Summary
Background
Recurrence of hepatocellular carcinoma (HCC) after surgery is frequent, and is an important factor adversely influencing the long-term survival of patients. This prospective study evaluated whether adjuvant chemotherapy with oral tegafur/uracil (UFT) reduces the recurrence rate of HCC. In addition, expression of thymidylate synthase (TS) and dihydropyrimidine dehydrogenase (DPD) were investigated in resected tumors and nontumorous tissues, and the relationship between their expression and the effectiveness of UFT was examined.
Methods
A total of 117 patients who underwent curative hepatic resection for HCC were randomly allocated to UFT 400 mg/d (n = 24, UFT group) or surgery alone (n = 56, control group). The primary endpoint was the recurrence-free survival rate, and the secondary endpoint was the overall survival rate. Expression of the DPD and TS genes were quantified with TaqMan reverse transcription-polymerase chain reaction assay using β-actin as an internal standard. The cut-off value was set at the mean value of TS and DPD expression.
Results
Among the 61 patients in the UFT group, 37 patients (60.6%) discontinued UFT within 1 month. Recurrence-free survival (p = 0.16) and overall survival (p = 0.29) were similar in the two groups. In the UFT group, recurrence-free survival did not differ significantly between high-TS (TS > 3.6) and high-DPD (DPD > 8.9; n = 10), and low-TS (TS ≤ 3.6) and low-DPD (DPD ≤ 8.9; n = 9) groups. However, there was a significant difference between the two groups in overall survival (p = 0.04).
Conclusion
Peroral UFT administration fails to prolong the recurrence-free rates and overall survival rates, in comparison with surgery alone. However, oral administration of UFT may improve the survival of HCC patients when the levels of TS and DPD mRNA are low in the tumor tissue.
Keywords
dihydropyrimidine dehydrogenase;hepatocellular carcinoma;thymidylate synthase
1. Introduction
Although the safety of hepatic resection for hepatocellular carcinoma (HCC) has been improved,1 ; 2 the cancer frequently recurs after surgery, and this is an important factor adversely influencing long-term survival.3 ; 4 The remaining liver is the predominant site of recurrence. Various efforts to reduce the risk of recurrence after hepatic resection have been attempted, including different regimens of adjuvant chemotherapy and neoadjuvant chemotherapy.5 ; 6 However, their effectiveness for prevention of HCC recurrence is controversial.
Uracil-tegafur (UFT, Taiho Pharmaceutical Co., Tokyo, Japan), an oral fluorinated pyrimidine chemotherapeutic agent, has been used for adjuvant chemotherapy against several tumor types (particularly colorectal and lung cancer).7 ; 8 Although this is effective in some HCC patients, most patients do not receive the benefit from the agent.9 ; 10 Therefore, prediction of the sensitivity of each patient with HCC to this agent is important for avoiding unnecessary administration.
Thymidylate synthase (TS) and dihydropyrimidine dehydrogenase (DPD) are key enzymes in the regulation of 5-fluorouracil (5-FU) metabolism. TS is an essential enzyme for DNA synthesis, and is inhibited by the 5-FU metabolite 5-fluoro-2′-deoxyuridylate 5′-monophosphate. Several studies have demonstrated a correlation between high levels of TS and resistance to 5-FU.11 ; 12 However, DPD, which is both an initial and rate-limiting enzyme in the catabolism of 5-FU, has been reported to play an important role in 5-FU pharmacokinetics; > 80% of administered 5-FU is converted to the inactive metabolite 5-fluoro-5, 6-dihydrouracil through a catabolic pathway.13 An association between high DPD levels and 5-FU resistance has been reported.14 ; 15 Recently, basic and clinical researchers have demonstrated a correlation between the antitumor effect of 5-FU and the levels of TS mRNA or DPD mRNA in tumors by using Taqman reverse-transcription polymerase chain reaction (RT-PCR).16 However, there have been no reports of correlations between the quantitative amounts of TS mRNA and DPD mRNA and survival duration in patients with HCC receiving radical surgery combined with adjuvant oral UFT chemotherapy.
