Summary
Objective
Carcinoid tumors of the lung are rare, and account for 1% of all primary tumors of the lung. This study was undertaken to investigate the histological characteristics and clinical behavior of carcinoid tumors of the lung.
Methods
We have retrospectively reviewed the hospital records of 11 consecutive patients undergoing surgical treatment for carcinoid tumors of the lung between 1992 and 2007.
Results
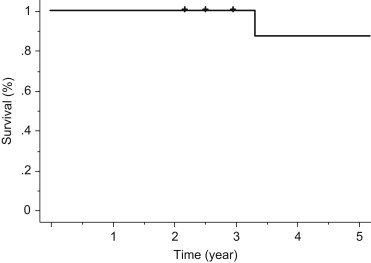
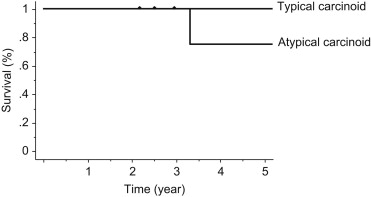
Patients with carcinoid tumors accounted for 0.8% (11 of 1319) of the patients undergoing surgical treatment for nonsmall cell lung cancer. The group comprised six males and five females with a mean age at presentation of 58.6 years (range 27–78 years). All of the operations were lobectomies, including two sleeve lobectomies. Six patients had typical and five had atypical carcinoid tumors. Seven patients had stage IA disease, two had stage IB, one had stage IIA, and one had stage IIIA. Recurrent tumors developed in two of the five patients affected by atypical carcinoid tumors, but none of the six patients with typical carcinoid tumors. Overall, the 5-year survival rate of patients with both typical and atypical carcinoid tumors was 90.9%.
Conclusion
Survival of carcinoid tumors was favorable. In this analysis, two patients with atypical carcinoid had postoperative recurrences. Recurrence was more common among patients with atypical carcinoid tumors.
Keywords
carcinoid tumor;lung cancer;surgery
1. Introduction
The distinction between typical and atypical carcinoid tumors was first described by Engelbreth-Holm1; the histological criteria for this distinction were later established by Arrigoni et al.2 The initial classification of these tumors established by the World Health Organization (WHO)3 in 1982 has been amended various times. As a result of clinical and prognostic disputes, new histological criteria proposed by Travis et al4 to separate typical and atypical carcinoid tumors have recently been considered and accepted by the WHO and the International Association for the Study of Lung Cancer (IASLC) in the 1999 classification of lung tumors.5 At present, the investigation of these pathological processes centers on the causes of their specific differentiation, behavior, and treatment options.
We review our experience with 11 patients and investigated the histological characteristics and clinical behavior of carcinoid tumors of the lung.
2. Patients and methods
2.1. Patients
We retrospectively reviewed our experience with the surgical treatment of both typical and atypical carcinoid tumors of the lung. Eleven patients who underwent thoracotomy between 1992 and 2007 at the Kitakyushu Municipal Medical Center, Fukuoka, and had a final histologic diagnosis of a carcinoid tumor of the lung were analyzed. Thirty-day mortality was defined as a fatality within 30 days after pulmonary resection, and in-hospital mortality (not including 30-day mortality) was defined as a fatality occurring at any time during the postoperative hospital stay.
Tumors were classified according to the current WHO/IASLC criteria for neuroendocrine tumors.6 Typical carcinoids are defined as tumors greater than 5 mm in diameter, with carcinoid morphology and less than two mitoses per 2 mm2, and lacking necrosis. Tumors with a mitosis rate of 2 or more but less than 10 per 2 mm2, with or without necrosis, were classified as atypical carcinoids. The final pathological stage was classified according to the general rules for the clinical and pathological record of lung cancer (the seventh edition).7 A complete resection, classified as R0, was defined as the pathological demonstration of negative tissue margins and assessment by the surgeon that all detectable diseases had been removed.
