Abstract
Traditionally, hydrogen sulfide (H2S) was simply considered as a toxic and foul smelling gas, but recently H2S been brought into the spot light of cardiovascular research and development. Since the 1990s, H2S has been mounting evidence of physiological properties such as immune modification, vascular relaxation, attenuation of oxidative stress, inflammatory mitigation, and angiogenesis. H2S has since been recognized as the third physiological gaseous signaling molecule, along with CO and NO [65,66]. H2S is produced endogenously through several key enzymes, including cystathionine β-lyase (CBE), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (MST)/cysteine aminotransferase (CAT). These specific enzymes are expressed accordingly in various organ systems and CSE is the predominant H2S-producing enzyme in the cardiovascular system.
The cystathionine γ-lyase (CSE)/H2S pathway has demonstrated various cardioprotective effects, including anti-atherosclerosis, anti-hypertension, pro-angiogenesis, and attenuation of myocardial ischemia–reperfusion injury. CSE exhibits its anti-atherosclerotic effect through 3 mechanisms, namely reduction of chemotactic factor inter cellular adhesion molecule-1 (ICAM-1) and CX3CR1, inhibition of macrophage lipid uptake, and induction of smooth muscle cell apoptosis via MAPK pathway. The CSE/H2S pathways anti-hypertensive properties are demonstrated via aortic vasodilation through several mechanisms, including the direct stimulation of KATP channels of vascular smooth muscle cells (VSMCs), induction of MAPK pathway, and reduction of homocysteine buildup. Also, CSE/H2S pathway plays an important role in angiogenesis, particularly in increased endothelial cell growth and migration, and in increased vascular network length. In myocardial ischemia–reperfusion injuries, CSE/H2S pathway has shown a clear cardioprotective effect by preserving mitochondria function, increasing antioxidant production, and decreasing infarction injury size.
However, CSE/H2S pathways role in inflammation mitigation is still clouded, due to both pro and anti-inflammatory results presented in the literature, depending on the concentration and form of H2S used in specific experiment models.
Abbreviations
Akt, protein kinase B;BCA, brachiocephalic artery;CAM, chorioallantoic membrane;CAT, cysteine aminotransferase;CBS, cystathionine β-lyase;CLP, cecal ligation and puncture;CSE, cystathionine γ-lyase;CSE KO, CSE knock out;CTO, chronic total occlusion;CX3CL1, chemokine (C-X3-C Motif) ligand 1;CX3CR1, CX3C chemokine receptor 1;EC, endothelial cell;ERK, extracellular signal-regulated kinase;GAPDH, glyceraldehyde 3-phosphate dehydrogenase;GSH-Px, glutathione peroxidase;GYY4137, morpholin-4-Ium-4-methoxyphenyl(morpholino) phosphinodithioate;H2S, hydrogen sulfide;HUVECs, human umbilical vein endothelial cells;ICAM-1, inter cellular adhesion molecule-1;IMT, intima–media complex thickness;LPS, lipopolysaccharide;l-NAME, NG-nitro-l-arginine methyl ester;MAPK, mitogen-activated protein kinase;MPO, myeloperoxidase;NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells;Nrf2, nuclear factor erythroid 2-related factor 2;oxLDL, oxidized low density lipoprotein;PAG, DL-propagylglycine;PPAR-γ, peroxisome proliferator-activated receptor;PTPN1, protein tyrosine phosphatase, non-receptor type 1;ROS, reactive oxygen species;SAH, S-adenosylhomocysteine;SAM, S-adenosylmethionine;SMCs, smooth muscle cells;SOD, superoxide dismutase;S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-Yl)-phenyl ester;VEGF, vascular endothelial growth factor;VSMCs, vascular smooth muscle cells;MST, 3-mercaptopyruvate sulfurtransferase
Keywords
Cystathionine γ-lyase;Hydrogen sulfide;Atherosclerosis;Vasorelaxation;Angiogenesis;Ischemia–reperfusion injury
1. Introduction
Hydrogen sulfide (H2S) has long been known for its toxic and foul smelling properties, but has recently gained significant attention for its physiological regulatory role in the human body. Since the late 1990s, H2S has continuously been proven to exhibit distinctive functions regarding vascular tone [10]; [11]; [12]; [13]; [14]; [15]; [16]; [17]; [18]; [19]; [20]; [21]; [22]; [23]; [24]; [25]; [26] ; [27], inflammatory process [51]; [52]; [53]; [54]; [55]; [56]; [57]; [58]; [59]; [60]; [61]; [62]; [63] ; [64], post-myocardial infarction remodeling [37]; [38]; [39]; [40]; [41]; [42]; [43]; [44]; [45]; [46]; [47]; [48]; [49] ; [50], angiogenesis [29]; [30]; [31]; [32] ; [33], and other physiological processes. Hence, H2S has since been included in a family of physiological gaseous signaling molecule along with CO and NO [65] ; [66] as small molecules that can freely pass through cell membranes to directly exert biological function by interacting with cellular components.
H2S is produced intrinsically through 3 major enzymes in our body, namely the CSE, CBS, and 3-MST/CAT pathways. This review provides an overview of the innate production of H2S and several aspects of H2S function with a cardiocytoprotective focus, particularly on the CSE/H2S pathway. The CSE/H2S pathway has recently become one of the heated focuses of cardiovascular disease pathophysiology research.
2. Hydrogen sulfide in nature
H2S is a naturally occurring colorless, corrosive, and flammable gaseous compound with the characteristic foul odor of rotten eggs under room temperature at low concentrations up to 30 ppm. The detectable order threshold for H2S is as low as 1 ppm. The H2S gas is produced naturally by anaerobic breakdown of sulfur-containing organic matter and is often associated with petroleum and natural gas refinement, chemical manufacture, and waste disposal industries.
Biologically, H2S is considered a broad-spectrum poison, acting on several systems in the body, particularly devastating in the nervous system. H2S is classified as a chemical asphyxiant, which forms a complex bond to the ferric moiety, causing inhibition of mitochondrial cytochrome oxidase, and thereby arresting aerobic metabolism, similar to cyanide toxicity. H2S toxicity is further enhanced by its high lipid solubility which allows it to penetrate easily through phospholipid membranes. At concentrations above 100 ppm, H2S affects a persons perception of smell by causing a rapid temporary paralysis of the olfactory nerves. At concentrations greater than 700 ppm, H2S may cause sudden death [65] ; [66].
3. Physiological production of H2S
H2S can be detected in a wide range of tissues and organs including the brain, thoracic aorta, lungs, liver, kidney, ileum, pancreatic islets, uterus, placenta and umbilical cord, and several other organs, suggesting a pleotropic role for the gaseous transmitter [65] ; [66]. Endogenous H2S produced in the human body has been attributed to three key enzyme pathways, namely the cystathionine γ-lyase (CSE), cystathionine β-lyase (CBS), and 3-mercaptopyruvate sulfurtransferase (MST)/cysteine aminotransferase(CAT) pathways. The CSE enzyme is expressed in the cardiovascular system, predominantly the myocardium and vascular smooth muscle cells (VMSCs). The CBS enzyme can be found mostly in the central nervous system. MST and CAT are primarily expressed in the brain and vascular endothelium [67]; [68]; [69]; [70]; [71]; [72]; [73]; [74]; [75]; [76] ; [77].