The aim of this prospective study was to evaluate whether adjuvant chemotherapy with oral UFT reduces the recurrence rate of HCC. In addition, the expression of TS and DPD was investigated in resected tumors, and its relationship with the effectiveness of UFT was examined.
2. Methods
One hundred and seventeen patients with HCC who had undergone initial curative hepatic resection at Dokkyo Medical University Hospital, Tochigi, Japan between August 2002 and February 2006 were enrolled in a prospective randomized study of postoperative adjuvant chemotherapy. The inclusive criteria were: (1) absence of residual or recurrent tumors at 1 month after curative surgery; (2) patient age 15–75 years; (3) liver function Child-Pugh class A or B; and (4) absence of severe renal or cardiac disease. All patients were informed of the nature and risks of the study, and written informed consent was obtained from all of them. Patients were allocated randomly to either a UFT group or a control group using the minimization method.17 UFT (400 mg/d) was administered orally for at least 1 year in the UFT group, the administration of UFT started at 1 month after surgery. The control group received surgery alone. No patient received radiation or chemotherapy before surgery or other chemotherapy during the study period.
The primary end point was the recurrence-free survival rate, and the secondary end point was overall survival rate. The follow-up study protocol was uniform for all patients, and included a serum α-fetoprotein assay and an ultrasonography performed every 4 weeks, dynamic computed tomography performed every 3 months, and chest radiograph performed every 6 months for the 1st year, and the intervals of these examinations were gradually increased after the 1st year. When recurrence was confirmed using appropriate imaging, adjuvant chemotherapy was stopped and the disease was treated accordingly with treatment modalities such as reoperation, transarterial chemoembolization, or systemic chemotherapy. If patients had less than four recurrent HCCs, they underwent reoperation on the basis of Makuuchis criteria. However, if patients had more than three recurrent HCCs, they underwent transarterial chemoembolization, radio-frequency ablation, or systemic chemotherapy instead of reoperation.
2.1. Measurement of TS and DPD activities
Tumor tissue and adjacent normal tissue were obtained from surgically resected samples and stored at −80°C until assayed.
TS and DPD gene expression was quantified with RT-PCR assay (Hoffman-La Roche, Inc., Nutley, NJ, USA) using β-actin as an internal standard, and expressed as the TS: β-actin or DPD: β-actin mRNA ratio. The cut-off value was set at the median value of TS or DPD expression.
2.2. Statistical analysis
Data are expressed as mean ± SD. The Mann–Whitney U test or Chi-square test was used where appropriate for comparing clinical parameters between the two groups. Recurrence-free and overall survival curves were obtained using the Kaplan–Meier method, and survival rates were compared between the groups using the Wilcoxon test. For all tests, differences at p < 0.05 were considered significant.
3. Results
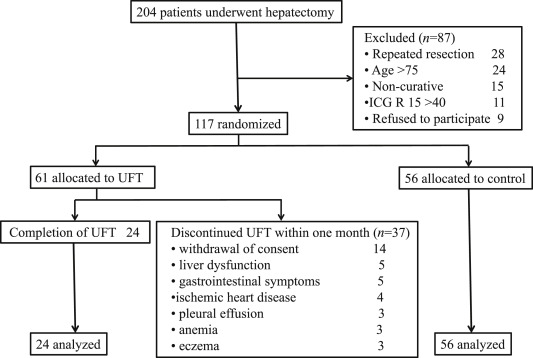
A total of 117 patients who underwent curative hepatic resection for HCC were randomly allocated to UFT 400 mg/d (n = 61 UFT group) or surgery alone (n = 56, control group). Treatment with UFT was permanently discontinued in 37 patients because of withdrawal of consent (n = 14), liver dysfunction (n = 5), gastrointestinal symptoms (n = 5), ischemic heart disease (n = 4), pleural effusion (n = 3), anemia (n = 3), and eczema (n = 3) within 1 month after the start of administration. There were a lot of side effects due to administration of UFT in patients with HCC. The remaining 24 patients were analyzed. No adverse effects occurred in these patients, and the characteristics of the two groups were similar ( Figure 1).
