Summary
Catamenial pneumothorax (CP) is a rare entity of spontaneous, recurring pneumothorax in females. Although it has been known to be associated with thoracic endometriosis, varying clinical course and the lack of consistent intraoperative findings have led to conflicting etiological theories. We herein discuss the etiology, clinical course, and surgical treatment of three patients with CP. Three females (aged 40 years, 28 years, and 34 years) had recurrent right-sided spontaneous pneumothoraces that coincided with their menses. They had undergone video-assisted thoracoscopic surgery (VATS) previously. Blueberry spots in the right diaphragm were detected in all three cases. Two patients had recurrence, postoperatively. The other patient, who received luteinizing hormone-releasing hormone analog therapy for an abdominal endometriosis in the perioperative period and postoperative chemical pleurodesis to prevent recurrence, has been free of recurrence for 15 months, postoperatively. However, pelvic endometriosis was detected in this patient only. Therefore, CP should be suspected in ovulating females with spontaneous pneumothorax, even in the absence of any symptoms associated with pelvic endometriosis. In addition, while performing VATS, careful inspection of the diaphragmatic surface is important. In complicated cases, hormonal suppression therapy and chemical pleurodesis might also be helpful adjunct modalities.
Keywords
catamenial pneumothorax;pneumothorax;surgical treatment
1. Introduction
Catamenial pneumothorax (CP) is a type of spontaneous recurring pneumothorax that coincides with the menses in females. The first report on CP was published in 1958,1 and since then it has always been considered as a usual condition for various reasons, especially due to its high recurrence rate.2 In the past decade, the accuracy of detecting CP has improved and subsequently more number of cases are being reported, comprising almost one-third of all spontaneous pneumothorax cases in females.3 ; 4
The CP is generally considered to be the most frequent presentation of thoracic endometriosis syndrome, although histological findings of endometriosis during surgery are rare.5 The etiology of CP is most likely to be multifactorial in origin, involving a combination of different mechanisms.6; 7 ; 8
The optimal management of CP also remains unclear, as the traditional treatments for pneumothorax are often unsuccessful. The purpose of this study is to report three typical cases of CP and to discuss the pathogenesis of CP.
2. Case reports
We performed a retrospective review from January 2001 to December 2011 of all female patients undergoing surgery for spontaneous pneumothorax at the Kitakyushu Municipal Medical Center (Fukuoka, Japan). Fourteen patients with recurrent spontaneous pneumothorax received surgical treatment. Of these, three (21.4%) were classified as having CP. The diagnosis of CP was made based on preoperative symptoms that coincided with their menses as well as on the intraoperative pathological findings.
2.1. Case 1
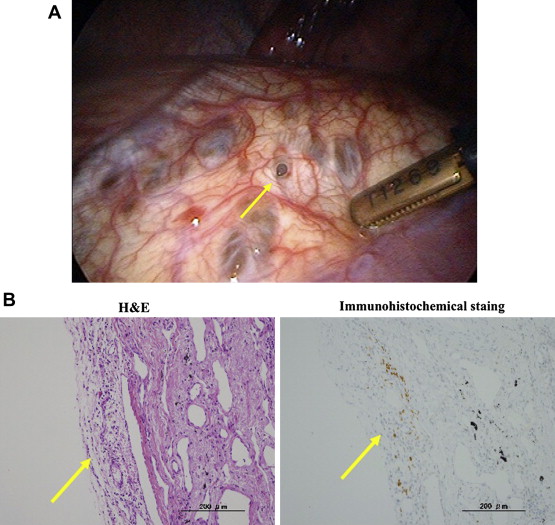
In December 2008, a healthy 40-year-old female had a right-sided spontaneous pneumothorax and was treated with a thoracic tube. Because of a recurrence after conservative treatment, video-assisted thoracoscopic surgery (VATS) was performed. We noted a fenestration-like lesion in the tendinous part of the diaphragm (Fig. 1A) and a subpleural bulla in the apex of the right lung. A partial resection of the diaphragm and a bullectomy were performed. A histological examination of the resected tissues confirmed an endometrial implant in the bulla, but not in the diaphragm. The fenestration-like lesion had no fenestration microscopically, and only some hemosiderin-laden macrophages were observed. Immunohistochemical staining was positive for the estrogen receptor within the stroma of the endometrial implant in the bulla (Fig. 1B). The patient had a recurrence that coincided with her menses in February 2009. Chemical pleurodesis using OK432 was performed twice after thoracic drainage. After recovery, the patient has been symptom-free for 47 months.