Physiological H2S is produced predominantly through the metabolic pathways of sulfur-containing amino acids. Beginning with methionine, methionine loses a methyl group through the SAM–SAH pathway to form homocysteine. CBS then converts homocysteine to cystathionine and CSE converts cystathionine to l-cysteine and α-ketobutyrate [67]; [68]; [69]; [70]; [71]; [72]; [73]; [74]; [75]; [76] ; [77].
Both CSE and CBS are cytosolic pyroxidal-5′-phosphatedependent enzymes that use l-cysteine as their principal substrate to form H2S. CBS hydrolyzes l-cysteine to form l-serine and H2S. CSE reacts with dimerized l-cysteine to form thiocysteine, pyruvate, and NH3. Thiocysteine in turn may nonenzymatically break down to form pyruvate and H2S. Alternatively, thiocysteine may engage in a CSE-mediated reaction with another thiol compound (R-SH) to form Cys-S-R and H2S [67]; [68]; [69]; [70]; [71]; [72]; [73]; [74]; [75]; [76] ; [77].
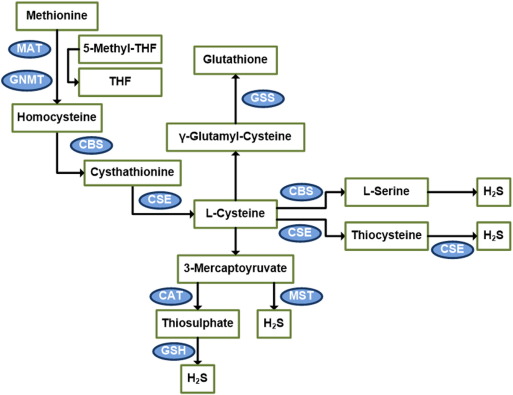
On the other hand, MST and CAT are both cytosolic and mitochondrial enzymes, with a majority distribution in the mitochondria. CAT catalyzes l-cysteine and α-ketoglutarate to form 3-mercaptopyruvate and l-glutamate. 3-mercaptopyruvate may be desulfurized by MST to form pyruvate and H2S. Alternatively, 3-mercaptopyruvate can be converted by CAT to thiosulfate and pyruvate. Thiosulfate may then oxidize GSH to form GSSG, SO32 −, and H2S [67]; [68]; [69]; [70]; [71]; [72]; [73]; [74]; [75]; [76] ; [77] (Fig. 1).
|
|
|
Fig. 1. Diagram of the transsulfuration pathway. The majority of endogenous H2S is produced through the transsulfuration pathway, which utilizes methionine-derived l-cysteine as substrate for CSE and CBE enzymes to form H2S. An alternative route exists where l-cysteine is first converted to 3-mercaptopyruvate before reacting with MST enzyme to form H2S. MST = mercaptopyruvatesulfurtransferase, CAT = cysteine aminotransferase, GNMT = glycine N-methyltransferase, GSS = Glutathione synthase, CSAD = cysteine sulfinic acid decarboxylase. |
Once H2S is formed, it is quickly broken down through chemical and enzymatic pathways. Methemoglobin may react with H2S to form sulfhemoglobin, which acts as a metabolic sink for H2S. Thiol-S-methyltransferase may methylate H2S to form dimethylsulfide and methanethiol. Furthermore, H2S may be rapidly oxidized to thiosulfate in the mitochondria and subsequently be converted to sulfite and sulfate.
4. Physiological concentration of H2S
The range of H2S concentrations offered by the current literature has proven to be considerably inconsistent. H2S concentration in the human blood and tissues shown in literature ranges from 2–160 μM [11]; [25]; [40]; [43]; [78]; [79]; [80] ; [81]. Not only is this range so large that it encompasses a difference of several orders of magnitude, the entire detected range may itself be questioned for its validity. Free H2S under the before mentioned concentrations is well above the detectable order threshold and yet our blood does not smell of the characteristic rotten egg smell of H2S. The most widely used colorimetric detection method of methylene blue and sulfite-sensitive ion-selective electrodes are imperfect in its design. The detection assays are conducted in the presence of Fe3 + in highly acidic environments, which may liberate multiple acid-labile sulfur species to generate exaggeratedly high values of detected H2S. Other detections methods such as sulfide-sensitive fluorescent dye detection, gas chromatography, monobromobimane, and polarographic electrodes have made improvements in detection but differ greatly with detection limits and accuracy [67]; [95]; [96]; [97] ; [98].
However, all the currently utilized detection methods all require the disruption of normal tissue physiology. Measuring H2S from homogenates of tissue samples disregards the actual physiological compartmentalization of H2S and cannot be used to reflect the localized concentration of H2S in vivo. Recent developments in reaction-based fluorescent probe imaging have begun to detect H2S in living cells and shedding light on the spatial distribution and localized concentration of H2S [95]; [96]; [97]; [98]; [99]; [100]; [101]; [102]; [103]; [104] ; [105].
5. Cystathionine γ-lyase (CSE)
The crucial enzyme for cardiovascular physiological production of H2S is cystathionine γ-lyase (CSE), which is the predominantly expressed in the myocardium and vascular smooth muscle cells. The murine CSE gene can be traced to a 1.8 kb full-length cDNA containing an open reading frame of 1197 bp, which encodes a 43.6 kDa protein. The CSE gene is identified as a 35 kb mouse genomic fragment through λ genomic library screening. The CSE gene contains promoter regions, 12 exons, ranging in size from 53 to 579 bp and spanning over 30 kb, and exon/intron boundaries that are conserved with rat and human CSE [106] (Table 1).
| Sample | Subject | H2S concentration (μM) |
|---|---|---|
| Serum | Mouse [55] | 23 |
| Rat [40] ; [55] | 30–46 | |
| Blood | Mouse [53]; [78]; [88]; [89]; [90]; [91]; [92]; [93] ; [94] | 7–80 |
| Rat [34]; [82]; [83]; [84]; [85]; [86]; [87] ; [88] | 7–63 | |
| Human [11]; [34]; [40]; [43]; [78]; [79]; [80] ; [81] | 2–110 | |
| Brain | Rat [11] ; [34] | 50–160 |
| Human [11] ; [34] | 50–160 |
The CSE protein (EC = 4.4.1.1) is a pyridoxal-phosphate-dependent enzyme that can be found in high concentrations particularly in the cytoplasm of heart cells and vascular smooth muscle cells. CSE has an optimal functional pH of 8.2 and pharmacokinetic parameters KM = 0.5 mM for l-cystathionine, KM = 5.4 mM for homocysteine, and KM = 3.5 mM for cysteine. CSE is directly regulated by calmodulin and has broad substrate specificity.