2.2. Patient follow-up
The records of the patients were reviewed for preoperative symptoms, surgical procedures performed, and postoperative complications. The patients were followed up at 1- or 3-month intervals for 5 years or more. Physical examinations, routine hematological analyses, chest radiographies, computed tomography (CT) scans of the chest and upper abdomen, magnetic resonance images of the brain, and bone scintigraphy were performed for evaluations of recurrence.
The survival rates were calculated using the Kaplan–Meier method, and the significance of the differences in these rates was evaluated with the log-rank test.
3. Results
Patients with carcinoid tumors accounted for 0.8% (11 of 1319) of the patients undergoing surgical treatment for nonsmall cell lung cancer. The patients' characteristics are presented in Table 1. The group comprised six males (55%) and five females (45%) with a mean age at presentation of 58.6 years (range 27–78 years). Symptoms were present in four (36%) patients and included cough (n = 3) and hemoptysis (n = 1). Seven (64%) patients were asymptomatic at presentation. Carcinoid syndrome was not observed in any of the patients. The tumor was in the right lung in seven patients and in the left lung in four. Seven tumors were peripherally located. The other four tumors were centrally located. All operations performed were lobectomies including two sleeve lobectomies. All patients had R0 resections. Six patients (55%) had typical and five (45%) had atypical carcinoid tumors ( Table 2). Seven patients had pathological stage IA disease, two had stage IB, one had stage IIA, and one had stage IIIA. The overall 5-year survival of patients with both typical and atypical carcinoid tumors of the lung was 90.9% (Fig. 1). Both the 30-day and in-hospital mortality were 0%. Recurrent tumors developed in two of the five patients affected by atypical carcinoid, but none of the six patients with typical carcinoid tumors (Fig. 2). One patient developed mediastinal lymph node metastasis at 20 months after the operation, and she underwent radiotherapy to the mediastinum (60 Gy). Brain metastasis subsequently developed at 25 months and gamma knife therapy was performed. However, the patient was found to have lung metastasis and pleuritis carcinomatosa at 28 months, and died at 40 months postoperatively. Another patient is still alive 30 months postoperatively despite liver metastasis. He suffered from a cerebral infarction after being the discharged and thereafter was followed without any particular treatment.
| No. of patients (%) | ||
|---|---|---|
| Gender | Male | 5 (45) |
| Female | 6 (55) | |
| Age (y) | 27–78 | (mean = 58.6) |
| Symptom | Cough | 3 (27) |
| Hemosputum | 1 (9) | |
| Asymptomatic | 7 (64) | |
| Location | Rt upper lobe | 5 (45) |
| Rt middle lobe | 1 (9) | |
| Rt lower lobe | 1 (9) | |
| Lt upper lobe | 4 (36) | |
| Lt lower lobe | 0 | |
| Central type | 4 (36) | |
| Peripheral type | 7 (64) | |
| Histology | Typical carcinoid | 6 (55) |
| Atypical carcinoid | 5 (45) | |
| Procedure | Lobectomya | 11 (100) |
| Curability | Complete resection | 11 (100) |
| Incomplete resection | 0 (0) | |
| p T stage | T1 | 8 (73) |
| T2 | 3 (27) | |
| p N stage | N0 | 9 (82) |
| N1 | 1 (9) | |
| N2 | 1 (9) | |
| p stage IA | IA | 7 (64) |
| IB | 2 (18) | |
| IIA | 1 (9) | |
| IIIA | 1 (9) | |
| Total | 11 |
Lt = left; Rt = right.
a. Including two sleeve lobectomies.