|
|
|
Figure 1. (A) Intraoperative findings of a perforation-like lesion in the tendinous part of the diaphragm. (B) A focus of an endometrial implant of the visceral pleura. Immunohistochemical analysis showed strong nuclear staining for the estrogen receptor (100×). H&E = hematoxylin and eosin. |
2.2. Case 2
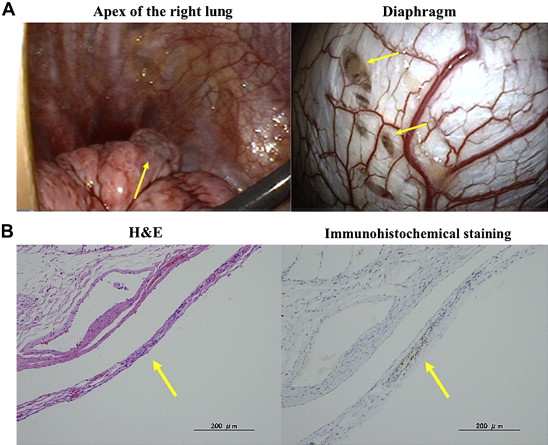
In January 2009, a healthy 28-year-old female had a right-sided spontaneous pneumothorax and was treated with a thoracic tube. Because of recurrence after conservative treatment, VATS was performed. We noted several blueberry spot-like lesions without fenestration in the tendinous part of the diaphragm (Fig. 2A) and a whitish subpleural change in the apex of the right lung. A partial resection of the diaphragm and apical resection were performed. The histological examination confirmed an endometrial implant in the subpleural change, but not in the diaphragm. The blueberry spot-like lesions had only some hemosiderin-laden macrophages. Immunohistochemical staining was positive for the estrogen receptor within the stroma of the endometrial implant in the regions of subpleural change (Fig. 2B). The patient had a recurrence that coincided with her menses in March 2009. Chemical pleurodesis using OK432 was performed once after the thoracic drainage. After recovery, the patient has been symptom-free for 35 months.
|
|
|
Figure 2. (A) Intraoperative findings of blueberry spot-like lesions in the tendinous part of the diaphragm. (B) A focus of an endometrial implant of the visceral pleura. Immunohistochemical analysis showed strong nuclear staining for the estrogen receptor (100×). H&E = hematoxylin and eosin. |
2.3. Case 3
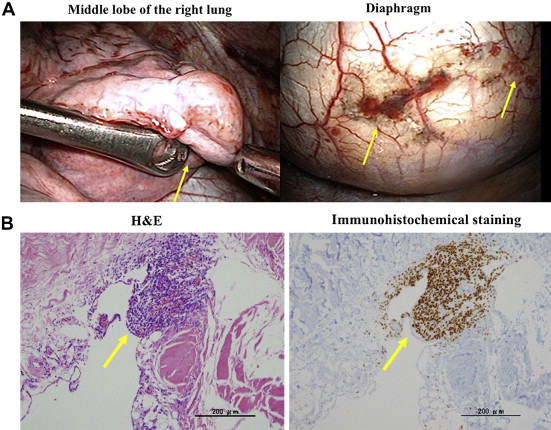
In June 2011, a 34-year-old female receiving luteinizing hormone-releasing hormone analog therapy for abdominal endometriosis had a right-sided spontaneous pneumothorax and was treated with a thoracic tube. Because of recurrence after conservative treatment, VATS was performed. We noted several blueberry spots without fenestration in the tendinous part of the diaphragm (Fig. 3A) and a whitish subpleural change and atelectasis in the middle lobe of the right lung. A partial resection of the diaphragm and the middle lobe was performed. A histological examination confirmed an endometrial implant, where stromal cells constantly admixed with extravasated erythrocytes, and hemosiderin-laden macrophages were observed in the diaphragm without fenestration, but not in the region of subpleural change. Immunohistochemical staining was positive for the estrogen receptor within the stroma of the endometrial implant in the diaphragm (Fig. 3B). Postoperatively, chemical pleurodesis using OK432 was performed once to prevent recurrence. After recovery, the luteinizing hormone-releasing hormone analog therapy was continued, and the patient has been symptom-free for 15 months.