CSE catalyzes the last step in the trans-sulfuration pathway, using homocysteine and cysteine as substrates to form lanthionine and hydrogen sulfide. Further, CSE also acts as cysteine-protein sulfhydrase to regulate the functions of sulfur-containing protein such as those of GAPDH, PTPN1 and NF-κB.
The CSE gene is also highly expressed in adult mouse liver and kidney. In newborn mouse liver and kidney, CSE expression steadily increases and peaks at approximately 3 weeks of age. From there on, CSE expression in the liver remains constant while CSE expression decreases dramatically in the kidney [106].
Through mouse CSE cDNA cloning and sequencing, GeneBank® accession number AY083352, the analysis has shown that the mouse CSE ORF is 92.5% identical with the rat sequence and is 81.6% identical with the human sequence [106].
6. Atherosclerosis
With respect to cardiovascular diseases, we now focus on the H2S forming enzyme CSE and cardiovascular pathophysiology. Atherosclerosis is a vascular pathology characterized by plaque formation in large and medium sized vessel. These plaques increase vessel rigidity, decrease vascular flow, and may lead to thrombosis in vital organs causing detrimental effects. Atherosclerosis is the result of a chronic inflammation process beginning with vascular remodeling, endothelial dysfunction, smooth muscle cell proliferation and migration, accumulation of cholesterol-rich lipoproteins. Atherosclerotic progression is then followed by recruitment of lipid-laden macrophages, also known as foam cells, to form a fibrous cap of collagen and smooth muscle cells, and a necrotic core rich in lipids [1]. Atherosclerotic lesion rupture may ensue as the result of mechanical damage, overwhelming plaque burden, or insufficiency of smooth muscle cell collagen formation to maintain plague integrity. This lesion rupture releases matrix metalloproteins and necrotic lipid components causing an inflammatory and coagulative vascular response.
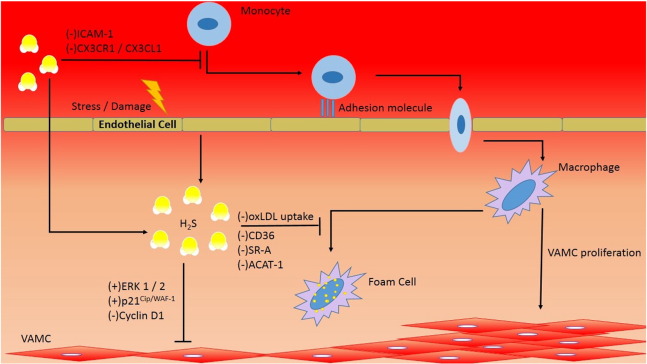
CSE is suggested to exhibit its anti-atherosclerotic effect through 3 mechanisms, namely the reduction of chemotactic factors inter cellular adhesion molecule-1 (ICAM-1) and CX3CR1, inhibition of macrophage lipid uptake, and induction of smooth muscle cell apoptosis via MAPK pathway [3]; [4] ; [5] (Fig. 2).
|
|
|
Fig. 2. H2S function in atherosclerosis. The precipitating factors of atherosclerosis include endothelial damage, vascular inflammation, and smooth muscle proliferation. H2S functions to attenuate leukocyte chemotaxis through down regulation of ICAM-1 and CX3CR1/CX3CL1. Moreover, H2S inhibits foam cell formation through down regulating CD36, SR-A, and ACAT-1 surface markers and decreases oxLDL uptake in macrophage. Further, H2S induces vascular smooth muscle apoptosis through up regulating ERK 1/2 and p21Cip/WAF-1 and by down regulating cyclin D1. |
The circulating form of ICAM-1 has been evaluated as a predictor of cardiovascular risk and is a marker for vascular inflammation in atherosclerosis. ICAM-1 is minimally expressed on normal endothelium, but can be found in significant quantities in atherosclerotic plaques [7]. Proliferation of ICAM-1 is associated with chemotaxis of inflammatory cells. Mani et al. [1] demonstrated that CSE knock out (CSE KO) mice fed with atherogenic diet displayed a 30-fold increase in ICAM-1 expression and increased atherosclerotic plaque size compared to wild type control.
In a separate experiment, overexpression of CSE yielded a significant reduction in CX3CR1 and CX3CL1 expression, as well as reduction in PPAR-γ dependent CX3CR1-mediated chemotaxis in stimulated macrophages. In atherogenic-diet mouse model, the CSE inhibitor DL-propagylglycine(PAG) treatment group displayed significant aortic atherosclerosis, including a significant increase in brachiocephalic artery (BCA) plaque size, common carotid artery intima–media complex thickness (IMT), and aortic arch IMT assessed by ultrasound biomicrocopy compared to control group. Histology analysis also revealed an obvious rise in BCA plaque area and lipid core [2].
Foam cell accumulation is a key part of atherosclerotic plaque formation. In terms of macrophage-derived foam cell formation, H2S exerts inhibitory effect through the down regulation of SR-A, CD36, and ACAT-1 expression, which attenuates oxidized low density lipoprotein (ox-LDL) binding and uptake by macrophages [9]. In support, PAG treatment exacerbates the before mentioned factors towards foam cell formation [8].
Vascular smooth muscle cell (VSMC) proliferation and migration is crucial in the formation of atherosclerotic plaques. In H2S treatment or CSE overexpression in cultured smooth muscle cells (SMCs), SMCs have shown increased phosphorylation of extracellular signal-regulated kinase (ERK) 1/2, altered expression of cyclin D1 and p21Cip/WAF-1, activation of caspase 3, and ultimately lead to cell apoptosis. ERK is a member of mammalian mitogen-activated protein kinase (MAPK) family and is involved in regulation of cell metabolism and gene expression particularly associated with growth, stress response, and apoptosis [6]; [10]; [11]; [35] ; [73]. Additively, CSE-KO mice has shown increased SMC proliferation rate in vitro and in vivo, reduced phosphorylation of ERK1/2 in mesenteric SMCs and mesenteric artery tissue, and increased susceptibility to apoptosis induced by exogenous H2S at physiologically relevant concentrations [10].
7. Vasorelaxation
Hypertension is a chronic medical condition where the arterial blood pressure is elevated above 140/90 mm Hg. In all hypertensive illnesses, 90–95% of cases are categorized as primary hypertension, with risk factors such as advanced age, high sodium intake, obesity, stagnant lifestyle, excessive alcohol intake, stress, caffeine consumption, vitamin D deficiency, and a slew of genetic and environmental factors. Hypertension, as the term implies, puts increased pressure, or stress on the heart, which could lead to coronary arterial diseases, hypertensive heart diseases, peripheral arterial diseases, chronic kidney diseases, stroke, aneurysms, among other diseases.