| Case | Age | Gender | Symptoms | Final histology | TNM | Stage | Operation | Tumor size | Location (mm) | Duration of observation (d) | Recurrence | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | F | (–) | Typical | T1aN0M0 | IA | LUL | 15 | Central | 2066 | (–) | Alive |
| 2 | 43 | M | (–) | Typical | T1aN0M0 | IA | RUL | 13 | Peripheral | 804 | (–) | Alive |
| 3 | 47 | M | Cough | Typical | T1aN0M0 | IA | RLL | 20 | Peripheral | 3723 | (–) | Alive |
| 4 | 54 | F | (–) | Typical | T1aN0M0 | IA | LUL | 11 | Peripheral | 1089 | (–) | Alive |
| 5 | 61 | M | (–) | Typical | T1bN0M0 | IA | RML | 27 | Central | 2452 | (–) | Alive |
| 6 | 70 | F | Cough | Typical | T2aN0M0 | IB | LUSL | 10 | Central | 2297 | (–) | Alive |
| 7 | 59 | M | (–) | Atypical | T1aN1M0 | IIA | RUL | 19 | Peripheral | 2158 | (–) | Alive |
| 8 | 63 | F | (–) | Atypical | T1aN0M0 | IA | LUL | 11 | Peripheral | 4894 | (–) | Alive |
| 9 | 69 | F | Hemosputum | Atypical | T1aN0M0 | IA | RUSL | 6 | Central | 2194 | (–) | Alive |
| 10 | 74 | F | (–) | Atypical | T2aN2M0 | IIIA | RUL | 24 | Peripheral | 1214 | Brain, lung, mediastinal | Dead |
| 11 | 78 | M | Cough | Atypical | T2aN0M0 | IB | RUL | 20 | Peripheral | 926 | liver | Alive |
RUL: right upper lobectomy; LUL: left upper lobectomy; RUSL: right upper sleeve lobectomy; RLL: right lower lobectomy; RML: right middle lobectomy; LUSL: left upper sleeve lobectomy.
|
|
|
Figure 1. Survival curve of operation for carcinoid tumors. Hash marks represent censored patients. |
|
|
|
Figure 2. Survival curve of operation for typical and atypical carcinoid tumors. Hash marks represent censored patients. |
4. Discussion
Carcinoid tumors of the lung comprise 1–2% of all lung malignancies. Historically called bronchial adenomas, they were thought to be benign tumors.8; 9 ; 10 The recognition of a more progressive variety of carcinoid tumors, the atypical carcinoid tumors, suggested that they might all be malignant.2; 4; 11; 12 ; 13 Currently, typical and atypical carcinoids are considered to be part of a spectrum of malignant neoplasms with neuroendocrine differentiation along with large cell neuroendocrine carcinoma and small cell carcinoma.4; 5 ; 12
In general, patients with typical carcinoid tumors have good prognoses, with >87% of patients surviving for 10 years. In contrast, approximately 25–69% of patients with atypical carcinoid tumors survive for 5 years, and many develop widespread disease.2; 4; 10; 13; 14; 15; 16; 17; 18; 19 ; 20 Multivariate analyses from several studies have suggested that the pathological stage and atypical histology are the most important factors affecting survival.4; 13; 15 ; 18 By contrast, according to the data of 661 patients with typical or atypical tumors of the lung, no difference in survival was observed between typical and atypical carcinoid in pathological stage IA, although a difference was found in stage IB. However, in a multivariate analysis, a factor of typical or atypical carcinoid tumors of the lung is one of the significant factors influencing survival,21 and surgical treatment for early-stage atypical carcinoid tumors might provide a better outcome. In this study, postoperative recurrence and tumor-related death were seen for atypical carcinoid tumors but not for typical carcinoid tumors. However, two of five atypical carcinoid tumors of the lung were pathological stage IA, and both patients had no postoperative recurrence.
The radiological studies in our patients showed a predominance of peripheral nodules (64%). Central nodules were seen in 36% of the patients. Choplin et al22 reported that a round or ovoid, lobulated peripheral mass was the most common radiologic presenting feature of atypical carcinoid tumors (80%). Wilkins et al23 revealed that 60% of their patients with peripheral bronchial carcinoid tumors had atypical carcinoid tumors. Our data showed that 57% of peripheral carcinoid tumors of the lung were atypical carcinoid tumors. Forster et al24 suggested that tumor size may be a prognostic factor for the differentiation of neuroendocrine lung tumors. They concluded that any tumor measuring more than 2.5 cm on a CT scan is more likely to be an atypical carcinoid tumor than a typical carcinoid tumor.
