary intervention (PCI) is an effective therapy for atherosclerotic cardiovascular disease [1]. During the last decade, PCI has resulted in a better prognosis; it has decreased restenosis [2]. Furthermore, PCI appeared to have a positive influence on subjective health status [3]; [4]; [5]; [6] ; [7]. Thereafter, subjective health status became an increasingly important parameter to assess the effect of PCI in clinical practice [8]; [9]; [10]; [11] ; [12].
Until now, a clear conceptual framework that describes specific predictors for long-term subjective health status after PCI is lacking. Only a few studies have reported on medical and psychosocial predictors of (subjective) health status post-PCI [13]; [14]; [15]; [16]; [17] ; [18]. Some of these studies found baseline subjective health status to be an important predictor of subjective health status post-PCI. [13]; [14]; [17] ; [18] Furthermore, age [13], male gender, previous coronary artery bypass grafting (CABG), renal impairment [17], and smoking [16] were predictors of health status post-PCI. An important limitation of these studies was the short duration of follow-up; all these studies had a follow-up of 12 months only [13]; [14]; [15]; [16] ; [17].
Medical and psychosocial predictors of subjective health status at longer term are still unknown. The overall goal of this study is to bridge this gap in knowledge and gain insight in this domain. Specifically, this studys aim is to determine medical and psychosocial predictors of poor subjective health status over a 10 years period post-PCI. We hypothesized that the medical predictors, as found in the few previous studies (with a maximum follow-up of 1 year) up, will also be significant predictors at 10 years follow-up. Therefore, we selected these predictors as candidate predictors in this study.
2. Methods
2.1. Inclusion/Exclusion
The initial study population of this study was part of the RESEARCH registry. The design of the RESEARCH registry has been published elsewhere [19]. Briefly, the RESEARCH registry is a single-center registry evaluating the safety and efficacy of sirolimus-eluting stent (SES) implantation in patients treated in daily clinical practice.
In this study, 1411 consecutive patients treated with PCI with implantation of bare metal stents (BMSs) (October 2001 to April 2002) or SES (April 2002 to October 2002) were included.
2.2. Assessment procedure
For this study, of the 1411 patients, all surviving patients were contacted by mail at 6 months (referred to as baseline in the remainder of the paper) and 10 years post-PCI. They were asked to complete questionnaires about anginal status and medication usage, and the 36-item Short-Form Health Survey (SF-36), assessing subjective health status. At each assessment moment, a reminder was sent to patients who did not return their questionnaire. Only patients (N = 1055) who did not die or were not lost over 10 years follow-up were selected. The assessment at 6 months was chosen to ascertain a stable medical condition. A similar approach has been adopted in other studies of PCI patients [20].
Information about the in-hospital outcomes was obtained from our hospital electronic clinical database maintained at our institution and by reviewing the hospital records for those discharged to referring hospitals. The survival status at each assessment moment was obtained from the Municipal Civil Registries. The referring physicians and institutions were contacted whenever necessary for additional information.
This study was not subject to the Dutch Medical Research Involving Human Subjects Act. Therefore, approval from the local research ethics committee to conduct this retrospective follow-up study was not required at the time of enrollment. Moreover, the study was conducted according to the Helsinki Declaration [21]. All patients consented participation in this study.
2.3. Baseline variables
Demographic variables included gender and age. Information about clinical variables was prospectively collected at the time of the procedure and recorded in the institutional database. Clinical variables included hypertension, smoking, diabetes mellitus, family history of CAD, indication for PCI, type of stent, previous myocardial infarction, previous CABG, previous PCI, multi-vessel disease, and body mass index [BMI]. Multivessel disease was defined as PCI in more than one coronary artery or in the left main. BMI was categorized in three groups: BMI < 25 kg/m2, BMI 25–30 kg/m2 and BMI ≥ 30 kg/m2.
2.4. Subjective health status
The subjective health status was assessed with the SF-36 Health Survey. The SF-36 assesses 8 health status domains: physical functioning, role limitations due to physical problems, bodily pain, general health, social functioning, role limitations due to emotional functioning, mental health, and vitality. The score range is from 0 to 100. A higher score on the SF-36 sub domains represents an improved functioning; a high score on the bodily pain scale indicates the absence of pain [22]. Good reliability and validity have been reported for the Dutch version of the SF-36, with Cronbach α ranging from 0.65 to 0.96 for all subscales [23].