The CSE/H2S pathway has been shown to exhibit aortic vasodilation effect through several mechanisms, including the direct stimulation of KATP channels of VSMC, induction of MAPK pathway, and reduction of homocysteine buildup, ultimately leading to vasodilation and lowering of blood pressure.
The primary vasodilatory effect of H2S can be attributed to the direct stimulation of KATP channels with subsequent hyperpolarization of aortic vascular smooth muscle in the mesenteric artery, aorta, and portal vein in rat animal model [10]; [11]; [12]; [13]; [14]; [15]; [16]; [17]; [18]; [19]; [20]; [21]; [22]; [23]; [24]; [25]; [26]; [27] ; [28]. According to Zhao et al., intravenous bolus H2S injection triggered roughly a 30 second transient decrease in mean arterial blood pressure of 12–30 mm Hg [13]. This blood pressure lowering effect can be mimicked by penacidil, a KATP channel opener, and antagonized by glibenclamide, a KATP channel blocker. Kohn et al. [14] have shown that the rat aortic ring contraction has a positive dose response to serotonin at 1–10 μmol/L and that combined incubation with PAG at 10 mmol/L for 30 min produces a more intensive contraction. Further evidence provided by Zhong et al. [15] shows that hypertension can be introduced in Wistar rats by oral administration NG-nitro-l-arginine methyl ester (l-NAME), which significantly inhibited CSE expression and H2S synthesis in the thoracic aorta. Other supportive observations include PAG treatment significantly increasing blood pressure in Sprague–Dawley rats and mice [11] ; [21], and the down regulation of CSE/H2S pathway during the development of hypertension in spontaneously hypertensive rat (SHR) models.
In CSE KO mice, besides an evident 80% reduction in H2S level in the serum, heart, and aorta, there is observed prominent hypertension of approximately 18 mm Hg higher than age-matched control at 12 weeks of age and reduced endothelium-dependent vasorelaxation [11]. However, CSE KO mice conversely showed a heightened sensitivity to exogenous H2S-induced vasorelaxation and greater H2S-induced decrease in blood pressure [13].
Additively, CSEs apoptotic effects on VSMC also contribute to the lowering of blood pressure. The apoptotic effect of CSE/H2S pathway has already been described in the previous section. With reduced total VSMC number, the reduction of VMSC contractile force results in an overall decreased blood pressure [10] ; [11].
CSEs antihypertensive effect is also associated with the reduction of homocysteine buildup. Homocysteine is a metabolite of methionine that can be further metabolized to form H2S in the transsulfuration pathway [17] ; [18]. Hyperhomocysteinemia, or elevated serum homocysteine levels, is an independent and graded risk factor associated with cardiovascular diseases, including hypertension. In hyperhomocysteinemia rat model, both serum CSE activity and H2S levels in the myocardium are significantly decreased [18]. In a separate study, the 10-week-old male CSE KO mice group has shown elevated plasma homocysteine and l-cysteine levels about 18 and 0.8 times, respectively, compared to age-matched wild type control [17].
8. Angiogenesis
Angiogenesis is a complex biological process characterized by extracellular matrix remodeling and changes in endothelial cell (EC) behavior that lead to increased growth, migration, and assembly into capillary structures [30]; [31]; [32] ; [33].
In vitro study evidence shows H2Ss proangiogenic effect, Cai et al. [29] demonstrated that RF/6A transformed EC displayed an up to 100% increase in cell proliferation and up to 30% increase in migration under H2S 6–600 μM concentration. In a separate study, exposure of human umbilical vein endothelial cells (HUVECs) to H2S at 60 μM promotes an observable EC growth with 2-fold increase in cell number and sustained increase in ERK1/2 phosphorylation [30]. Furthermore, H2S at 60 μM enhances capillary-like structure formation of ECs cultured on reduced growth factor Matrigel by roughly 34%. EC also displayed an approximately 4 fold increased cell migration under 60 μM H2S as compared to control.
Another supportive evidence regarding CSE proangiogenic effect is related to VEGF, a well-established stimulator of angiogenesis. CSE siRNA transfected EC cells display an average decrease of CSE protein by 59.8 ± 6.7% and also showing attenuated MAPK, ERK1/2, and p38 pathways. CSE siRNA transfected EC cells stimulated with VEGF displays a 60% decreased cell migration compared to controls under the same conditions. Further, aortic ring explants from CSE KO shows a roughly 80% decreased new microvessel formation compared to WT control under both vehicle and VEGF stimulation [31].
In vivo study demonstrates the angiogenic effect of H2S in chick chorioallantoic membrane (CAM) model. Vascular network length is measured after 48 h of treatment with H2S and PAG. Membranes treated with 240 pmol/egg H2S show an average 40% increase in vessel length while administration of 300 μmol/egg PAG displayed a roughly 50% decrease in vessel branching points and 25% decrease in vessel length as compared to control [31]. It has long been suspected but only recently brought into light that H2S and NO may in fact share common pathways regarding angiogenesis and vasorelaxation [26] ; [27].
Lastly, angiogenesis can be arguably gauged through wound healing. In rat burn wound model, wound closure after 1 month shows 5% significant improvement in animal receiving daily topical H2S compared to WT control. In CSE KO mice, would area were consistently larger than WT control, suggesting a proangiogenic effect of CSE [30].
9. Myocardial ischemia and reperfusion injuries
Ischemia is a vascular insult characterized by interruption of arterial blood supply to tissues and organs, causing a shortage of oxygen, glucose, and other essential components required for cellular metabolism and well-being. Causes for ischemia include vasoconstriction, embolism, thrombosis, or trauma. Ischemia causes tissue damage through an excessive build-up of metabolic waste, mitochondrial damage, disruption of cellular membranes, and dispersion of autolyzing and proteolytic enzymes, which causes general dysfunction and ultimately death of the affected tissues and organs. If a vascular insult is met with immediate medical attention, there is a highly likely chance that blood supply may be restored to the ischemic tissues and organs to prevent total loss of function. However, the reperfusion of ischemic tissues and organs may cause another type of damage known as reperfusion injury. Reperfusion injury is mostly a type of compounded microvascular injury where the reintroduced blood oxygen causes increased production of free radicals and reactive oxygen species which damage cellular components and the inflammatory response to the damaged tissue causes swelling and obstruction which may lead to further ischemia.
In myocardial ischemia and reperfusion injuries, CSE/H2S pathway has shown a clear cardioprotective effect in reduced ischemic infarction lesion and increased cell survival [36]. Johansen et al. showed that exogenous H2S administration protects against ischemia–reperfusion injury in a dose-dependent manner observable through the reduction in rat myocardial infarction size [37]. Elrod et al. further demonstrated that direct administration of NaHS at 50 mg/kg during reperfusion injury in an in vivo mouse model preserves mitochondrial function which leads to a substantial reduction of infarct size [38].
