Summary
Background
We aimed to investigate whether disruption of the circadian rhythm in rotating shift work (RSW) workers would change anorectal motility and cardiac autonomic function. We also determined whether sleep and psychological status (e.g., anxiety and depression) would affect anorectal motility in RSW workers.
Methods
Sixteen RSW workers and 11 control individuals were involved in the study. All study participants underwent anorectal manometry and spectral analysis of heart rate variability. All participants completed three questionnaires: the Pittsburgh Sleep Quality Index (PSQI), the State-Trait Anxiety Inventory (STAI) questionnaire, and the Taiwanese Depression Questionnaire (TDQ).
Results
The RSW workers had a lower threshold volume for maximal urge (p = 0.006) and greater rectal compliance (p = 0.02), compared to the controls. The RSW workers had a greater PSQI score (p = 0.002) and TDQ score (p = 0.003), compared to the controls. The RSW workers had a significantly increased low-frequency power percentage (LF%), compared to the controls (p = 0.03). The RSW workers had a significant correlation between the resting anal sphincter pressure and high-frequency power percentage (HF%; r = –0.62, p = 0.01), and between the R-R interval and the threshold for maximal urge (r = 0.51, p = 0.04). The PSQI score was significantly correlated with the threshold volume for urge (r = 0.55, p = 0.03) and for compliance (r = 0.51, p = 0.04) in the RSW workers.
Conclusion
Rotating shift workers have anorectal dysmotility and cardiac sympathetic hyperactivity. Anorectal dysmotility in RSW workers has a close relationship with cardiac autonomic dysfunction, sleep disturbance, and depression, but not with anxiety.
Keywords
Anorectal motility ; Heart rate variability ; Shift work ; Sleep
Introduction
Disruption of the circadian rhythm in rotating shift work (RSW) workers has an impact on physical and mental health [1] . There is increasing evidence that RSW can lead to a variety of gastrointestinal symptoms [2] , and possibly result in functional bowel disorders [3] . The effect of RSW on gastrointestinal disturbances is significantly associated with the type and duration of shift work [3] ; [4] ; the effect is particularly more severe in people on RSW than other types of shifts [3] . Furthermore, the effect of shift work on gastrointestinal symptoms is associated with sleep disturbances and psychological disorders, which suggests that poor sleep may deteriorate these shift work-induced symptoms [5] ; [6] . However, other studies have demonstrated that the association between irritable bowel syndrome and RSW is independent of sleep disturbance [3] .
Spectral analysis of heart rate variability (HRV) is a sophisticated and noninvasive tool for the identification of autonomic nervous system (ANS) control of the heart. Heart rate variability can be categorized into a high-frequency power component (HF; 0.15–0.40 Hz) and a low-frequency power component (LF; 0.04–0.15 Hz) [7] . The HF component and R-R interval are equivalent to respiratory sinus arrhythmia and are regarded as the vagal control of heart rate; the sympathetic and parasympathetic nerves jointly contribute to LF [8] . The low-frequency power percentage (LF%), high-frequency power percentage (HF%), and the ratio of LF to HF (LF/HF) may reflect sympathovagal balance, whereas LF% and LF/HF also represent sympathetic modulation [9] ; [10] . The physiological explanation for very-low-frequency power (VLF) is less defined [7] . The variance in the HRV represents the parasympathetic effect [11] . Spectral analysis of HRV has shown reliable reproducibility in physiological and pathophysiological studies on cardiac autonomic function [12] ; [13] ; [14] . An earlier study with HRV has shown that shift work is associated with sympathetic hyperactivity [15] . A further study shows that the presence of sympathetic hyperactivity occurs more in people working permanently on night shifts [16] . In addition, we have previously demonstrated that the application of HRV can represent the autonomic control of gastrointestinal functions [17] . The 5-minute HRV has been widely utilized in our previous studies, and is adequate for representing ANS function in clinical studies [12] ; [13] ; [18] ; [19] . By applying anorectal manometry, we have previously demonstrated that rectal compliance was correlated significantly with poor sleep, as determined by the Pittsburgh Sleep Quality Index (PSQI) score, which suggests that poor sleep may predispose previously healthy individuals to constipation [13] ; [20] ; [21] . In this study, we aimed to test the hypothesis that the disruption of the circadian rhythm by RSW would affect anorectal motility and cardiac autonomic function. We also investigated whether sleep and psychological statuses (i.e., anxiety and depression) would predispose an individual to altered anorectal motility relevant to RSW.