|
|
|
Figure 3. (A) The intraoperative findings of diaphragmatic endometriosis. (B) A focus of an endometrial implant in the tendinous part of the diaphragm. Immunohistochemical analysis showed strong nuclear staining for the estrogen receptor (100×). |
The characteristics of the three patients are presented in Table 1. There were no complications associated with these treatments. The mean duration of thoracic tube drainage was 3.3 days (range: 2–4 days). Hormonal treatment was performed in only one patient, who had already been receiving luteinizing hormone-releasing hormone analog therapy prior to the surgery. Pelvic endometriosis was also detected only in this patient. The others received a gynecological evaluation postoperatively, but pelvic endometriosis was not detected.
| Case | Age (y) | Pelvic endometriosis | Pathological findings | Surgical treatment | Chest tube drainage (d) | Hormonal treatment | Recurrence | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | No | Visceral pleural implant | ED, PL | 2 | No | Yes | 47 |
| 2 | 28 | No | Visceral pleural implant | ED, PL | 2 | No | Yes | 35 |
| 3 | 34 | Yes | Diaphragmatic implant | ED, PL, P | 4 | Yes | No | 15 |
ED = excision of diaphragm; P = pleurodesis; PL = partial lung resection.
3. Discussion
The CP involves females of reproductive age and occurs within 72 hours from the onset of menses. The right side is involved in most cases (95%), and symptoms often develop in a recurrent manner.5 ; 9 Several hypotheses about the pathogenesis of CP have been put forth, including the following: (1) spontaneous rupture of blebs; (2) alveolar rupture caused by prostaglandin-induced bronchiolar constriction; (3) fenestration of endometrial implants of visceral pleura with subsequent air leakage; and (4) in the absence of the cervical mucus plug, passage of air from the genital tract through congenital or acquired diaphragmatic defects. Thus, it is classically thought that CP is a typical presentation of thoracic endometriosis.5 ; 9 By contrast, CP can also occur in the presence of diaphragmatic or thoracic endometriosis.10
Endometriosis seems to play the most important role in the development of CP, although an association with pelvic endometriosis has been reported in 20–70% of patients.4 In our experience, one of three patients with CP had pelvic endometriosis. It seems that diaphragmatic defects are strictly correlated with endometriosis. Diaphragmatic defects have been detected in 29–66% of CP patients.4 ; 7 In a previous study, Marshall et al11 reported concomitant diaphragmatic defects and endometrial implants, but the association was not widely observed. In our case series, one patient had histological diaphragmatic implantation.
The exact pathogenesis of thoracic endometriosis remains controversial. The coelomic metaplasia theory has been favored for many years; however, it cannot explain the possible intrapulmonary localization of the disease or its right-side predominance.5 ; 12 Another theory has suggested that the pathogenesis results from the transplantation of endometrium to ectopic sites after lymphatic or vascular embolization or retrograde menstruation.5 ; 12 This last mechanism could explain the known right-sided predominance of the disease, which was confirmed in our experience. It is well-known that there exists a preferential flow of peritoneal fluids and endometrial tissue from the pelvis along the right paracolic gutter up to the subphrenic space.10 Furthermore, the anatomy of the two upper abdominal quadrants is different. The large, solid, and compressible viscera of the left upper quadrant cannot exert such activity.10
The surgical standard treatment for CP also remains controversial. Although some studies have reported that pleural abrasion alone is necessary for the management of CP,5 others have reported that apical blebs and parenchymal implants should be resected, and diaphragmatic fenestration should be excised or closed.13 Moreover, because of the high recurrence rate, it was reported that an additional procedure to specifically address the risk of basilar recurrence on the diaphragm should be performed.14 ; 15 A polyglactin mesh placed on the diaphragm appears to augment pleurodesis in this difficult area.7 Another option is pleurodesis using OK432 or talc, as we used in Case 3. Although we have so far experienced no recurrence in our patient who underwent postoperative chemical pleurodesis, it is unclear whether the potential risks of pleurodesis outweigh the benefits in this young population. There were endometrial implants in the lungs in Cases 2 and 3. Their resection margins were 2 cm or more, which we regarded as sufficient. Although we could not find any residual endometrial implants macroscopically, we could not explain the causes of their recurrences. Further studies including a large number of patients with a longer follow-up time are thus needed.