|
|
|
Figure 1. Flow chart of patient allocation. |
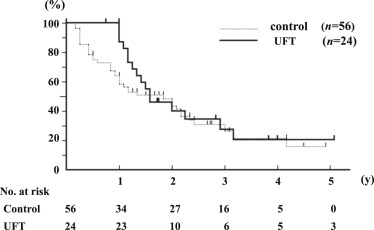
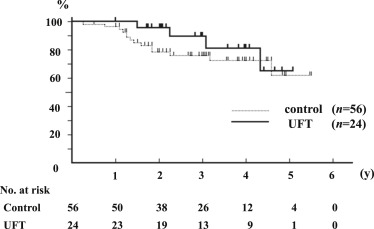
Median follow-up was 2.8 years (range, 0.5–5.5 years). Recurrence-free survival (p = 0.16) and overall survival (p = 0.29) were similar in the two groups. The recurrence-free survival rates at 1 year and 3 years were 87% and 27.5% in the UFT group, and 58.5% and 25.4% in the control group, respectively ( Figure 2). The overall survival rate at 3 years and 5 years were 89.3% and 64.3% in the UFT group, and 75.9% and 62.2% in the control group, respectively (Figure 3).
|
|
|
Figure 2. Recurrence-free and overall survival curves after curative resection of HCC. Recurrence-free survival curves after curative resection of HCC in the UFT group and control group (p = 0.16). |
|
|
|
Figure 3. Recurrence-free and overall survival curves after curative resection of HCC. Overall survival curves after curative resection of HCC in the UFT Group and Control Group (p = 0.29). |
3.1. Levels of TS and DPD mRNA in tumors and adjacent tissue
The relative gene expressions of TS and DPD in tumor tissues were observed to be 2.16- and 3.34-fold higher compared with adjacent control tissues.
Measurements of TS and DPD expression in the UFT group were available for 21 samples, and the ranges were 0.03–17.8 and 0.51–32.1, respectively. Similarly, measurement of TS and DPD expression in the control group were available for 35 samples, and the ranges were 0.06–23.4 and 0.18–40.3, respectively.
The respective cut-off values of TS and DPD expression in the UFT group were 3.6 and 8.91, and those in the control group were 3.08 and 8.41 (difference not significant).
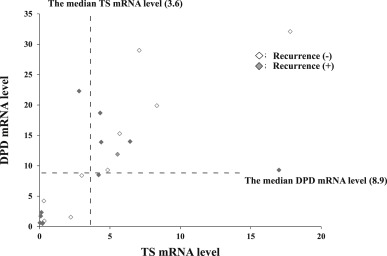
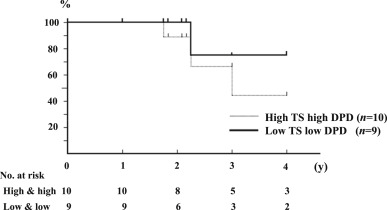
In the UFT group, the recurrence-free rate did not differ significantly between the high-TS and high-DPD (n = 10). and low-TS and low-DPD groups (n = 9; Figure 4). However, there was a significant difference between the two groups in overall survival (p = 0.04; Figure 5).
|
|
|
Figure 4. Relationships between recurrences and, TS mRNA and DPD mRNA levels in 21 samples of HCC tissues in the UFT group. The dotted lines indicate the median value of each mRNA level. |
|
|
|
Figure 5. Overall survival curve in the UFT Group with low-TS and low-DPD (TS ≤ 3.6, DPD ≤ 8.9), and high-TS and high-DPD (TS > 3.6, DPD > 8.9; p = 0.04). |
4. Discussion
The response to conventional fluoropyrimidine derivatives can be inadequate in patients with HCC. Because a poor response is caused by the increased activity of the 5-FU-metabolizing enzyme DPD in the tumor tissue, inhibition of DPD could lead to prolongation of the active concentrations of 5-FU. Fluoropyrimidine derivatives combined with a DPD inhibitor are termed DPD inhibitory fluoropyrimidine. UFT was the first such DPD inhibitory fluoropyrimidine to be applied, and is now widely used.