Methods
Participants
All study participants provided written informed consent and were interviewed about their work status, general health, and gastrointestinal symptoms before the study. Sixteen RSW workers and 11 control study participants who only worked days participated in this study. The definition of RSW was judged and qualified on the work with rotation between day shift and night shift for at least 3 rotating nights/mo [22] . No study participant had any history of an underlying medical condition, previous gastrointestinal surgery, gastrointestinal symptoms, or clinical conditions that affected HRV. Individuals with poor communication or impaired hearing were excluded. All individuals were enrolled from a community and/or university population by public advertisement. The study protocol was reviewed and approved by the research ethics committee of Buddhist Tzu Chi General Hospital (Hualien, Taiwan). The study recruitment period was between January 2012 and August 2012.
Anorectal manometry
All study participants were instructed to evacuate the rectum and received a Fleets enema before the test. The probe was a 4.5-mm diameter, solid state catheter with multiple pressure transducers (Sandhill Scientific, Inc., Highlands Ranch, CO, USA) and a lumen for balloon inflation. A 5-cm balloon was tied to the distal end of the catheter. The lubricated catheter was introduced into the rectum as the study participants lay on their left side with their hips and knees flexed to 90°. The average resting and squeeze pressures (i.e., maximum and sustained) were recorded by the stationary pull-through technique. The threshold volume for rectoanal inhibitory reflexes (RAIR) was assessed by distending the rectal balloon in progressive 10-mL decrements, starting at 60 mL, until anal sphincter relaxation occurred at a lower volume of distension. Rectal sensation was evaluated using a rectal balloon inflated at an interval of 10 mL until the individual reported the first sensation. The balloon volume was then increased by steps of 30 mL so that the study participants experienced the sensation of the urge to defecate and maximum distension. The threshold volumes for inducing these sensations were recorded. Rectal compliance for each balloon distention was derived from the slope of the volume–pressure curve. Our laboratory has reported normal values for anorectal manometry [23] .
Processing of electrocardiogram signal and analysis of HRV
The detailed procedures for HRV analysis have been previously reported [24] . In brief, an electrocardiogram (ECG) was monitored for 5 minutes in the daytime, while the individual lay quietly and breathed normally. The ECG signals were recorded using an analog-to-digital converter with a sampling rate of 256 Hz. Frequency-domain analysis was performed using a nonparametric method of fast Fourier transformation (FFT). The direct current component was deleted, and a Hamming window was used to attenuate the leakage effect [14] . For each time segment (i.e., 288 seconds, 2048 data points), our algorithm estimated the power spectrum density based on FFT. The power spectrum was subsequently quantified into standard frequency-domain measurements [7] , which include total variance, LF, HF, LF/HF, and LF% [7] . Variance, LF, HF, and LF/HF were logarithmically transformed to correct the skewness of the distribution [14] .
Subjective sleep and psychological assessment
Sleep disturbance was assessed using the PSQI, which has been validated as differentiating “poor” from “good” sleep [25] . The PSQI allows the assessment of sleep disturbances along seven dimensions: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. Each dimension is rated on a four-point scale (0–3, with “3” indicating a more profound effect), which are summed to yield a global score. A global score of >5 indicates “poor sleep.” Levels of anxiety were assessed by applying the State-Trait Anxiety Inventory (STAI) questionnaire [26] . Normal adults without anxiety have scores of <40 [26] . Levels of depression were evaluated by applying the Taiwanese Depression Questionnaire (TDQ) [27] . A global score of >18 suggests the presence of depression.
Statistical analysis
Data were expressed as the mean ± the standard error of the mean (SEM). Statistical comparisons of variables between groups were assessed using Mann–Whitney U test. Differences in categorical variables between groups were determined using the Chi-square test or Fisher’s exact test. Correlations were studied using the Pearson test. The p value was set at 0.05 for all statistical analyses. Statistical analyses were conducted using SPSS statistical software, version 11.0, for Windows (SPSS, Inc, Chicago, IL, USA).