2.5. Outcome
The clinical end point was defined as poor subjective health status at 10-year follow-up. As recommended by others [9] ; [24], to enhance clinical interpretability, we dichotomized the health status sub domains into: poor health status (i.e. the lowest tertile) versus good health status (i.e. the other two tertiles).
2.6. Statistical analysis
Discrete variables were compared with the χ2 test and continuous variables with Student t test. Univariable and multivariable logistic regression analyses were done to examine the predictors of poor subjective health status for all of the eight health status subscales. Predictors were ranked in a forward stepwise logistic regression model.
To determine predictors of poor subjective health status 10 years post-PCI, analyses were done on data of those patients who completed the SF-36 questionnaire at both baseline and 10 years post-PCI (N = 573). In multivariable analyses, we used all baseline characteristics, and baseline SF-36 scores of the corresponding SF-36 sub domain. All tests were 2-tailed, using a probability value of p < 0.05. For logistic regression analyses, odds ratios (ORs) and their corresponding 95% confidence intervals (95% CIs) were reported. All data were analyzed using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL).
3. Results
3.1. Participants (flow)
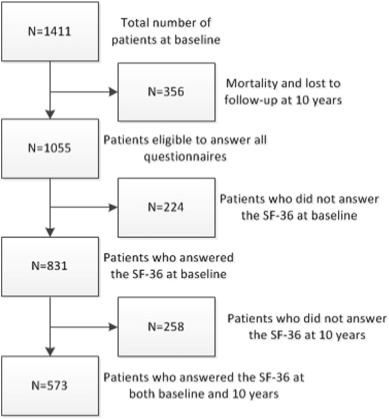
Fig. 1 shows the patient flow throughout the study. At the start of the study, 1411 participants were contacted of whom 356 were lost during 10 years follow-up. Of these 356 patients, 322 died and 34 were lost to follow-up. Of these 1055 eligible patients 573 patients filled in questionnaires at both baseline and 10 years post-PCI.
|
|
|
Fig. 1. Flowchart of patient selection. |
3.2. Baseline characteristics
Baseline characteristics of the 573 patients who responded at baseline and 10 years vs. the 482 patients who did not respond at both baseline and 10 years are listed in Table 1. Overall, between these two groups, baseline characteristics are comparable, apart from three exceptions: patients who responded at both baseline and 10 years have lower rate of multivessel disease (47% vs. 54%; p = 0.021), lower rate of diabetes mellitus (11% vs. 18%; p = 0.001), and their BMI is lower (27 ± 3.6 vs. 28 ± 6.7; p = 0.009).
| Patients who responded at baseline and 10 years | Patients who did not respond at both baseline and 10 years | P | |
|---|---|---|---|
| No. of patients | 573 | 482 | |
| Male | 427 (75%) | 342 (71%) | 0.19 |
| Age ± SD | 60 ± 9.6 | 59 ± 11.6 | 0.33 |
| Cardiovascular history | |||
| Previous MI | 215 (38%) | 165 (34%) | 0.24 |
| Previous CABG | 50 (9%) | 35 (7%) | 0.38 |
| Previous PCI | 103 (18%) | 105 (22%) | 0.12 |
| Multivessel disease | 267 (47%) | 259 (54%) | 0.02 |
| Risk factors | |||
| Hypertension | 188 (33%) | 167 (35%) | 0.53 |
| Family history | 186 (33%) | 162 (34%) | 0.69 |
| Current smoker | 214 (37%) | 201 (42%) | 0.15 |
| Diabetes mellitus | 62 (11%) | 85 (18%) | 0.001 |
| Body mass index | 27 ± 3.6 | 28 ± 6.7 | 0.01 |
| Indication for PCI | 0.92 | ||
| Stable angina | 279 (49%) | 229 (48%) | |
| Unstable angina | 198 (35%) | 172 (36%) | |
| Acute myocardial infarction | 96 (17%) | 81 (17%) | |
| Type of stent | 0.75 | ||
| Bare metal stent | 298 (52%) | 246 (51%) | |
| Sirolimus-eluting stent | 275 (48%) | 236 (49%) |
Compared with the 1055 eligible patients who were alive 10 years post-PCI, baseline characteristics of the 34 patients who were lost to follow-up did not differ significantly, except for indication of PCI. Furthermore, compared with the 1055 patients who were alive after 10 years follow-up, patients who died differed significantly in all baseline characteristics.