H2S exerts cardioprotective effects against ischemia–reperfusion injury through up regulating Bcl-2/Bax ratio to preserve mitochondrial function and by enhancing antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) to scavenge reactive oxygen species (ROS), both ultimately result in increased cell survival [39]. Moreover, H2S serves to activate the Akt–Nrf2 pathway to promote antioxidant and anti-apoptotic molecule expression and by inflammatory inhibition through NF-KB dependent path, both leading to tissue survival [39].
In support of CSE cardioprotective effects, Huang et al. showed that in an ischemia–reperfusion post-conditioning mouse model, administration of 2 mM PAG prior to global ischemia resulted in an infarct size of 35.9 ± 6.4% compared to infarct size without PAG treatment of 18.3 ± 5.7% [40]. Similarly, Zhu et al. proved that in myocardial ischemia rat model, infarct size was 52.9 ± 3.5% in vehicle-treated, 62.9 ± 7.6% in PAG-treated, and 43.4 ± 2.8% in NaHS-treated (P < 0.05 vs. vehicle) groups [41].
10. Inflammation
A debatable representation of the CSE/H2S pathway is on its effects regarding inflammation. There are separate studies citing both pro and anti-inflammatory effects of CSE/H2S pathway. Regarding the pro-inflammatory effect of CSE/H2S pathway, several researchers have demonstrated that in mouse models of hind paw edema [51], lipopolysaccharide (LPS) induced endotoxemia [52], and cecal ligation and puncture (CLP) induced sepsis [53] ; [54], administration of H2S donor drug leads to an increased Myeloperoxidase (MPO) expression, a marker for tissue neutrophil infiltration activity, and upregulation of NF-KB, which results in a histologically evident increase in tissue inflammation area. Whereas the administration or pretreatment of with PAG resulted in reduced MPO activity and less tissue inflammation. The same pattern can be seen in caerulein-induced pancreatitis mouse model [55]. Further, in CSE KO mouse caerulein-induced pancreatitis model, the CSE KO mice showed reduced acute pancreatitis inflammation and diminished associated lung injury compared to wild type mouse [56].
However, other researchers have demonstrated anti-inflammatory effect of H2S/CSE pathway. Research with slow releasing H2S donors such as GYY4137 [6] ; [57] and S-diclofenac [58] demonstrated an anti-inflammatory effect with reduced NF-KB, TNF-α, and MPO expression in LPS-induced inflammation models. Another research showed that pretreatment or administration of PAG in LPS-treated rats aggregate liver damage, displaying increased serum AST, liver MPO, and decreased liver GSH [60]. The existing contradicting observations portray the yet understood physiological nature of CSE/H2S pathway, and more research is needed to shed light on this complex pathway. Several researchers have postulated that there may be a biphasic affect of H2S in relation to either the stage of inflammation or the time-concentration of H2S.
11. Summary
H2S is received as the third gaseous physiological transmitter behind CO and NO and plays an important role in several organ systems in the body. The CSE/H2S pathway demonstrates cardiovascular anti-atherogenic effect, vessel dilatory effect, angiogenic effect, inflammatory mediator effect, cardioprotective effect against ischemia–reperfusion injury, and regulates cellular function and humeral response among others. Although portions of the underlying molecular mechanism of the CSE/H2S pathway are yet to be clarified, there is continual mounting evidence and research to provide further insight. To date, researchers seem to offer a bell-shaped dose–response curve to CSE/H2S effect: at physiological concentrations, H2S seems to exhibit cytoprotective effects whereas at higher concentrations, H2S demonstrates detrimental or cytotoxic effect. Furthermore, recent studies performed with slow releasing H2S donors in GYY4137 and S-diclofenac seem to emulate physiological conditions more closely and may provide further insight into the CSE/H2S pathway. There is much effort devoted to clinical disease model research in correlation with the CSE/H2S pathway, such as in coronary artery disease, ischemic cardiomyopathy, and chronic total occlusion (CTO) of the coronary artery among other life threatening cardiovascular diseases. In one recent study, new insight into H2S' cardioprotective effects includes the attenuation of CD11b+Gr-1+ myeloid migration to reduce post infarct inflammation [107]. Furthermore, observational data from our group shows that in CTO reperfusion injury model, H2S production after recanalization decreases by approximately 25% in the first 24 hour period, followed by a slow increase to approximately 50% compared to CTO controls. Unsurprisingly, though the decrease in H2S and vascular pathology, such as ischemia–reperfusion injury, hypertension, and atherosclerosis, is observed, we have yet to grasp the full scope of the CSE/H2S pathway. A clearer understanding of the CSE/H2S is key to understanding vascular microenvironment changes and foundation for building future therapeutic strategies against cardiovascular diseases.
Source of funding
This work was supported by State Key Development Program for Basic Research of China (No. 2011CB503905), National Key Technology Support Program (No. 2011BAI11B10), and Major Program of National Natural Science Foundation of China (No.81230007).
Disclosures
None.
References
- [1] S. Mani, H. Li, A. Untereiner, et al.; Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis; Circulation, 127 (2013), pp. 2523–2534
- [2] H. Zhang, C. Guo, D. Wu, et al.; Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CXCR1 and CX3CL1 expression; PLoS One, 7 (7) (2012), p. e41147 https://doi.org/10.1371/journal.phone.0041147
- [3] S. Xu, Z. Liu, P. Liu; Targeting hydrogen sulfide as a promising therapeutic strategy for atherosclerosis; Int J Cardiol, 172 (2) (2014), pp. 313–317 https://doi.org/10.1016/j.ijcard.2014.01.068