Results
This study included 16 RSW workers and 11 controls. Table 1 summarizes the demographic characteristics of both groups. There were no significant differences between the groups in sex or age. The body mass index was similar between the two groups.
| Variable | Controls (n = 11) | RSW (n = 16) | p |
|---|---|---|---|
| Age (y) | 23.4 (0.5) | 24.4 (0.6) | 0.3 |
| Sex (female, %) | 6 (55) | 8 (50) | 0.56 |
| Height (cm) | 164 (2) | 165 (2) | 0.65 |
| Weight (kg) | 60.7 (0.8) | 60.0 (0.7) | 0.55 |
| BMI (kg/m2 ) | 23.0 (1.0) | 22.2 (0.9) | 0.34 |
Data are expressed as the mean (standard error of the mean) or by the percentage (%).
BMI = body mass index; RSW = rotating shift work.
HRV analysis
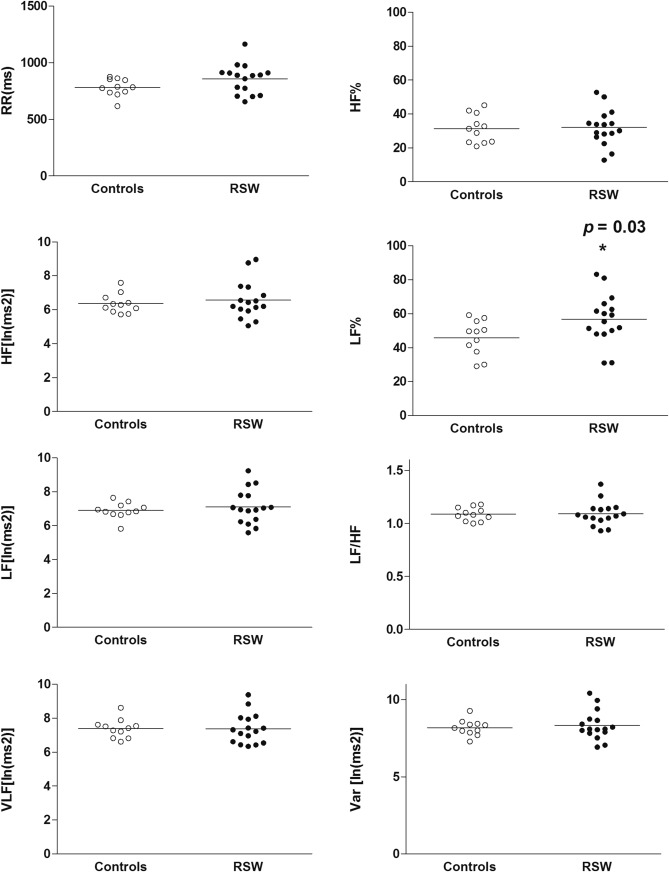
Figure 1 provides the quantitative analysis of the standard parameters of HRV by group. We found that RSW workers had a significantly increased LF%, compared to the controls (p = 0.03). No other HRV parameters showed statistically significant differences between RSW and control participants.
|
|
|
Figure 1. Quantitative analysis of frequency-domain parameters of heart rate variability such as RR, variance in the R-R interval (Var), high-frequency power (HF), normalized HF [HF%; in normalized units (nu)], low-frequency power (LF), normalized LF (LF%; in nu), very-low-frequency power, and the ratio of LF to HF (LF/HF) in the controls and rotating shift work (RSW) workers. Values are expressed as the mean ± the standard error of the mean. *p = 0.03, controls versus RSW workers. HF% = high-frequency power percentage; LF% = low-frequency power percentage; R-R indicate R-R interval = RR interval = the R wave-to-R wave interval = beat-to-beat interval = Inter-beat Interval (IBI). |
Anorectal manometric results
All individuals underwent anorectal manometry without any adverse effects. Anal sphincter pressure did not differ between the controls and the RSW workers (Table 2 ). There was no group difference for the anal sphincter length and the threshold volume for RAIR. The RSW workers had a lower threshold volume for maximal urge (p = 0.006), compared to the controls ( Table 2 ). Rectal compliance was significantly greater in the RSW workers than in the controls (p = 0.02; Table 2 ).