Adjuvant oral chemotherapy with UFT has been shown to be effective against HCC recurrence,9 ; 10 but negative results of adjuvant chemotherapy in terms of overall survival have also been reported.18 In the present study, we expected that adjuvant chemotherapy with UFT after hepatic resection might effectively kill residual microscopic tumor cells in the remnant liver and circulation. However, contrary to expectation, there were no significant differences in the recurrence-free and overall survival rates between the UFT group and control group. Although no adverse effects occurred in the UFT group, our data are similar to the results of another prospective study by Hasegawa et al.18
Our study is the first to have examined the expression of both TS and DPD in the same specimens of HCC to evaluate the efficacy of UFT in HCC patients. DPD expression levels vary among different types of cancer. In HCC, expression of DPD mRNA and TS mRNA was generally higher than those of other types of cancers (mean ± SD value = 1.34 ± 1.79 and 2.81 ± 3.13, respectively).19 It has also been suggested that the response to 5-FU varies depending on the type of cancer, and is related to the expression levels of 5-FU-related enzymes. Theoretically, the lower activities of TS and DPD in tumors produce the better response to 5-FU.20; 21 ; 22 In fact, low TS patients who received hepatic artery infusion chemotherapy had 4.1 times higher response for colorectal liver metastases than those of high TS patients.23 Furthermore, in patients with neck and head cancer, complete responders had a significantly lower DPD activity than those of partial or no responders.24 Miyoshi et al8 have also reported that adjuvant chemotherapy with UFT might improve the survival of patients with nonsmall cell lung cancer when TS levels in tumor tissues are low. Therefore, although a previous study18 revealed that there was no difference between the UFT group and the control group in overall survival, the low-TS and low-DPD group would have a higher overall survival rate than those of control group when investigation of the TS and DPD expression was performed.
Conversely, Mizutani et al25 have reported that higher TS activity in primary-cultured RCC was predictive of higher sensitivity to 5-FU. Similarly, higher TS expression was reported to be significantly associated with longer survival in patients with stage II colon cancer.26 One possible reason for these conflicting results might be as follows. Although excessively high expression of TS in tumor tissue would reduce the sensitivity to 5-FU in highly proliferative cancer such as HCC, moderately high expression of TS in a tumor would sensitize other types of cancer to 5-FU. The present study also revealed that the expression of TS mRNA was higher than those of other types of cancers.19
Our results revealed no significant difference in overall survival rates between the UFT group and control group. However, low-TS and low-DPD patients group who were treated with UFT had a significant better survival rate than those of high-TS and high-DPD patients group who were also treated with UFT (p = 0.04). Therefore, TS and DPD activity is considered to be an important determinant of the response to fluoropyrimidine drugs, and may be an independent predictor of outcome.
Because the results, which were demonstrated with quantitative RT-PCR, showed the TS or DPD/β-actin mRNA ratio, there might be a discrepancy between the mRNA expression levels and TS/DPD enzyme activities. In addition, it would be better to define TS/DPD cut-off values using receiver operating characteristic curve analyses than those of median TS/DPD expression levels. In fact, although there was no significant difference between low-TS and low-DPD, and high-TS and high-DPD patients groups in the recurrence rate (Figure 4), there was a significant difference between these two groups in overall survival (Figure 5). However, a previous study demonstrated that DPD protein in the liver cytosol correlated with the activity of enzyme.27 Therefore, it would be acceptable to use TS/DPD mRNA levels as prognostic markers of HCC patients receiving UFT adjuvant therapy, instead for TS/DPD enzyme activities. In addition, a possible explanation for this discrepancy between recurrence-free and overall survival is based on a hypothesis that there is a difference between carcinogenesis and tumor progress in patients with HCC. Even if UFT treatment might be effective for suppressing tumor progression in patients with low-TS and low-DPD, there might be no relationship between UFT treatment and HCC recurrence in the residual liver after liver resection for HCC. In fact, both TS and DPD expression levels were lower in the control tissues than those in the tumor tissues.