- [4] C. Bauer, J. Boyle, K. Porter, C. Peers; Modulation of Ca2 + signaling in human vascular endothelial cells by hydrogen sulfide; Atherosclerosis, 209 (2010), pp. 374–380
- [5] X. Wang, F. Wang, S.J. You, et al.; Dysregulation of cystathionine γ-lyase(CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophages; Cell Signal, 25 (2013), pp. 2255–2262
- [6] Z. Liu, Y. Han, L. Li, et al.; The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E−/− mice; Br J Pharmacol, 169 (2013), pp. 1795–1809
- [7] Y. Wang, X. Zhao, H. Jin, et al.; Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice; Arterioscler Thromb Vasc Biol, 29 (2009), pp. 173–179
- [8] Z.Z. Zhao, Z. Wang, G.H. Li, et al.; Hydrogen sulfide inhibits macrophage-derived foam cell formation; Exp Biol Med, 236 (2011), pp. 169–176
- [9] Y. Zhang, Z.H. Tang, Z. Ren, et al.; Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases; Mol Cell Biol, 33 (6) (2013), pp. 1104–1113
- [10] X.B. Wang, H.F. Jin, C.S. Tang, J.B. Du; Significance of endogenous sulphur-containing gases in the cardiovascular system; Clin Exp Pharmacol Physiol, 37 (2010), pp. 745–752
- [11] G. Yang, L. Wu, S. Bryan, N. Khaper, S. Mani, R. Wang; Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells; Cardiovasc Res, 86 (2010), pp. 487–495
- [12] C. Wagner; Hydrogen sulfide: a new gaseous signal molecule and blood pressure regulator; J Nephrol, 22 (2009), pp. 173–176
- [13] W.M. Zhao, J. Zhang, Y.J. Lu, R. Wang; The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener; EMBO J, 20 (21) (2001), pp. 6008–6016
- [14] C. Kohn, J. Schleifenbaum, I.A. Szijarto, et al.; Differential effects of cystationine-γ-lyase-dependent vasodilatory H2S in periadventitial vasoregulation of rat and mouse aortas; PLoS One, 7 (8) (2012), p. e41951
- [15] G. Zhong, F. Chen, Y. Cheng, C. Tang, J. Du; The role hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase; J Hypertens, 21 (2003), pp. 1879–1885
- [16] S.C. Bir, C.G. Kevil; Sulfane sustains vascular health: insight into cystathionine γ-lyase function; Circulation, 127 (2013), pp. 2472–2474
- [17] D. Yang, L. Wu, B. Jiang, et al.; H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase; Science, 322 (5901) (2008), pp. 587–590
- [18] L. Chang, B. Geng, F. Yu, et al.; Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats; Amino Acids, 34 (2008), pp. 573–585
- [19] X.H. Li, J.B. Du, L. Shi, et al.; Down-regulation of endogenous hydrogen sulfide pathway in pulmonary hypertension and pulmonary vascular structural remodeling induced by high pulmonary blood flow in rats; Circ J, 69 (2005), pp. 1418–1424
- [20] R. Wang; Hydrogen sulfide: a new EDRF; Kidney Int, 76 (2009), pp. 700–704
- [21] A. Roy, A. Khan, M. Islam, M.C. Prieto, D.S. Majid; Interdependency of cystathionine γ-lyase and cystationine β-synthase in hydrogen sulfide-induced blood pressure regulation in rats; Am J Hypertens, 25 (1) (2012), pp. 74–81
- [22] B. Baragatti, E. Ciofini, D. Sodini, S. Luin, F. Scebba, F. Coceani; Hydrogen sulfide in the mouse ductus arteriosus: a naturally occurring relaxant with potential EDHF function; Am J Physiol Heart Circ Physiol, 304 (2013), pp. H927–H934
- [23] G. Meng, Y. Ma, L. Xie, A. Ferro, Y. Ji; Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases; Br J Pharmacol (Sept 10 2014) https://doi.org/10.1111/bph.12900 [epub ahead of print]
- [24] G. Tang, G. Yang, B. Jiang, Y. Ju, L. Wu, R. Wang; H2S is an endothelium-derived hyperpolarizing factor; Antioxid Redox Signal, 19 (14) (2013), pp. 1634–1644
- [25] M. Bhatia; Hydrogen sulfide as a vasodilator; IUBMB Life, 57 (2005), pp. 603–606
- [26] A. King, D. Polhemus, S. Bhushan, et al.; Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent; Proc Natl Acad Sci U S A, 111 (8) (2014), pp. 3182–3187
- [27] Z. Altaany, G. Yang, R. Wang; Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells; J Cell Mol Med, 17 (7) (2013), pp. 879–888
- [28] J. Beltowski; Endogenous hydrogen sulfide in perivascular adipose tissue: role in the regulation of vascular tone in physiology and pathology; Can J Physiol Pharmacol, 91 (2013), pp. 889–898
- [29] W.J. Cai, M.J. Wang, P.K. Moore, H.M. Jin, T. Yao, Y.C. Zhu; The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation; Cardiovasc Res, 76 (1) (2007), pp. 29–40
- [30] A. Papapetropoulos, A. Pyriochou, Z. Altaany, et al.; Hydrogen sulfide is an endogenous stimulator of angiogenesis; Proc Natl Acad Sci U S A, 106 (51) (2009), pp. 21972–21977
- [31] C. Coletta, A. Papapetropoulos, K. Erdelyi, et al.; Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation; Proc Natl Acad Sci U S A, 109 (23) (2012), pp. 9161–9166
- [32] C. Szabo, A. Papapetropoulos; Hydrogen sulphide and angiogenesis: mechanisms and applications; Br J Pharmacol, 164 (2011), pp. 853–865
- [33] C. Kohn, G. Dubrovska, Y. Huang, M. Gollasch; Hydrogen sulfide: potent regulator of vascular tone and stimulator of angiogenesis; Int J Biomed Sci, 8 (2) (2012), pp. 81–86
- [34] G. Yang, W. Yang, L. Wu, R. Wang; H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells; J Biol Chem, 282 (22) (2007), pp. 16567–16576
- [35] G. Yang, L. Wu, R. Wang; Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells; FASEB J, 20 (2005), pp. 553–555
- [36] N. Sen, B. Paul, M. Gadalla, et al.; Hydrogen sulfide-linked sulfhydration of NF-κB mediates it antiapoptotic actions; Mol Cell, 45 (2012), pp. 13–24
- [37] D. Johansen, K. Ytrehus, G.F. Baxter; Endogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury — evidence for a role of KATP channels; Basic Res Cardiol, 101 (1) (2006), pp. 53–60
- [38] J.W. Elrod, J.W. Calvert, J. Morrison, et al.; Hydrogen sulfide attenuates myocardial ischemia–reperfusion injury by preservation of mitochondrial function; Proc Natl Acad Sci U S A, 104 (39) (2007), pp. 15560–15565
- [39] L.L. Pan, X.H. Liu, Q.H. Gong, H.B. Yang, Y.Z. Zhu; Role of cystathionine γ-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy?; Antioxid Redox Signal, 17 (2012), pp. 106–118
