Summary
Objective
Massive hemothorax in patients on extracorporeal membrane oxygenation (ECMO) is potentially life threatening and remains a medical challenge. In this study, we present the clinical results of using aggressive management to treat a consecutive series of patients on ECMO whose conditions were complicated by massive hemothorax.
Methods
Between November 2003 and February 2010, 14 adult patients on ECMO developed massive hemothorax that was unrelated to the cannulation problems of the ECMO circuit at National Taiwan University Hospital, Taipei, Taiwan. Information was obtained regarding patient demographics, disease course, and treatment. Aggressive treatment of hemothorax included blood component therapy, chest tube drainage, pleural epinephrine irrigation, and surgical intervention. The criteria for surgical intervention, video-assisted thoracoscopic surgery (VATS), or open-window thoracostomy included one-third or more of the thoracic cavity that had accumulated blood clots resulting in a compromised cardiopulmonary status, continuous blood loss > 300 mL/hour for 4 hours or more, or continued bleeding for 24 hours after persistent blood transfusion.
Results
All hemothoraces were unilateral. With coagulopathic correction, control of bleeding was obtained in two patients after decompression of the pleural cavity, four patients after pleural epinephrine irrigation, and eight of 14 patients required surgical intervention for blood clot evacuation. There were no specific findings except blood clot accumulation in each of the patients who underwent thoracotomy or VATS. Three of the eight patients required multiple operations to treat persistent bleeding. The in-hospital mortality rate was 36% (5 of 14 patients); one patient died of intractable bleeding and four deaths were related to multiple organ failure. Blood transfusion (Mann-Whitney U test; p = 0.039) and comorbidities such as bacteremia, septic shock, diabetic mellitus, and immunocompromised status (Fisher exact test; p = 0.031) were found to be significant and independent predictors of mortality. However, other factors such as age, complicated pneumothorax, and ECMO circuit duration were not statistically correlated with mortality.
Conclusion
ECMO-related massive hemothorax usually occurred unilaterally and presented as a life-threatening condition. With intensive treatment, nearly two-thirds of the patients were saved. The most significant risk factor for mortality was the presence of a comorbidity such as sepsis, diabetic mellitus, or immunocompromised status.
Keywords
extracorporeal membrane oxygenation;hemothorax
1. Introduction
Extracorporeal membrane oxygenation (ECMO) is internationally used as a viable, life-saving cardiopulmonary intervention that to treat a variety of critical ill patients.1; 2; 3 ; 4 However, the complication of massive hemothorax in patients undergoing ECMO remains a medical challenge and is potentially life threatening because of the underlying problems of bleeding tendency,5 associated multiple organ failure, and the difficulty of performing the surgical techniques required for its treatment.6 Although hemothorax has been recognized as a clinical entity for centuries, the surgical strategies for its management, especially when complicated by ECMO, are rarely described in detail in the medical literature. On review of the literature, only adjustments to the heparin dosage6 and the drainage of pleural hemorrhage7 are mentioned. This study summarizes the multimodal approach and related prognostic factors for treating patients with ECMO-related massive hemothorax.
2. Patients and methods
2.1. Study design
This retrospective cohort study, which was approved by the institutional research board of our institution, included all of the consecutive adult ECMO patients who were treated at the National Taiwan University Hospital between November 2003 and February 2010. In general, the indication for ECMO was severe cardiopulmonary disorder when all other management options failed.
2.2. Case selection and hemothorax management
Patients with massive hemothorax that met the criteria of rapid accumulation of pleural hematoma and the shifting of the mediastinal structures that were recognizable on bedside imaging were included in this study. In total, 14 consecutive adult ECMO patients with massive hemothorax (unrelated to ECMO cannulation or cardiopulmonary resuscitation) were enrolled into this study. The medical records included each patients sex, event that resulted in pleural hemorrhage, and specific interventions that were administered with respect to variations in chest tube output and blood transfusion requirements. Daily anteroposterior chest radiographs were obtained during ECMO support for all 14 patients. Sonograms were used to verify any radiographically suspected pleural fluid and on follow-up examinations of any hemorrhage.