The assessment of the biological behavior of bronchial carcinoid tumors is not always accurate. Atypical carcinoid tumors present more often at a more advanced stage, are more likely to recur, and are associated with lower 5- and 10-year survival rates than typical carcinoid tumors. These findings reveal the need for more accurate differentiation between histological subtypes. Strict application criteria are mandatory to allow for distinction between the typical and atypical subtypes, which could influence surgical management.
It has been reported that the appropriate surgical management of bronchial carcinoid tumors is suggested by their recurrence and survival patterns.4; 19; 25; 26; 27; 28; 29 ; 30 Limited resection such as wedge resection or segmentectomy for peripheral tumors and isolated bronchial sleeve resection or sleeve lobectomy for central tumors should be considered when feasible for early-stage typical bronchial carcinoid tumors because local recurrence is unlikely and survival is excellent. We did not investigate the use of endobronchial laser therapy for localized central typical carcinoids. Because of the excellent results of standard surgical therapy for such tumors and the risk of local recurrence when laser modalities are used, we favor resection except in patients who are deemed to be at excessive risk.
Long-term survival and local recurrence are both unfavorably affected by the finding of atypical histology. We think a more aggressive surgical approach including formal lobectomy (or pneumonectomy when indicated) and lymph node dissection should be performed when this histology is identified. The potential benefits of postoperative adjuvant chemotherapy and/or radiation therapy also should be considered, especially for atypical carcinoid tumors.
5. Conclusion
This study suggested that survival of carcinoid tumors of the lung was favorable. Atypical carcinoid tumors showed a more malignant potential in their clinical behavior.
References
- 1 J. Engelbreth-Holm; Benign bronchial adenomas; Acta Chir Scand, 90 (1944), pp. 383–409
- 2 M.G. Arrigoni, L.B. Woolner, P.E. Bernatz; Atypical carcinoid tumor of the lung; Thorac Cardiovasc Surg, 64 (1972), pp. 413–421
- 3 The World Health Organization Histological Typing of Lung Tumors. 2nd ed. Am J Clin Pathol. 1982;vol. 77:123–136.
- 4 W.D. Travis, W. Rush, D.B. Flieder, et al.; Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid; Am J Surg Pathol, 22 (1998), pp. 934–944
- 5 W.D. Travis, L.H. Sobin; Histologic Typing of Lung and Pleural Tumours; International Histologic Classification of Tumours (No. 1); Springer-Verlag, New York, NY (1999)
- 6 W.D. Travis, B. Corrin, Y. Shimosato, E. Brambilla; The Histological Typing of Lung and Pleural Tumours. WHO/IASLC Classification of Lung and Pleural Tumours; (3rd ed.)Springer Verlag, Berlin (1999)
- 7 The Japan Lung Cancer Society. General Rule for Clinical and Pathological Record of Lung Cancer. 7th ed.
- 8 D.G. Davila, W.F. Dunn, H.D. Tazelaar, P.C. Pairolero; Bronchial carcinoid tumors; Mayo Clin Proc, 68 (1993), pp. 795–803
- 9 D.H. Harpole Jr., J.M. Feldman, S. Buchanan, W.G. Young, W.G. Wolfe; Bronchial carcinoid tumors: a retrospective analysis of 126 patients; Ann Thorac Surg, 54 (1992), pp. 50–54
- 10 M. Torre, M. Barberis, B. Barbieri, E. Bonacina, P. Belloni; Typical and atypical bronchial carcinoids; Respir Med, 83 (1989), pp. 305–308
- 11 J. Engelbreth-Holm; Bengn bronchial adenomas; Acta Chir Scand, 90 (1944), pp. 383–421