3.3. Predictors of poor subjective health status
Univariable analyses showed that SF-36 score at baseline significantly predicted all 8 sub domains, whereas acute myocardial infarction as indication for PCI significantly predicted 7 sub domains. Other significant predictors were gender, age, and previous PCI (all predicting 6 sub domains), obesity (predicting 5 sub domains), and smoking and diabetes mellitus (predicting 4 sub domains). (Appendix 1).
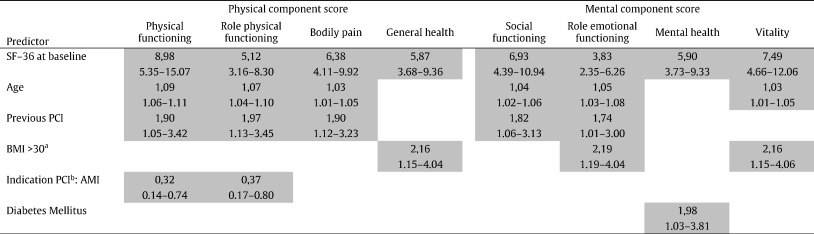
Multivariable analyses again showed that SF-36 at baseline significantly predicted all 8 sub domains. Age significantly predicted 6 sub domains, previous PCI 5 sub domains, obesity 3 sub domains, acute myocardial infarction as indication for PCI 2 sub domains, and diabetes mellitus 1 sub domain. (Table 2).
|
Abbreviations: PCI, percutaneous coronary intervention; BMI, body mass index. AMI, acute myocardial infarction. Outcome measure: odds ratios and 95% CI. Darks cells indicate significant results. Predictors tested in model: male gender, age, hypertension, current smoker, diabetes mellitus, family history, old MI, previous CABG, previous PCI, multivessel disease, BMI, indication PCI, type of stent, SF-36 at baseline. aReference: BMI < 25. bReference: Indication PCI: stable angina pectoris.
|
|
Table 2. Multivariable analyses |
4. Discussion
Besides the SF-36 scores at baseline, age and prior PCI were predictors of a broad range of sub domains of subjective health status 10 years post-PCI. The SF-36 score at baseline significantly predicted all 8 sub domains of the SF-36. Thus SF-36 at baseline can be regarded as a predictor of long-term subjective health status post-PCI.
Our results were partly similar to previous studies, which had a shorter follow-up interval. These studies also found that baseline subjective health status was an important predictor for health status post-PCI [13]; [14]; [17] ; [18]. In other studies, age predicted health status post-PCI as well [13]; [17] ; [18]. This was also found in studies with CABG patients [25]; [26]; [27]; [28]; [29] ; [30] or patients with coronary artery disease [31]. Furthermore, obesity and diabetes mellitus were previously found to be predictors of health status [26]; [27]; [28]; [30] ; [32]. However, this was not found in studies that measured health status post-PCI.
In contrast with other studies [13] ; [17] and with trends in the general population [33], female gender was not a predictor of subjective health status 10 years post-PCI. This result has not been previously found and is hard to explain. In contrast, for CABG patients, gender was still predictive for subjective health status, even after long follow-up duration [25]; [26] ; [27].
The results of this study have implication in clinical practice. Low SF-36 score, older age, and prior PCI were predictors of long-term poor subjective health. Both age and prior PCI could not be modified. However, clinicians could be alert to the health status in these patients. In contrast, the SF-36 score could be modified. First, patients have to get optimal medical therapy. Optimal medical therapy gives patients a better health status [6]; [7] ; [10]. Second, physical health status gets better by cardiac rehabilitation [2]. So cardiologists have to refer their patients to cardiac rehabilitation. Last, mental health status could be influenced by psychosocial support of e.g. social workers.