In conclusion, adjuvant chemotherapy with UFT may not be useful for HCC patients. However, when TS and DPD activity is low in tumor tissue, oral administration of UFT after surgery might improve survival. Because the results of this study were based on a small number of patients, further prospective randomized controlled studies with larger numbers of cases will be required to verify our results. In addition, because there was a high withdraw rate from the UFT group in the study, use of UFT should be seriously considered in the following studies.
Acknowledgments
We received no funding/grant support for this study.
References
- 1 H.Q. Wang, J. Yang, L.N. Yan, X.W. Zhang, J.Y. Yang; Liver resection in hepatitis B related-hepatocellular carcinoma: clinical outcomes and safety in elderly patients; World J Gastroenterol, 20 (2014), pp. 6620–6625
- 2 T. Kamiyama, M. Tahara, K. Nakanishi, et al.; Long-term outcome of laparoscopic hepatectomy in patients with hepatocellular carcinoma; Hepatogastroenterology, 61 (2014), pp. 405–409
- 3 S.K. Kwon, S.S. Yun, H.J. Kim, D.S. Lee; The risk factor of early recurrence after hepatectomy in hepatocellular carcinoma; Ann Surg Treat Res, 86 (2014), pp. 283–288
- 4 C.C. Yeh, J.T. Lin, L.B. Jeng, et al.; Nonsteroidal anti-inflammatory drugs are associated with reduced risk of early hepatocellular carcinoma recurrence after curative liver resection: a nationwide cohort study; Ann Surg, 261 (2015), pp. 521–526
- 5 H.Y. Shi, S.N. Wang, S.C. Wang, S.C. Chuang, C.M. Chen, K.T. Lee; Preoperative transarterial chemoembolization and resection for hepatocellular carcinoma: a nationwide Taiwan database analysis of long-term outcome predictors; J Surg Oncol, 109 (2014), pp. 487–493
- 6 Z. Wang, G. Zhang, J. Wu, M. Jia; Adjuvant therapy for hepatocellular carcinoma: current situation and prospect; Drug Discov Ther, 7 (2013), pp. 137–143
- 7 Y. Shimada, T. Hamaguchi, J. Mizusawa, et al.; Randomized phase III trial of adjuvant chemotherapy with oral uracil and tegafur plus leucovorin versus intravenous fluorouracil and levofolinate in patients with stage III colorectal cancer who have undergone Japanese D2/D3 lymph node dissection: final results of JCOG0205; Eur J Cancer, 50 (2014), pp. 2231–2240
- 8 T. Miyoshi, K. Kondo, H. Toba, et al.; Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase expression in tumor tissue, regarding the efficacy of postoperatively administered UFT (tegafur+uracil) in patients with non-small cell lung cancer; Anticancer Res, 27 (2007), pp. 2641–2648
- 9 T. Ishikawa, T. Ichida, Y. Ishimoto, et al.; Complete remission of multiple hepatocellular carcinomas associated with hepatitis C virus-related, decompensated liver cirrhosis by oral administration of enteric-coated tegafur/uracil; Am J Gastroenterol, 94 (1999), pp. 1682–1685
- 10 T. Ishikawa, T. Ichida, Y. Sugitani, et al.; Improved survival with oral administration of enteric-coated tegafur/uracil for advanced stage IV-A hepatocellular carcinoma; J Gastroenterol Hepatol, 16 (2001), pp. 452–459
- 11 T. Takenoue, H. Nagawa, K. Matsuda, et al.; Relation between thymidylate synthase expression and survival in colon carcinoma, and determination of appropriate application of 5-fluorouracil by immunohistochemical method; Ann Surg Oncol, 7 (2000), pp. 193–198