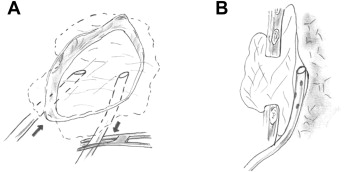
The patients with ECMO-related massive hemothorax were initially managed by simultaneous replacement of blood volume and drainage of hemothorax by chest tube thoracostomy. The volume of blood initially drained from the chest tube and the rate of continuous blood loss were used to decide the amount of replacement intravenous fluid. Low-dose heparin infusion as part of the ECMO circuit (100 U/kg heparin) was discontinued and all subjects were prescribed 500 mg q8h tranexamic acid via intravascular injection to control bleeding. We also used pleural epinephrine irrigation through the chest tube at a rate of 5 mg epinephrine/1000 mL normal saline. Two hundred mL was infused each time and retained for 15 minutes and then released, and the procedure was repeated no more than five times. In addition, the surgical indications included continuous blood loss > 300 mL/hour over a period of 4 hours, persistent blood transfusion requirements, continued bleeding for 24 hours, or, more often, the accumulation of blood clots in one-third or more of the chest cavity that resulted in compromised cardiopulmonary status. The surgical strategies included video-assisted thoracoscopic surgery (VATS) for detecting the source of the bleeding and evacuating the blood clots. Hemostasis was achieved by clip ligation and electrocautery. VATS was converted to open-window thoracostomy with dressing and packing if control of the refractory bleeding failed. The wet dressings were frequently changed for postoperative hemostasis, and continuous pleural epinephrine irrigation was administered through a chest tube (Fig. 1).
|
|
|
Figure 1. Schematic illustration showing soaked dressings in an open-window thoracostomy and epinephrine irrigation through tubes between the dressings and the lung. A) Front view showing the dressings in an open-window thoracostomy with intermittent epinephrine irrigation and the retention of hemostasis. B) Lateral view showing epinephrine irrigation through chest tubes that were placed between the lung parenchyma and the dressings. |
2.3. ECMO circuit management
At our institution, the ECMO circuit consists of a centrifugal pump, a hollow-fiber microporous membrane oxygenator with an integrated heater, and percutaneous thin-wall cannula (CB2505; Medtronic Inc., Anaheim, CA, USA), all of which are coated with a heparin-bound Carmeda Bioactive Surface. A silicone membrane oxygenator (I-4500-2A; Medtronic BioMedicus, Inc, Minneapolis, Minn, U.S.A.) is used in the ECMO circuit when prolonged ECMO support is required because the microporous membrane oxygenator cannot last for long periods of time. Heparin (100 U/kg) was administered to the patient before cannulation, and then continuous heparin infusion was used to keep the whole blood activated clotting time (ACT) in the range of 160–220 seconds (normal: ≤ 120 seconds) in order to prevent ECMO-related hemolysis or thrombotic complications. In addition, the patients were maintained on paralytics and narcotics during ECMO support to reduce stress and the oxygen demands. Under ECMO support, the mechanical ventilator setting was adjusted according to the lung-protection ventilator strategy (lowest FiO2 to maintain SpO2 ≥ 90%, ventilator rate 6–10/min, pressure control mode with peak inspiratory pressure ≤ 35 cm H2O, and positive end-expiratory pressure maintained at 15 cm H2O) used to prevent ventilator-induced lung injury, the complications of oxygen toxicity, and to maximize the recruitment of functional residual lung capacity. Continuous online circuit monitors were able to display the hematocrit, pre- and postoxygenated blood oxygen saturation level (BioTrend; Medtronic Inc., Anaheim, CA), blood flow rate, and the prepump, preoxygenated, and postoxygenated pressure. After adequate improvement of the patients clinical hemodynamic status was achieved, attempts were made to wean the patient off the ECMO system.
2.4. Statistical analysis
The demographic and clinical characteristics of the patients are presented as the means ± standard deviations (SD) or as proportions. The Mann-Whitney U test was used to assess the impact of blood loss, blood transfusion, white blood cell count, platelet count, prothrombin time, partial thromboplastin time, C-reactive protein level, age, mean tidal volume, and PaO2/FiO2 ratio between the surviving and deceased patients. Noncontinuous data, including chest X-ray findings, sex, and comorbidities such as bacteremia, septic shock, bleeding tendency, diabetic mellitus, and immunocompromised status were evaluated using the Fisher exact test. The primary clinical outcome was survival and discharge from the hospital. All statistical analyses were performed using Stata 8.0 software (SAS Corporation, College Station, TX, USA). Statistical significance was defined as a p < 0.05.
3. Results
Among our 14 patients (12 male, 2 female; median age: 39 years; range: 16–82 years), the indications for ECMO support was status asthmaticus in one patient, adult respiratory distress syndrome (ARDS) in nine patients, lung transplantation with primary graft dysfunction in two patients, acute myocardial infarction in one patient, and unstable hemodynamics after the repair of a cardiac penetration injury in one patient (Table 1). Hemothorax in each patient in this study was unilateral (right side, 4; left side, 9). Among the lung transplant and cardiac trauma patients, hemothorax was not contributed to the previous surgery during the subsequent operation for blood clot evacuation. Nine of the 14 patients (64%) survived to hospital discharge; one death was attributed to refractory bleeding and four deaths were attributed to multiple organ failure. Among these 14 patients, the mean ECMO circuit duration was 570 ± 436 hours (range: 45–1242 hours). Five patients, including three survivors, received unusually long periods of ECMO support (> 900 hours) due to severe ARDS and pneumothorax with prolonged air leak. The median interval between the start of ECMO and onset of pleural hemorrhage was 12 days (range: 2–42 days).