The strength of this study is the long duration of follow-up and the focus on post-PCI patients. This is the first study that determined predictors of subjective health status 10 years post-PCI. Previous studies that determined predictors of health status post-PCI had a maximum follow-up duration of 1 year [13]; [14]; [15]; [16] ; [17]. Furthermore, previous studies reported on predictors of health status after acute coronary syndrome or reported patients with coronary artery disease and not on post-PCI patients [7]; [11]; [31]; [34]; [35]; [36] ; [37]. None of these studies had a follow-up duration of 10 years. The study with the longest follow-up had a follow-up of 28 months [36].
Regarding CABG patients, several studies determined predictors of health status [25]; [26]; [27]; [28]; [29]; [30]; [32]; [38]; [39]; [40]; [41] ; [42]. Some studies had a follow-up duration of several years [25]; [26]; [27]; [28] ; [41]. Especially Herlitz et al. have studies with long follow-up duration, with a maximum of 15 years [27]. This study is therefore unique due to the 10 years' follow-up in PCI patients.
This study had several potential limitations. First, in this study two types of stents were used: SES and BMS. Patients were not randomized in regard of their type of stent. However, the strength of this study is that no exclusion criterion was applied. This means that the study population was representative for the population in the daily practice of interventional cardiology. To close the gap between research and clinical practice, it has been advocated to conduct research in daily practice settings [8]. Second, only 54% of the 1055 eligible patients responded on the SF-36 at both baseline and 10 years post-PCI. It is a well-known problem that there are many non-responders in a long registry. Other studies have this problem as well [25] ; [26]. Third, baseline characteristics of patients who responded at both baseline and 10 years post-PCI and patients who did not respond at both baseline and 10 years post-PCI were not completely similar. Responders had lower rate of multivessel disease and diabetes mellitus, and they had a lower BMI. Fourth, there may also be predictors that are not included in our analyses but that have a predictive value.
Further studies with a comparable long-term follow-up interval are needed to replicate our findings in order to develop a framework of predictors of long-term subjective health status after PCI.
In conclusion, we found that SF-36 scores at baseline, age, and previous PCI are significant predictors of subjective health status 10 years post-PCI. Assessment of health status at baseline is a useful indicator to predict long-term subjective health status.
The following is the supplementary data related to this article.
Appendix 1.
Univariable analyses.
Conflict of interest
The authors have no competing interests to report.
Acknowledgments
We thank Laurence C Walhout, BsC for providing language help.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- [1] S.C. Smith Jr., J.T. Dove, A.K. Jacobs, et al.; ACC/AHA guidelines of percutaneous coronary interventions (revision of the 1993 PTCA guidelines)—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty); J. Am. Coll. Cardiol., 37 (2001), pp. 2215–2239
- [2] S. Windecker, S. Stortecky, G.G. Stefanini, et al.; Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis; BMJ, 348 (2014), p. g3859
- [3] B. Brorsson, S.J. Bernstein, R.H. Brook, L. Werko; Quality of life of chronic stable angina patients 4 years after coronary angioplasty or coronary artery bypass surgery; J. Intern. Med., 249 (2001), pp. 47–57
- [4] E.D. Folland, P.M. Hartigan, A.F. Parisi; Percutaneous transluminal coronary angioplasty versus medical therapy for stable angina pectoris: outcomes for patients with double-vessel versus single-vessel coronary artery disease in a Veterans Affairs Cooperative randomized trial. Veterans Affairs ACME Investigators; J. Am. Coll. Cardiol., 29 (1997), pp. 1505–1511