| Variable | Controls (n = 11) | RSW (n = 16) | p |
|---|---|---|---|
| Threshold volume (mL) | |||
| First sensation | 30.5 (4.0) | 40.7 (4.0) | 0.08 |
| Urge | 103.2 (7.9) | 87.2 (3.9) | 0.09 |
| Maximal | 159.1 (7.1) | 126.7 (6.7) | 0.006 |
| RAIR | 20.9 (0.9) | 21.3 (0.9) | 0.79 |
| Anal sphincter pressure (mmHg) | |||
| Resting | 51.9 (3.5) | 54.8 (4.4) | 0.6 |
| Maximal | 60.5 (14.5) | 91.6 (13.9) | 0.14 |
| Sustained squeeze | 1111.6 (14.9) | 145.5 (12.1) | 0.09 |
| Length of anal sphincter (cm) | 2.0 (1.1) | 2.1(1.2) | 0.48 |
| Compliance (mL/mmHg) | 3.2 (0.4) | 5.6 (0.9) | 0.02 |
Data are expressed as the mean (standard error of the mean) or by the percentage (%).
RAIR = rectoanal inhibitory reflex; RSW = rotating shift work.
Sleep disturbances and psychological status
Table 3 shows the results of sleep function, anxiety, and depression for all participants. Sleep disturbance was present more often in the RSW workers (11/16) than in the controls (0/11) (p < 0.001; Table 3 ). The PSQI score was significantly greater in the RSW workers than in the controls (p = 0.002; Table 3 ). Significant depression occurred more often in the RSW workers (5/16) than in the controls (0/11) (p = 0.04; Table 3 ). The RSW workers had significantly greater TDQ scores than the controls (p = 0.003). There was no significant difference in the presence of anxiety between the RSW workers (11/16) and the healthy participants (6/11) (p = 0.69; Table 3 ). In addition, there was no difference in the STAI anxiety scores between the RSW workers and the controls (p = 0.88; Table 3 ).
| Variable | Controls (n = 11) | RSW (n = 16) | p |
|---|---|---|---|
| Sleep disturbance | |||
| Score | 3.3 (0.5) | 5.9 (0.6) | 0.002 |
| Prevalence (%) | 0 | 69 | 0.001 |
| Anxiety | |||
| Score | 40.2 (1.5) | 42.3 (1.2) | 0.88 |
| Prevalence (%) | 55 | 69 | 0.69 |
| Depression | |||
| Score | 7.6 (1.4) | 14.9 (1.7) | 0.003 |
| Prevalence (%) | 0 | 31 | 0.05 |
Data are expressed as the mean (standard error of the mean) or by the percentage (%).
RSW = rotating shift work.
Association of HRV, sleep, and psychosocial status to anorectal manometry in RSW
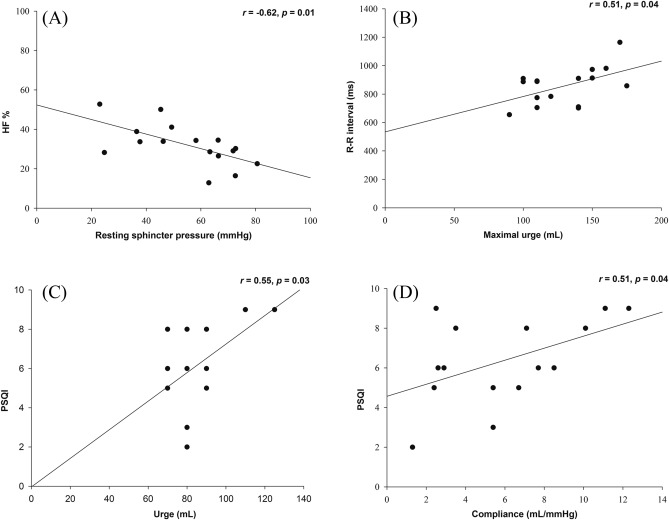
In RSW workers, there was a significant correlation between the resting anal sphincter pressure and HF% (r = –0.62, p = 0.01; Figure 2 A). The R-R interval correlated well with the threshold volume for maximal urge (r = 0.51, p = 0.04; Figure 2 B). There was a lack of correlation between anorectal manometry and psychological status (i.e., depression and anxiety) in RSW workers; despite this, the PSQI was significantly correlated with the threshold volume for urge (r = 0.55, p = 0.03; Figure 2 C) and compliance (r = 0.51, p = 0.04; Figure 2 D).