- 12 W.D. Travis, R.I. Linnoila, M.G. Tsokos, et al.; Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases; Am J Surg Pathol, 15 (1991), pp. 529–553
- 13 P.M. Gould, J.A. Bonner, T.E. Sawyer, C. Deschamps, C.M. Lange, H. Li; Bronchial carcinoid tumors: importance of prognostic factors that influence patterns of recurrence and overall survival; Radiology, 208 (1998), pp. 181–185
- 14 S.E. Mills, P.H. Cooper, A.N. Walker, I.L. Kron; Atypical carcinoid tumor of the lung. A clinicopathologic study of 17 cases; Am J Surg Pathol, 6 (1982), pp. 643–654
- 15 B.C. McCaughan, N. Martini, M.S. Bains; Bronchial carcinoids. Review of 124 cases; J Thorac Cardiovasc Surg, 89 (1985), pp. 8–17
- 16 T. Akiba, T. Naruke, H. Kondo, et al.; Carcinoid tumor of the lung: clinicopathological study of 32 cases; Jpn J Clin Oncol, 22 (1992), pp. 92–95
- 17 S. Attar, J.E. Miller, J. Hankins, et al.; Bronchial adenoma: a review of 51 patients; Ann Thorac Surg, 40 (1985), pp. 126–132
- 18 D.H. Harpole Jr., J.M. Feldman, S. Buchanan, W.G. Young, W.G. Wolfe; Bronchial carcinoid tumors: a retrospective analysis of 126 patients; Ann Thorac Surg, 54 (1992), pp. 50–54 discussion 54–55
- 19 A.J. Schreurs, C.J. Westermann, J.M. van den Bosch, et al.; A twenty-five-year follow-up of ninety-three resected typical carcinoid tumors of the lung; J Thorac Cardiovasc Surg, 104 (1992), pp. 1470–1475
- 20 P. Vadasz, G. Palffy, M. Egervary, Z. Schaff; Diagnosis and treatment of bronchial carcinoid tumors: clinical and pathological review of 120 operated patients; Eur J Cardiothorac Surg, 7 (1993), pp. 8–11
- 21 M. García-Yuste, M.J. Matilla, A. Cueto, et al.; Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung for the Spanish Society of Pneumonology and Thoracic Surgery (EMETNE-SEPAR). Typical and atypical carcinoid tumours: analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung; Eur J Cardiothorac Surg, 31 (2007), pp. 192–197
- 22 R.H. Choplin, E.H. Kawamoto, R.B. Dyer, K.R. Geisinger, S.E. Mills, T.L. Pope; Atypical carcinoid of the lung: radiographic features; AJR Am J Roentgenol, 146 (1986), pp. 665–668
- 23 E.W. Wilkins Jr., H.C. Grillo, A.C. Moncure, J.G. Scannell; Changing times in surgical management of bronchopulmonary carcinoid tumor; Ann Thorac Surg, 38 (1984), pp. 339–344
- 24 B.B. Forster, N.L. Müller, R.R. Miller, B. Nelems, K.G. Evans; Neuroendocrine carcinomas of the lung: clinical, radiologic, and pathologic correlation; Radiology, 170 (1989), pp. 441–445
- 25 R. Hurt, M. Bate; Carcinoid tumours of the bronchus: a 33 year experience; Thorax, 39 (1984), pp. 617–623
- 26 G. Stamatis, L. Freitag, D. Greschuchna; Limited and radical resection for tracheal and bronchopulmonary carcinoid tumour; Eur J Cardiothorac Surg, 4 (1994), pp. 527–532
- 27 C.-H. Marty-Ane, V. Costes, J.-L. Pujol, M. Alauzen, P. Baldet, H. Mary; Carcinoid tumors of the lung: do atypical features require aggressive management?; Ann Thorac Surg, 59 (1995), pp. 78–83
- 28 T.S. Chughtai, J.E. Morin, N.M. Sherner, J.A. Wilson, D.S. Mulder; Bronchial carcinoid – twenty years' experience defines a selective surgical approach; Surgery, 122 (1997), pp. 801–808
- 29 R. Shah, S. Sabanathan, J. Mearns, J. Richardson, C. Goulden; Carcinoid tumour of the lung; J Cardiovasc Surg, 38 (1997), pp. 187–189
- 30 X. Ducrocq, P. Thomas, G. Massard, et al.; Operative risk and prognostic factors of typical bronchial carcinoid tumors; Ann Thorac Surg, 65 (1998), pp. 1410–1414
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?