- [5] C.S. Rihal, D.L. Raco, B.J. Gersh, S. Yusuf; Indications for coronary artery bypass surgery and percutaneous coronary intervention in chronic stable angina: review of the evidence and methodological considerations; Circulation, 108 (2003), pp. 2439–2445
- [6] W.E. Strauss, T. Fortin, P. Hartigan, E.D. Folland, A.F. Parisi; A comparison of quality of life scores in patients with angina pectoris after angioplasty compared with after medical therapy. Outcomes of a randomized clinical trial. Veterans Affairs Study of Angioplasty compared to Medical Therapy Investigators; Circulation, 92 (1995), pp. 1710–1719
- [7] W.S. Weintraub, J.A. Spertus, P. Kolm, et al.; Effect of PCI on quality of life in patients with stable coronary disease; N. Engl. J. Med., 359 (2008), pp. 677–687
- [8] H.M. Krumholz, E.D. Peterson, J.Z. Ayanian, et al.; Report of the National Heart, Lung, and Blood Institute working group on outcomes research in cardiovascular disease; Circulation, 111 (2005), pp. 3158–3166
- [9] J.A. Spertus, P. Jones, M. McDonell, V. Fan, S.D. Fihn; Health status predicts long-term outcome in outpatients with coronary disease; Circulation, 106 (2002), pp. 43–49
- [10] R.T. van Domburg, J. Daemen, S.S. Pedersen, et al.; Short- and long-term health related quality-of-life and anginal status after randomisation to coronary stenting versus bypass surgery for the treatment of multivessel disease: results of the Arterial Revascularisation Therapy Study (ARTS); EuroIntervention, 3 (2008), pp. 506–511
- [11] S. Hofer, S. Doering, G. Rumpold, N. Oldridge, W. Benzer; Determinants of health-related quality of life in patients with coronary artery disease; Eur. J. Cardiovasc. Prev. Rehabil., 13 (2006), pp. 398–406
- [12] R.J. Gibbons, K. Chatterjee, J. Daley, et al.; ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina); J. Am. Coll. Cardiol., 33 (1999), pp. 2092–2197
- [13] J.A. Spertus, A.C. Salisbury, P.G. Jones, D.G. Conaway, R.C. Thompson; Predictors of quality-of-life benefit after percutaneous coronary intervention; Circulation, 110 (2004), pp. 3789–3794
- [14] A.S. de Quadros, T.C. Lima, A.P. Rodrigues, et al.; Quality of life and health status after percutaneous coronary intervention in stable angina patients: results from the real-world practice; Catheter. Cardiovasc. Interv., 77 (2011), pp. 954–960
- [15] W.S. Poston, C.K. Haddock, M. Conard, J.A. Spertus; Impact of obesity on disease-specific health status after percutaneous coronary intervention in coronary disease patients; Int. J. Obes. Relat. Metab. Disord., 28 (2004), pp. 1011–1017
- [16] C.K. Haddock, W.S. Poston, J.E. Taylor, M. Conard, J. Spertus; Smoking and health outcomes after percutaneous coronary intervention; Am. Heart J., 145 (2003), pp. 652–657
- [17] S.S. Pedersen, A.T. Ong, P.A. Lemos, R.A. Erdman, P.W. Serruys, R.T. van Domburg; Risk factors for impaired health status differ in women and men treated with percutaneous coronary intervention in the drug-eluting stent era; J. Psychosom. Res., 61 (2006), pp. 11–17
- [18] I.S. Nash, L.H. Curtis, H. Rubin; Predictors of patient-reported physical and mental health 6 months after percutaneous coronary revascularization; Am. Heart J., 138 (1999), pp. 422–429
- [19] P.A. Lemos, C.H. Lee, M. Degertekin, et al.; Early outcome after sirolimus-eluting stent implantation in patients with acute coronary syndromes: insights from the Rapamycin-Eluting Stent Evaluated at Rotterdam Cardiology Hospital (RESEARCH) registry; J. Am. Coll. Cardiol., 41 (2003), pp. 2093–2099
- [20] W.S. Poston, C.K. Haddock, M.W. Conard, P. Jones, J. Spertus; Assessing depression in the cardiac patient. When is the appropriate time to assess depression in the patient undergoing coronary revascularization?; Behav. Modif., 27 (2003), pp. 26–36
- [21] M.D. Goodyear, K. Krleza-Jeric, T. Lemmens; The declaration of Helsinki; BMJ, 335 (2007), pp. 624–625
- [22] J.E. Ware Jr., C.D. Sherbourne; The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection; Med. Care, 30 (1992), pp. 473–483