|
|
|
Figure 2. The correlations between anorectal motility, heart rate variability, and sleep in workers on rotating shift work (RSW). (A) There is a significant correlation between the resting anal sphincter pressure and HF% (r = –0.62, p = 0.01); (B) R-R interval is correlated with the threshold volume for maximal urge (r = 0.51, p = 0.04); (C and D) PSQI score is significantly correlated with the threshold volume for urge (r = 0.55, p = 0.03) and compliance (r = 0.51, p = 0.04). HF% = high-frequency power percentage; LF% = low-frequency power percentage; PSQI = Pittsburg Sleep Quality Index; R-R indicate R-R interval = RR interval = the R wave-to-R wave interval = beat-to-beat interval = Inter-beat Interval (IBI). |
Discussion
Autonomic nervous system regulation of the heart rate, as evidenced by noninvasive measurements of HRV, was applied in this study to detect changes in the ANS functions in RSW workers. In this study, we determined various parameters of HRV in a cohort of RSW workers and healthy controls in whom the differences in autonomic regulation were further investigated. In agreement with other studies [15] ; [28] , we observed a predominant shift in cardiac autonomic regulation toward sympathetic balance, as indicated by a significantly increase in LF%, compared to the healthy controls. Our investigation using a noninvasive method of HRV analysis is able to characterize the changes in the ANS functions in RSW workers, which suggests that such individuals may have a risk for long-term sympathetic hyperactivity.
Anorectal function can be evaluated by anorectal manometry which provides useful information with regard to anorectal physiology [29] ; [30] . The application of anorectal manometry allows the measurement of anal resting pressure, anal squeeze pressure, anal canal length, degree of inhibition of the anal sphincters, rectal sensation, rectal contractility, and the defecation dynamics. The utility of these physiologic results leads to an effective management, either medical or surgical, for defecation disorders such as fecal incontinence and constipation [31] . A limitation of this examination is that patients have difficulty in communicating during the procedure or tolerating the procedure.
In the present study, we investigated whether RSW is associated with an alteration in anorectal functioning. We found that RSW workers were characterized by a lower sensory threshold for anorectal balloon distension because people on RSW had a lower threshold volume for maximal urge, compared to healthy controls. The finding may be related to the fact that visceral perception is enhanced in people with poor sleep [32] , which was also commonly present in our RSW workers. We similarly observed that poor sleep may impair rectal compliance and urge perception by balloon distension inside the rectum. In addition, the association between sleep disturbance and enhanced visceral perception has been pathophysiologically implicated in functional bowel disorders [33] . Therefore, the present finding may support the mechanism that RSW potentially impairs anorectal functioning in healthy adult workers, which may possibly predispose a worker to the exacerbation of functional bowel symptoms that are highly prevalent in RSW [3] ; [6] .
Consistent with the findings of a previous investigation [6] , we observed that sleep disturbance occurred frequently in RSW workers. The explanation for increased sleep disturbance in RSW workers remains unclear. It is well accepted that the circadian rhythm, a self-regulated biological process, has an important role in maintaining normal sleep/wake function [34] . Therefore, it is conceivable that RSW may potentially result in vulnerability to circadian disruption by which a sleep disturbance may arise [35] . Furthermore, we found that RSW workers were more likely to have depression, compared to healthy workers. However, there was no group difference regarding the anxiety status. Our results were partially in agreement with a previous study on rotating shifts nurses, which showed a significant psychological disturbance regarding anxiety and depression [6] . However, the discrepancy in the presence of anxiety may be related to the differences in patient enrollment, the sex of the population studied, and the survey instrument.
In healthy adults, the internal anal sphincter at rest receives tonic discharge from sympathetic nerves [36] , inside of which exist excitatory and inhibitory neurons for mediating the motility of the internal anal sphincter [37] . In this study, we did not directly measure the local neural pathway that modulates anal sphincter function, although we showed a negative correlation between the resting sphincter pressure and HF% (i.e., an index for vagal activity), which suggests that vagal activity in rotating shift workers may potentially participate in the activity of resting anal sphincter pressure that relies on dual contributions from the tonic activities of the internal (75–85%) and external anal sphincters [38] . Therefore, our findings appear to support the evidence of joining activity from the vagal nerve in modulating anorectal motility [39] . In addition, we found a positive correlation between the R-R intervals and the threshold volume for maximal urge, which indicates that rotating shift workers with decreased threshold volume at maximal urge are more likely to have decreased R-R intervals (which is synonymous to increased sympathetic activity, as demonstrated in the RSW workers). With the aforementioned findings taken together, altered cardiac autonomic regulation and the link to changed anorectal function were evident in RSW workers; therefore, our study supports an early notion that anorectal motility is modulated by ANS [40] .