- 12 H. Zhao, Y. Zhao, Y. Guo, et al.; Clinical significance of the thymidylate synthase, dihydropyrimidine dehydrogenase, and thymidine phosphorylase mRNA expression in hepatocellular carcinoma patients receiving 5-fluorouracil-based transarterial chemoembolization treatment; Onco Targets Ther, 6 (2013), pp. 811–818
- 13 Z.H. Lu, R. Zhang, R.B. Diasio; Purification and characterization of dihydropyrimidine dehydrogenase from human liver; J Biol Chem, 267 (1992), pp. 17102–17109
- 14 W. Ichikawa, H. Uetake, Y. Shirota, et al.; Combination of dihydropyrimidine dehydrogenase and thymidylate synthase gene expression in primary tumors as predictive parameters for the efficacy of fluoropyrimidine-based chemotherapy for metastatic colorectal cancer; Clin Cancer Res, 9 (2003), pp. 786–791
- 15 L.H. Li, H. Dong, F. Zhao, et al.; The upregulation of dihydropyrimidine dehydrogenase in liver is involved in acquired resistance to 5-fluorouracil; Eur J Cancer, 49 (2013), pp. 1752–1760
- 16 H. Fujiwara, M. Terashima, T. Irinoda, et al.; Quantitative measurement of thymidylate synthase and dihydropyrimidine dehydrogenase mRNA level in gastric cancer by real-time RT-PCR; Jpn J Cancer Res, 93 (2002), pp. 1342–1350
- 17 D.R. Taves; Minimization: a new method of assigning patients to treatment and control groups; Clin Pharmacol Ther, 15 (1974), pp. 443–453
- 18 K. Hasegawa, T. Takayama, M. Ijichi, et al.; Uracil-tegafur as an adjuvant for hepatocellular carcinoma: randomized trial; Hepatology, 44 (2006), pp. 891–895
- 19 Y. Fukui, T. Oka, S. Nagayama, et al.; Thymidylate synthase, dihydropyrimidine dehydrogenase, orotate phosphoribosyltransferase mRNA and protein expression levels in solid tumors in large scale population analysis; Int J Mol Med, 22 (2008), pp. 709–716
- 20 G.J. Peters, C.L. Van der Wilt, B. van Triest, et al.; Thymidylate synthase and drug resistance; Eur J Cancer, 31 (1995), pp. 1299–1305
- 21 P.G. Johonston, E.R. Fisher, H.E. Rockette, et al.; The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer; J Clin Oncol, 12 (1994), pp. 2640–2647
- 22 A. Beck, M.C. Etienne, S. Cheradame, et al.; A role of dihydropyrimidine dehydrogenase and thymidylate synthase in tumor sensitivity to fluorouracil; Eur J Cancer, 30 (1994), pp. 1517–1522
- 23 M. Kornmann, K.H. Link, H.J. Lentz, et al.; Thymidylate synthase is a predictor for response and resistance in hepatic artery infusion chemotherapy; Cancer Lett, 118 (1997), pp. 29–35
- 24 M.C. Etienne, S. Cheradame, J.L. Fischel, et al.; Response to fluorouracil therapy in cancer patients. The role of tumoral dihydropyrimidine dehydrogenase activity; J Clin Oncol, 13 (1995), pp. 1663–1670
- 25 Y. Mizutani, H. Wada, O. Yoshida, et al.; Significance of thymidylate synthase activity in renal cell carcinoma; Clin Cancer Res, 9 (2003), pp. 1453–1460
- 26 M. Donada, S. Bonin, E. Nardon, et al.; Thymidylate synthase expression predicts longer survival in patients with stage II colon cancer treated with 5-flurouracil independently of microsatellite instability; J Cancer Res Clin Oncol, 137 (2011), pp. 201–210
- 27 Z. Lu, R. Zhang, R.B. Diasio; Population characteristics of hepatic dihydropyrimidine dehydrogenase activity, a key metabolic enzyme in 5-fluorouracil chemotherapy; Clin Pharmacol Ther, 58 (1995), pp. 512–522
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?