- [40] Y.E. Huang, Z.H. Tang, W. Xie, et al.; Endogenous hydrogen sulfide mediates the cardioprotection induced by ischemic postconditioning in the early reperfusion phase; Exp Ther Med, 4 (2012), pp. 1117–1123
- [41] Y.Z. Zhu, Z.J. Wang, P.Y. Ho, et al.; Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats; J Appl Physiol, 102 (2007), pp. 261–268
- [42] S. Givvimani, C. Munjal, R. Gargoum, et al.; Hydrogen sulfide mitigates transition from compensatory hypertrophy to heart failure; J Appl Physiol, 110 (2011), pp. 1093–1100
- [43] A. King, D. Lefer; Cytoprotective actions of hydrogen sulfide in ischaemia–reperfusion injury; Exp Physiol, 96 (9) (2011), pp. 840–846
- [44] Z.W. Lee, Y.L. Low, S. Huan, T. Wang, L.W. Deng; The cystathionine γ-lyase/hydrogen sulfide system maintains cellular glutathione status; Biochem J, 460 (3) (2014), pp. 425–435
- [45] J. Calvert, M. Elston, C.K. Nicholson, et al.; Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice; Circulation, 122 (2010), pp. 11–19
- [46] E.M. Bos, R. Wang, P.M. Snijder, et al.; Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress; J Am Soc Nephrol, 24 (2013), pp. 759–770
- [47] K. Kondo, S. Bhushan, A.L. King, et al.; H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase; Circulation, 217 (2013), pp. 1116–1127
- [48] J. Cui, L. Liu, J. Zou, et al.; Protective effect of endogenous hydrogen sulfide against oxidative stress in gastric ischemia–reperfusion injury; Exp Ther Med, 5 (2013), pp. 689–694
- [49] X. Yao, G. Tan, C. He, et al.; Hydrogen sulfide protects cardiomyocytes from myocardial ischemia–reperfusion injury by enhancing phosphorylation of apoptosis repressor with caspase recruitment domain; Tohoku J Exp Med, 226 (2012), pp. 275–285
- [50] Ida Tomoaki, T. Sawa, H. Ihara, et al.; Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling; Proc Natl Acad Sci U S A, 111 (21) (2014), pp. 7606–7611 https://doi.org/10.1073/pnas.1321232111
- [51] M. Bhatia, J. Sidhapuriwala, S.M. Moochhala, P.K. Moore; Hydrogen sulfide is a mediator of carrageenan-induced hindpaw edema in the rat; Br J Pharmacol, 145 (2005), pp. 141–144
- [52] L. Li, M. Bhatia, Y.Z. Zhu, et al.; Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse; FASEB J, 19 (9) (2005), pp. 1196–1198 https://doi.org/10.1096/fj.04-3583fje
- [53] H. Zhang, S.M. Moochhala, M. Bhatia; Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis; J Immunol, 181 (2008), pp. 4320–4331
- [54] H. Zhang, L. Zhi, P.K. Moore, M. Bhatia; The role of hydrogen sulfide in cecal ligation and puncture induced sepsis in the mouse; Am J Physiol Lung Cell Mol Physiol, 20 (2006), pp. 6008–6016
- [55] R. Tamizhselvi, P. Moore, M. Bhatia; Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: in vitro studies using isolated mouse pancreatic acinar cells; J Cell Mol Med, 11 (2007), pp. 315–326
- [56] A.D. Ang, J. Rivers-Auty, A. Hegde, I. Ishii, M. Bhatia; The effect of CSE gene deletion in caerulein-induced acute pancreatitis in the mouse; Am J Physiol Gastrointest Liver Physiol, 305 (10) (2013), pp. 712–721
- [57] L. Li, M. Salto-Tellez, C.H. Tan, M. Whiteman, P.K. Moore; GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in rats; Free Radic Biol Med, 47 (1) (2009), pp. 103–113
- [58] L. Li, G. Rossoni, A. Sparatore, L.C. Lee, P. Del Soldato, P.K. Moore; Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative; Free Radic Biol Med, 42 (5) (2007), pp. 706–719
- [59] S. Bekpinar, Y. Unlucerci, M. Uysal, F. Gurdol; Propargylglycine aggravates liver damage in LPS-treated rats: possible relation of nitrosative stress with the inhibition of H2S formation; Pharmacol Rep, 66 (2014), pp. 897–901
- [60] M. Bhatia; Role of hydrogen sulfide in the pathology of inflammation; Scientifica, 2012 (2012), p. 159680 https://doi.org/10.6064/2012/159680
- [61] A. Badiei, N. Muniraj, S. Chambers, M. Bhatia; Inhibition of hydrogen sulfide production by gene silencing attenuates inflammatory activity by downregulation of NF-κB and MAP kinase activity in LPS-activated RAW 264.7 cells; Biomed Res Int, 2014 (2014), p. 848570 https://doi.org/10.1155/2014/848570
- [62] Hui Yan, J. Du, C. Tang, G. Bin, H. Jiang; Changes in arterial hydrogen sulfide (H2S) content during septic shock and endotoxin shock in rats; J Infect, 47 (2003), pp. 155–160
- [63] V. Brancaleona, E. Mitidieri, R.J. Flower; Annexin A1 mediates hydrogen sulfide properties in the control of inflammation; J Pharmacol Exp Ther, 351 (2014), pp. 94–104
- [64] J. Wallace, R. Blackler, M.V. Chan, et al.; Anti-inflammatory and cytoprotective actions of hydrogen sulfide: translation to therapeutics; Antioxid Redox Signal, 22 (5) (2015 Feb 10), pp. 398–410 https://doi.org/10.1089/ars.2014.5901 Epub 2014 Apr 15
- [65] R. Wang; Twos company, threes a crowd: can H2S be the third endogenous gaseous transmitter?; FASEB J, 16 (2002), pp. 1792–1798
- [66] R. Wang; Physiological implication of hydrogen sulfide: a whiff exploration that blossomed; Physiol Rev, 92 (2) (2012), pp. 791–896
- [67] X.H. Yu, L.B. Cui, K. Wu, et al.; Hydrogen sulfide as a potent cardiovascular protective agent; Clin Chim Acta, 437 (2014), pp. 78–87
- [68] B. Olas; Hydrogen sulfide in hemostasis: friend or foe?; Chem Biol Interact, 217 (2014), pp. 49–56 https://doi.org/10.1016/j.cbi.2014.04.006 [Epub 2014 Apr 16]
- [69] A. Martelli, L. Testai, M.C. Breschi, et al.; Hydrogen sulphide: novel opportunity for drug discovery; Med Res Rev, 32 (6) (2012), pp. 1093–1130
- [70] D.J. Elsey, R.C. Fowkes, G. Baxter; Regulation of cardiovascular cell function by hydrogen sulfide (H2S); Cell Biochem Funct, 28 (2010), pp. 95–106
- [71] H. Kimura, N. Shibuya, Y. Kimura; Hydrogen sulfide is a signaling molecule and cytoprotectant; Antioxid Redox Signal, 17 (1) (2012), pp. 45–57
- [72] M.C. Vandiver, S.H. Snyder; Hydrogen sulfide: a gasotransmitter of clinical relevance; J Mol Med, 90 (2012), pp. 255–263
- [73] H. Kimura; Signaling molecules: hydrogen sulfide and polysulfide; Antioxid Redox Signal, 22 (5) (2015 Feb 10), pp. 362–376 https://doi.org/10.1089/ars.2014.5869 Epub 2014 Jun 25