Our results reconfirm the notion that poor sleep may have impact on anorectal function [21] . In RSW workers, we observed that increased sleep dysfunction, as determined by the PSQI, was associated with an increase in rectal compliance and in urge sensation. The first sensation did not achieve statistical significance, although this is likely because of the fact that only urge sensation is more relevant for mediating by visceral sensitization [41] . The rectal sensory data such as rectal sensation and compliance are obtained by using rectal balloon distension [42] . The measurement of rectal compliance together with the maximal tolerated volume may potentially identify factors leading to altered bowel habits and anorectal problems [38] . It has been demonstrated that increased rectal compliance occurs in physiological conditions such as megarectum and in patients with constipation [43] . The threshold volume for the urge to defecate may be absent or increased in chronic constipation [44] ; [45] . However, the cause–effect interrelationship between sensory impairment and chronic constipation remains unclear [38] . Therefore, the clinical implication of our study is that the presence of poor sleep in RSW workers may predispose them to constipation, and sleep disturbance in these workers may have an impact on daily bowel function.
There are some limitations in this study. First, the small sample size may have influenced the current results with a type II error. Second, although 5-minute HRV has been widely used in previous work, it may be more informative to record the HRV for a longer period. Furthermore, this study evaluated the association between anorectal function and sleep in RSW workers; therefore additional evaluation of occupation and socioeconomic status would help better explain current findings. Rotating shift workers already had sleep disturbance in their daily activity, which may have confounded the results of the current study. Therefore, further characterization of sleep quality in those individuals may help elucidate current findings.
In conclusion, our study indicated that circadian rhythm disruption due to RSW is highly associated with altered anorectal motility, greater sleep disturbance, and depression. Our findings indicate that RSW workers are more vulnerable to anorectal dysfunction, which may further impair bowel function and predispose them to constipation. Future studies with a larger sample size and different patterns of shift workers are required for further elucidation of the impact of shift work on physical function and psychosocial status.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgements
This study was supported by a grant, TCRD101-32 , from Buddhist Tzu Chi General Hospital , (Hualien, Taiwan). Chih-Hsun Yi, Tso-Tsai Liu and Wei-Yi Lei contributed to acquisition of data and research performance, analysis and interpretation of data, and approval of the final version of the manuscript; Jui-Sheng Hung and Chien-Lin Chen contributed to study concept and design, analysis and interpretation of data, drafting of the manuscript, statistical analysis, and approval of the final version of the manuscript.
References
- [1] C.M. Poissonnet, M. Veron; Health effects of work schedules in healthcare professions; J Clin Nurs, 9 (2000), pp. 13–23
- [2] H.R. Saberi, A.R. Moravveji; Gastrointestinal complaints in shift-working and day-working nurses in Iran; J Circadian Rhythms, 8 (2010), p. 9 https://doi.org/10.1186/1740-3391-8-9
- [3] B. Nojkov, J.H. Rubenstein, W.D. Chey, W.A. Hoogerwerf; The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses; Am J Gastroenterol, 105 (2010), pp. 842–847