It is therefore important to block the hormonal support from the ovary to the existing endometrial tissue, and to prevent further seeding, as part of the treatment. The literature contains a variety of reports on the use of oral contraceptives, progestational drugs, danazol, and gonadotropin-releasing hormone (GnRH) agonists, although controlled trials on the efficacy of these drugs in patients with CP are still lacking.5; 16; 17; 18; 19; 20; 21; 22 ; 23 As no specific drug has so far been shown to be superior to the others, the decision on which to use is influenced by the costs, side effects, duration of treatment, and the patients wish to become pregnant.5 That said, the experience in the past two decades has been the greatest with danazol and GnRH agonists. Among the various medical options available, GnRH agonists have been found to be the most effective in preventing CP recurrence when given for prolonged periods of up to 1 year.22; 24 ; 25 However, according to our experience, we treated only one patient with both estrogen–progesterone treatment and postoperative pleurodesis, and to date she has not demonstrated recurrence.
The endometrial foci of both the stroma and glands in different proportions were often with ecstatic glands and mostly lined by pseudostratified cuboidal to cylindrical epithelial cells. Immunohistochemically, most glands showed cytoplasmic positivity, with broad-spectrum cytokeratin, cytokeratin 7, and BER-ER4 staining, as well as strong nuclear staining for estrogen and progesterone receptors.26 In all of our cases, the stromal cells in the endometrial implants showed intense nuclear staining for the estrogen receptor. Therefore, while it seems to be difficult to diagnose CP in a histological examination using only hematoxylin and eosin staining, it is thought that immunochemical staining for estrogen receptor is effective to confirm the presence of endometrial implantation, because it is often difficult to detect a lesion macroscopically.
In conclusion, when a young female presents with pneumothorax during menses, CP should be suspected, and a careful investigation needs to be carried out to achieve a correct diagnosis. At the time of surgery, the diaphragm should be carefully inspected for any defects and endometrial implantation: all defects should be corrected and all nodules should be resected. Pleurodesis may be helpful to avoid a recurrence in patients with CP. Hormonal treatment also seems to be effective for enhancing the surgical results.
References
- 1 E.R. Maurer, J.A. Schaal, F.L. Mendez Jr.; Chronic recurring spontaneous pneumothorax due to endometriosis of the diaphragm; J Am Med Assoc, 168 (1958), pp. 2013–2014
- 2 M. Alifano, C. Jablonski, H. Hadiri, et al.; Catamenial and noncatamenial, endometriosis-related or nonendometriosis-related pneumothorax referred for surgery; Am J Resp Crit Care Med, 276 (2007), pp. 1048–1053
- 3 M. Alifano, R. Trisolini, A. Cancellieri, J.F. Regnard; Thoracic endometriosis: current knowledge; Ann Thorac Surg, 81 (2006), pp. 761–769
- 4 T. Shiraishi; Catamenial pneumothorax: report of a case and review of the Japanese and non-Japanese literature; Thorac Cardiovasc Surg, 39 (1991), pp. 304–307
- 5 J. Joseph, S.A. Sahn; Thoracic endometriosis syndrome: new observations from an analysis of 110 cases; Am J Med, 100 (1996), pp. 164–170