- [74] K. Zao, H. Li, S. Li; Regulation of cystathionine gamma-lyase/H2S system and its pathological implication; Front Biosci (Landmark Ed), 19 (2014), pp. 1355–1369
- [75] H. Kimura; The physiological role of hydrogen sulfide and beyond; Nitric Oxide, 41 (2014), pp. 4–10 https://doi.org/10.1016/j.niox.2014.01.002 [Epub 2014 Feb 1]
- [76] H. Kimura; Hydrogen sulfide: its production, release and function; Amino Acids, 41 (2011), pp. 113–121
- [77] L. Li, P. Rose, P.K. Moore; Hydrogen sulfide and cell signaling; Annu Rev Pharmacol Toxicol, 51 (2011), pp. 169–187
- [78] K. Wang, S. Ahmad, M. Cai, et al.; Dysregulation of hydrogen sulfide producing enzyme cystathionine γ-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia; Circulation, 127 (25) (2013), pp. 2514–2522 https://doi.org/10.1161/CIRCULATIONAHA.113.001631 [Epub 2013 May 23]
- [79] Y.H. Chen, W.Z. Yao, B. Geng, et al.; Endogenous hydrogen sulfide in patients with COPD; Chest, 128 (2005), pp. 3205–3211
- [80] Y.H. Chen, W.Z. Yao, J.Z. Gao, B. Geng, P.P. Wang, C.S. Tang; Serum hydrogen sulfide as a novel marker predicting bacterial involvement in patients with community-acquired lower respiratory tract infection; Respirology, 14 (2009), pp. 746–752
- [81] T. Goslar, T. Mars, M. Podbregar; Total plasma sulfide as a marker of shock severity in nonsurgical adult patients; Shock, 36 (2011), pp. 350–355
- [82] Z. Xu, G. Prathapasinghe, N. Wu, S.Y. Hwang, Y.L. Siow, O. Karmin; Ischemia–reperfusion reduces cystathionine-β-synthase-mediated hydrogen sulfide generation in the kidney; Am J Physiol Renal Physiol, 297 (2009), pp. F27–F35
- [83] W. Chai, Y. Wang, J.Y. Lin, et al.; Exogenous hydrogen sulfide protects against traumatic hemorrhagic shock via attenuation of oxidative stress; J Surg Res, 176 (2012), pp. 210–219
- [84] Y.Y. Mok, M.S. Atan, C. Yoke Ping, et al.; Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis; Br J Pharmacol, 142 (2004), pp. 881–889
- [85] L. Li, M. Whiteman, P.K. Moore; Dexamethasone inhibits lipopolysaccharide-induced hydrogen sulphide biosynthesis in intact cells and in an animal model of endotoxic shock; J Cell Mol Med, 13 (2009), pp. 2684–2692
- [86] M.A. Aminzadeh, N.D. Vaziri; Down regulation of the renal and hepatic hydrogen sulfide-producing enzymes and capacity in chronic kidney disease; Nephrol Dial Transplant, 27 (2012), pp. 498–504
- [87] Y.H. Chen, P.P. Wang, X.M. Wang, et al.; Involvement of endogenous hydrogen sulfide in airway responsiveness and inflammation of rat lung; Cytokines, 53 (2011), pp. 334–341
- [88] A. Geng, L. Chang, C. Pan, et al.; Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol; Biochem Biophys Res Commun, 38 (2004), pp. 756–763
- [89] P. Tripatara, N.S. Patel, V. Brancaleone, et al.; Charaterisation of cystathionine gamma-lyase/hydrogen sulphide pathway in ischemia/reperfusion injury of the mouse kidney: an in vivo study; Eur J Pharmacol, 606 (2009), pp. 205–209
- [90] B. Florian, R. Vintilescu, A.T. Balseann, et al.; Long-term hypothermia reduces infarct volume in aged rats after focal ischemia; Neurosci Lett, 432 (2008), pp. 180–185
- [91] J.N. Sidhapuriwala, A. Hegde, A.D. Ang, Y.Z. Zhu, M. Bhatia; Effects of S-propargyl-cysteine in caerulein-induced acute pancreatitis in mice; PLoS One, 7 (3) (2012), p. e32574 https://doi.org/10.1371/journal.pone.0032574
- [92] H. Zhang, L. Zhi, S.M. Moochhala, P.K. Moore, M. Bhatia; Endogenous hydrogen sulfide regulates leukocyte trafficking in cecal ligation and puncture-induced sepsis; J Leukoc Biol, 179 (2007), pp. 4153–4160
- [93] H. Zhang, S.W. Sio, S.M. Moochhala, M. Bhatia; Role of hydrogen sulfide in severe burn injury-induced inflammation in mice; Mol Med, 16 (2010), pp. 417–424
- [94] S.F. Ang, S.M. Moochhala, M. Bhatia; Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis; Crit Care Med, 38 (2010), pp. 619–628
- [95] A. Stein, S.M. Bailey; Redox biology of hydrogen sulfide: implications for physiology, pathophysiology, and pharmacology; Redox Biol, 1 (2013), pp. 32–39
- [96] K.R. Olson, E.R. DeLeon, F. Liu; Controversies and conundrums in hydrogen sulfide biology; Nitric Oxide, 41 (2014 Sep 15), pp. 11–26 https://doi.org/10.1016/j.niox.2014.05.012 Epub 2014 Jun 11
- [97] G.K. Kolluru, X. Shen, S.C. Bir, C.G. Kevil; Hydrogen sulfide chemical biology: pathophysiological roles and detection; Nitric Oxide, 35C (2013), pp. 5–20
- [98] O. McCook, P. Radermacher, C. Volani, et al.; H2S during circulatory shock: some unresolved questions; Nitric Oxide, 41 (2014), pp. 48–61
- [99] A.R. Lippert, E.J. New, C.J. Chang; Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells; J Am Chem Soc, 133 (2011), pp. 10078–10080
- [100] V.S. Lin, C.J. Chang; Fluorescent probes for sensing and imaging biological hydrogen sulfide; Curr Opin Chem Biol, 16 (2012), pp. 595–601
- [101] C. Wei, L. Wei, Z. Xi, Yi. Long; A FRET-based fluorescent probe for imaging H2S in living cells; Tetrahedron Lett, 54 (2013), pp. 6937–6939
- [102] Y. Zheng, M. Zhao, Q. Qiao, H. Liu, H. Lang, Z. Xu; A near-infrared fluorescent probe for hydrogen sulfide in living cells; Dyes Pigments, 98 (2013), pp. 367–371
- [103] K. Wang, H. Peng, B. Wang; Recent advances in thiol and sulfide reactive probes; J Cell Biochem, 115 (6) (2014), pp. 1007–1022 https://doi.org/10.1002/jcb.24762
- [104] A.R. Lippert; Designing reaction-based fluorescent probes for selective hydrogen sulfide detection; J Inorg Biochem, 133 (2014), pp. 136–142
- [105] V.S. Lin, A.R. Lippert, C.J. Chang; Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production; Proc Natl Acad Sci U S A, 110 (18) (2013), pp. 7131–7135
- [106] I. Ishii, N. Akahoshi, X.N. Yu, et al.; Murine cystathionine γ-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression; Biochem J, 381 (2004), pp. 113–123
- [107] Y. Zhang, H. Li, G. Zhao, et al.; Hydrogen sulfide attenuates the recruitment of CD11b+Gr-1+ myeloid cells and regulates Bax/Bcl-2 signaling in myocardial ischemia injury; Sci Rep, 4 (2014), p. 4774 https://doi.org/10.1038/srep04774
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?