- [4] K. Segawa, S. Nakazawa, Y. Tsukamoto, Y. Kurita, H. Goto, A. Fukui, et al.; Peptic ulcer is prevalent among shift workers; Dig Dis Sci, 32 (1987), pp. 449–453
- [5] G. Goldsmith, J.S. Levin; Effect of sleep quality on symptoms of irritable bowel syndrome; Dig Dis Sci, 38 (1993), pp. 1809–1814
- [6] W. Zhen Lu, K. Ann Gwee, K. Yu Ho; Functional bowel disorders in rotating shift nurses may be related to sleep disturbances; Eur J Gastroenterol Hepatol, 18 (2006), pp. 623–627
- [7] Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology; Circulation, 93 (1996), pp. 1043–1065
- [8] F.M. Fouad, R.C. Tarazi, C.M. Ferrario, S. Fighaly, C. Alicandri; Assessment of parasympathetic control of heart rate by a noninvasive method; Am J Physiol, 246 (1984), pp. H838–H842
- [9] D. Lucini, L. Dalla Vecchia, A. Porta, A. Malliani, M. Pagani; Non-invasive assessment of the changes in static and oscillatory components of peripheral pressure/flow relationships produced by moderate exercise in humans; J Hypertens, 15 (1997), pp. 1755–1760
- [10] M. Pagani, O. Rimoldi, P. Pizzinelli, R. Furlan, W. Crivellaro, D. Liberati, et al.; Assessment of the neural control of the circulation during psychological stress; J Auton Nerv Syst, 35 (1991), pp. 33–41
- [11] J.J. Goldberger, S. Challapalli, R. Tung, M.A. Parker, A.H. Kadish; Relationship of heart rate variability to parasympathetic effect; Circulation, 103 (2001), pp. 1977–1983
- [12] C.L. Chen, W.C. Orr, C.C. Yang, T.B. Kuo; Cardiac autonomic regulation differentiates reflux disease with and without erosive esophagitis; Scand J Gastroenterol, 41 (2006), pp. 1001–1006
- [13] K.Y. Chen, C.L. Chen, C.C. Yang, T.B. Kuo; Cardiac autonomic dysregulation in patients with acute hepatitis; Am J Med Sci, 332 (2006), pp. 164–167
- [14] T.B. Kuo, T. Lin, C.C. Yang, C.L. Li, C.F. Chen, P. Chou; Effect of aging on gender differences in neural control of heart rate; Am J Physiol, 277 (1999), pp. H2233–H2239
- [15] N. Ishii, T. Iwata, M. Dakeishi, K. Murata; Effects of shift work on autonomic and neuromotor functions in female nurses; J Occup Health, 46 (2004), pp. 352–358
- [16] M.H. Chung, T.B. Kuo, N. Hsu, K.R. Chuo, H. Chu, C.C. Yang; Comparison of sleep-related cardiac autonomic function between rotating-shift and permanent night-shift workers; Ind Health, 49 (2011), pp. 589–596
- [17] C.L. Chen, H.H. Lin, W.C. Orr, C.C. Yang, T.B. Kuo; Transfer function analysis of heart rate variability in response to water intake: correlation with gastric myoelectrical activity; J Appl Physiol, 96 (2004), pp. 2226–2230
- [18] C.L. Chen, W.C. Orr; Autonomic responses to heartburn induced by esophageal acid infusion; J Gastroenterol Hepatol, 19 (2004), pp. 922–926
- [19] C.L. Chen, C.C. Yang, T.B. Kuo; Transfer function analysis of heart rate variability correlated with gastric myoelectrical activity using a liquid nutritional meal compared to water: are they different?; Chin J Physiol, 50 (2007), pp. 271–276
- [20] K.A. Gwee; Disturbed sleep and disturbed bowel functions: implications for constipation in healthy individuals; J Neurogastroenterol Motil, 17 (2011), pp. 108–109
- [21] T.T. Liu, C.H. Yi, C.L. Chen, W.C. Orr; Impact of sleep dysfunction on anorectal motility in healthy humans; J Neurogastroenterol Motil, 17 (2011), pp. 180–184
- [22] E.S. Schernhammer, F. Laden, F.E. Speizer, W.C. Willett, D.J. Hunter, I. Kawachi, et al.; Night-shift work and risk of colorectal cancer in the nurses' health study; J Natl Cancer Inst, 95 (2003), pp. 825–828
- [23] T.T. Liu, C.L. Chen, C.H. Yi, P.C. Tung, C. CH; Gender difference in anorectal function in healthy young Taiwanese; Gastroenterol J Taiwan, 25 (2008), pp. 171–175