- 6 S. Korom, H. Canyurt, A. Missbach, et al.; Catamenial pneumothorax revisited: clinical approach and systematic review of the literature; J Thorac Cardiovasc Surg, 128 (2004), pp. 502–508
- 7 P. Bagan, F. Le Pimpec Barthes, J. Assouad, R. Souilamas, M. Riquet; Catamenial pneumothorax: retrospective study of surgical treatment; Ann Thorac Surg, 75 (2003), pp. 378–381
- 8 R.P. Shearin, N.G. Hepper, W.S. Payne; Recurrent spontaneous pneumothorax concurrent with menses; Mayo Clin Proc, 49 (1974), pp. 98–101
- 9 E. Carter, D. Ettensohn; Catamenial pneumothorax; Chest, 98 (1990), pp. 713–716
- 10 P. Kirschner; Porous diaphragm syndromes; Chest Surg Clin N Am, 8 (1998), pp. 449–472
- 11 M.B. Marshall, Z. Ahmed, J.C. Kucharczuk, L.R. Kaiser, J.B. Shrager; Catamenial pneumothorax: optimal hormonal and surgical management; Eur J Cardiothorac Surg, 27 (2005), pp. 662–666
- 12 D.L. Olive, L.B. Schwartz; Endometriosis; N Engl J Med, 328 (1993), pp. 1759–1769
- 13 P. Ciriaco, G. Negri, L. Libretti, et al.; Surgical treatment of catamenial pneumothorax: a single centre experience; Interact Cardiovasc Thorac Surg, 8 (2009), pp. 349–352
- 14 J.D. Martin Jr., A.E. Hauck; Endometriosis in the male; Am Surg, 51 (1985), pp. 426–430
- 15 C. Perrotin, S. Mussot, E. Fadel, A. Chapelier, P. Dartevelle; Catamenial pneumothorax. Failure of videothorascopic treatment; Presse Med, 31 (2002), pp. 402–404 [Article in French]
- 16 Y. Terada, F. Chen, T. Shoji, H. Itoh, H. Wada, S. Hitomi; A case of endobronchial endometriosis treated by subsegmentectomy; Chest, 115 (1999), pp. 1475–1478
- 17 Z. Yu, J.K. Fleischman, H.M. Rahman, A.F. Mesia, F. Rosner; Catamenial hemoptysis and pulmonary endometriosis: a case report; Mt Sinai J Med, 69 (2002), pp. 261–263
- 18 D.L. Elliot, A.F. Barker, L.M. Dixon; Catamenial hemoptysis. New methods of diagnosis and therapy; Chest, 87 (1985), pp. 687–688
- 19 O. Katoh, H. Yamada, Y. Aoki, S. Matsumoto, S. Kudo; Utility of angiograms in patients with catamenial hemoptysis; Chest, 98 (1990), pp. 1296–1297
- 20 B. Hope-Gill, B.V. Prathibha; Catamenial haemoptysis and clomiphene citrate therapy; Thorax, 58 (2003), pp. 89–90
- 21 P.H. Kuo, H.C. Wang, Y.S. Liaw, S.H. Kuo; Bronchoscopic and angiographic findings in tracheobronchial endometriosis; Thorax, 51 (1996), pp. 1060–1061
- 22 H. Slabbynck, M. Laureys, N. Impens, P. De Vroey, W. Schandevyl; Recurring catamenial pneumothorax treated with a Gn-RH analogue; Chest, 100 (1991), p. 851
- 23 I.M. Matalliotakis, A.G. Goumenou, G.E. Koumantakis, M.A. Neonaki, E.E. Koumantakis, A. Arici; Pulmonary endometriosis in a patient with unicornuate uterus and noncommunicating rudimentary horn; Fertil Steril, 78 (2002), pp. 183–185
- 24 H.F. Tripp, L.P. Thomas, J.A. Obney; Current therapy of catamenial pneumothorax; Heart Surg Forum, 1 (1998), pp. 146–149
- 25 H.F. Tripp, J.A. Obney; Consideration of anatomic defects in the etiology of catamenial pneumothorax; J Thorac Cardiovasc Surg, 117 (1999), pp. 632–633
- 26 D.B. Flieder, C.A. Moran, W.D. Travis, M.N. Koss, E.J. Mark; Pleuro-pulmonary endometriosis and pulmonary ectopic deciduosis: a clinicopathologic and immunohistochemical study of 10 cases with emphasis on diagnostic pitfalls; Hum Pathol, 29 (1998), pp. 1495–1503
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?