- [23] H.J. Smith, R. Taylor, A. Mitchell; A comparison of four quality of life instruments in cardiac patients: SF-36, QLI, QLMI, and SEIQoL; Heart, 84 (2000), pp. 390–394
- [24] J.S. Rumsfeld, D.J. Magid, M.E. Plomondon, et al.; History of depression, angina, and quality of life after acute coronary syndromes; Am. Heart J., 145 (2003), pp. 493–499
- [25] J. Herlitz, G. Brandrup-Wognsen, K. Caidahl, et al.; Improvement and factors associated with improvement in quality of life during 10 years after coronary artery bypass grafting; Coron. Artery Dis., 14 (2003), pp. 509–517
- [26] J. Herlitz, G. Brandrup-Wognsen, K. Caidahl, et al.; Determinants for an impaired quality of life 10 years after coronary artery bypass surgery; Int. J. Cardiol., 98 (2005), pp. 447–452
- [27] J. Herlitz, G. Brandrup-Wognsen, M.H. Evander, et al.; Quality of life 15 years after coronary artery bypass grafting; Coron. Artery Dis., 20 (2009), pp. 363–369
- [28] J. Herlitz, I. Wiklund, K. Caidahl, et al.; Determinants of an impaired quality of life five years after coronary artery bypass surgery; Heart, 81 (1999), pp. 342–346
- [29] J.S. Rumsfeld, P.M. Ho, D.J. Magid, et al.; Predictors of health-related quality of life after coronary artery bypass surgery; Ann. Thorac. Surg., 77 (2004), pp. 1508–1513
- [30] K.F. Welke, J.P. Stevens, W.C. Schults, E.C. Nelson, V.L. Beggs, W.C. Nugent; Patient characteristics can predict improvement in functional health after elective coronary artery bypass grafting; Ann. Thorac. Surg., 75 (2003), pp. 1849–1855 (discussion 55)
- [31] C.A. Beck, L. Joseph, P. Belisle, L. Pilote, Q. Investigators; Predictors of quality of life 6 months and 1 year after acute myocardial infarction; Am. Heart J., 142 (2001), pp. 271–279
- [32] V. Peric, M. Borzanovic, R. Stolic, et al.; Predictors of worsening of patients' quality of life six months after coronary artery bypass surgery; J. Card. Surg., 23 (2008), pp. 648–654
- [33] N.K. Aaronson, M. Muller, P.D. Cohen, et al.; Translation, validation, and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populations; J. Clin. Epidemiol., 51 (1998), pp. 1055–1068
- [34] C.C. Dias, P. Mateus, L. Santos, et al.; Acute coronary syndrome and predictors of quality of life; Rev. Port. Cardiol., 24 (2005), pp. 819–831
- [35] J.S. Rumsfeld, D.J. Magid, M.E. Plomondon, et al.; Predictors of quality of life following acute coronary syndromes; Am. J. Cardiol., 88 (2001), pp. 781–784
- [36] B. Silarova, I. Nagyova, J. Rosenberger, et al.; Sense of coherence as an independent predictor of health-related quality of life among coronary heart disease patients; Qual. Life Res., 21 (2012), pp. 1863–1871
- [37] E.N. Souza, A.S. Quadros, R. Maestri, C. Albarran, R. Sarmento-Leite; Predictors of quality of life change after an acute coronary event; Arq. Bras. Cardiol., 91 (2008), pp. 229–235 (52-9)
- [38] S. Al-Ruzzeh, T. Athanasiou, O. Mangoush, et al.; Predictors of poor mid-term health related quality of life after primary isolated coronary artery bypass grafting surgery; Heart, 91 (2005), pp. 1557–1562
- [39] O. Jarvinen, T. Saarinen, J. Julkunen, H. Huhtala, M.R. Tarkka; Changes in health-related quality of life and functional capacity following coronary artery bypass graft surgery; Eur. J. Cardiothorac. Surg., 24 (2003), pp. 750–756
- [40] V. Peric, M. Borzanovic, R. Stolic, et al.; Quality of life in patients related to gender differences before and after coronary artery bypass surgery; Interact. Cardiovasc. Thorac. Surg., 10 (2010), pp. 232–238
- [41] H. Sjoland, I. Wiklund, K. Caidahl, M. Hartford, T. Karlsson, J. Herlitz; Improvement in quality of life differs between women and men after coronary artery bypass surgery; J. Intern. Med., 245 (1999), pp. 445–454
- [42] V. Vaccarino, Z.Q. Lin, S.V. Kasl, et al.; Sex differences in health status after coronary artery bypass surgery; Circulation, 108 (2003), pp. 2642–2647
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?