- [24] T.B. Kuo, C.C. Yang, S.H. Chan; Selective activation of vasomotor component of SAP spectrum by nucleus reticularis ventrolateralis in rats; Am J Physiol, 272 (1997), pp. H485–H492
- [25] D.J. Buysse, C.F. Reynolds 3rd, T.H. Monk, S.R. Berman, D.J. Kupfer; The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research; Psychiatry Res, 28 (1989), pp. 193–213
- [26] C.D. Spielberger; State-trait anxiety inventory; Consulting Psychologists Press, Mountain View, CA (1983)
- [27] Y. Lee, M.J. Yang, T.J. Lai, N.M. Chiu, T.T. Chau; Development of the Taiwanese Depression Questionnaire; Chang Gung Med J, 23 (2000), pp. 688–694
- [28] H. Ito, M. Nozaki, T. Maruyama, Y. Kaji, Y. Tsuda; Shift work modifies the circadian patterns of heart rate variability in nurses; Int J Cardiol, 79 (2001), pp. 231–236
- [29] S.S. Rao, K.D. Welcher, R.E. Pelsang; Effects of biofeedback therapy on anorectal function in obstructive defecation; Dig Dis Sci, 42 (1997), pp. 2197–2205
- [30] A. Wald; Evaluation of anal sphincter defects; Am J Gastroenterol, 92 (1997), p. 907
- [31] S.S. Rao, R. Hatfield, E. Soffer, S. Rao, J. Beaty, J.L. Conklin; Manometric tests of anorectal function in healthy adults; Am J Gastroenterol, 94 (1999), pp. 773–783
- [32] W.C. Orr, C.L. Chen; Sleep and the gastrointestinal tract; Neurol Clin, 23 (2005), pp. 1007–1024
- [33] H. Mertz; Review article: visceral hypersensitivity; Aliment Pharmacol Ther, 17 (2003), pp. 623–633
- [34] A.D. Krystal; How the circadian rhythm affects sleep, wakefulness, and overall health: background for understanding shift work disorder; J Clin Psychiatry, 73 (2012), p. e05 https://doi.org/10.4088/JCP.11073br1
- [35] S.F. Niu, M.H. Chung, C.H. Chen, D. Hegney, A. O'Brien, K.R. Chou; The effect of shift rotation on employee cortisol profile, sleep quality, fatigue, and attention level: a systematic review; J Nurs Res, 19 (2011), pp. 68–81
- [36] B. Frenckner, T. Ihre; Influence of autonomic nerves on the internal and sphincter in man; Gut, 17 (1976), pp. 306–312
- [37] A. Carlstedt, S. Nordgren, S. Fasth, L. Appelgren, L. Hulten; Sympathetic nervous influence on the internal anal sphincter and rectum in man; Int J Colorectal Dis, 3 (1988), pp. 90–95
- [38] N.E. Diamant, M.A. Kamm, A. Wald, W.E. Whitehead; AGA technical review on anorectal testing techniques; Gastroenterology, 116 (1999), pp. 735–760
- [39] R. Radomirov, C. Ivancheva, A.F. Brading, D. Itzev, A. Rakovska, N. Negrev; Ascending and descending reflex motor activity of recto-anal region-cholinergic and nitrergic implications in a rat model; Brain Res Bull, 79 (2009), pp. 147–155
- [40] D. Wingate, M. Hongo, J. Kellow, G. Lindberg, A. Smout; Disorders of gastrointestinal motility: towards a new classification; J Gastroenterol Hepatol, 17 (Suppl) (2002), pp. S1–S14
- [41] F. Azpiroz, M. Bouin, M. Camilleri, E.A. Mayer, P. Poitras, J. Serra, et al.; Mechanisms of hypersensitivity in IBS and functional disorders; Neurogastroenterol Motil, 19 (2007), pp. 62–88
- [42] B.J. Caruana, A. Wald, J.P. Hinds, B.H. Eidelman; Anorectal sensory and motor function in neurogenic fecal incontinence. Comparison between multiple sclerosis and diabetes mellitus; Gastroenterology, 100 (1991), pp. 465–470
- [43] D. Waldron, K.L. Bowes, Y.J. Kingma, K.R. Cote; Colonic and anorectal motility in young women with severe idiopathic constipation; Gastroenterology, 95 (1988), pp. 1388–1394
- [44] A. De Medici, D. Badiali, E. Corazziari, G. Bausano, F. Anzini; Rectal sensitivity in chronic constipation; Dig Dis Sci, 34 (1989), pp. 747–753
- [45] N.W. Read, L. Abouzekry, M.G. Read, P. Howell, D. Ottewell, T.C. Donnelly; Anorectal function in elderly patients with fecal impaction; Gastroenterology, 89 (1985), pp. 959–966
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?