| Case No. | Age/ Sex | Diagnosis | Status while ECMO setup | Mode of ECMO support | Sites of cannulation (out/in) | Bleeding amount (ml/day) | Blood transfusion (ml/day) (component)* | Management modalities | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30/M | Status asthmaticus | 90 ml tidal volume | VV | RFV/ RIJV | 580 | 280 (R280) | Tube thoracostomy | S |
| 2 | 50/M | Liver abscess with ARDS, ARF | 100% O2 demand | VV | RFV/ RIJV | 1200 | 1060 (R440; W620) | VATS, OW thoracostomy | S |
| 3 | 52/F | Pulmonary lymphangiomyomatosis s/p LTx with ARDS, liver failure | s/p LTx with lung injury | VA | RFV/ RFA | 3390 | 4600 (R2315; F1280; P1005) | VATS | Mo |
| 4 | 20/M | Pneumonia with septic shock, ARDS, ARF | 100% O2 demand | VA then VV | LFV/ LFA | 2210 | 1845 (R1260; F585) | Tube thoracostomy | S |
| 5 | 38/M | VSD with Eisenmenger change s/p VSD repair and ASD fenestration, pneumonia, ARDS | ARDS with 100% O2 demand | VV | LFV/ RIJV | 2480 | 4755 (R2955; F1650; P150) | VATS | S |
| 6 | 22/M | Type I DM, empyema with sepsis, ARDS | 90% O2 demand | VA->VA+VV->VV | LFV/ LFA | 1360 | 2750 (R1430; F710; P500; C110) | VATS, OW thoracostomy | Mo |
| 7 | 54/M | Acute myocardial infarction with ARDS, ARF | IABP used | VV then VA | LFV/ LFA | 1350 | 7130 (R2915; F120; P1410; W2685) | VATS, OW thoracostomy | Mo |
| 8 | 36/M | Heart penetrating injury, necrotizing pneumonia with MOF | Cardiac tamponade with CPR | VV and VA | RA/ Aorta | 1080 | 10020 (R7120; C2900) | VATS, OW thoracostomy | Mo |
| 9 | 16/M | Septic arthritis, pneumonia with MOF | CPR | VA then VV | LFV/ LFA | 1350 | 4210 (R3680; P530) | VATS, OW thoracostomy | S |
| 10 | 28/M | ALL s/p BMT, CMV pneumonitis with ARDS | 100% O2 demand | VV | RFV/ RIJV | 4080 | 2620 (R2000; P500; C120) | VATS, OW thoracostomy | Mo |
| 11 | 30/F | Paraneoplastic pemphigus and bronchiolitis obliterans s/p LTx | s/p LTx with lung injury | VA then VV | LFV/ LFA | 1670 | 3485 (R1300; F1420; P765) | PEI | S |
| 12 | 56/M | Pneumonia with septic shock, ARDS, ARF | IABP used | VV | RFV/ RIJV | 700 | 1240 (R360; P880) | PEI | S |
| 13 | 34/M | Pneumonia with ARDS | 100% O2 demand | VV | RFV/ RIJV | 900 | 1450 (R700; F750) | PEI | S |
| 14 | 82/M | Pneumonia with septic shock, ARDS, ARF | 100% O2 demand | VV | LFV/ RIJV | 600 | 2590 (R1750; F330; P450; C60) | PEI | S |
ALL: Acute lymphoblastic leukemia; ARDS: acute respiratory distress syndrome; ARF: acute renal failure; ASD: atrial septal defect; BMT: bone marrow transplantation; CMV: Cytomegalovirus; DM: diabetic mellitus; F: female; LFA: left femoral artery; LFV: left femoral vein; LTx: lung transplantation; M: male; Mo: mortality; MOF: Multiple organ failure; OW: open window; PEI: Pleural epinephrine irrigation; RA: right atrium; RFA: right femoral artery; RFV: right femoral vein; RIJV: right internal jugular vein; S: survival; s/p: status post; VA: venoarterial; VATS: video-assisted thoracoscopic surgery; VSD: ventricular septal defect; VV: venovenous.
- component: C: cryoprecipitate; F: fresh frozen plasma; P: platelet; R: packed red blood cell; W: whole blood.
The mean daily amount of blood transfusion during the most active bleeding episode was 3431 ± 2623.1 mL/day. Blood transfusion (Mann-Whitney U test, p = 0.039) and the presence of a comorbidity (Fishers exact test, p = 0.031) were independent predictors of death (Table 2). However, 24-hour blood loss during the most active bleeding episode did not significantly influence the clinical outcome (p = 0.423; Table 2). With coagulopathic correction, control of bleeding was obtained in two patients after decompression of the pleural cavity. In addition, another four patients developed hemostasis without surgery after pleural epinephrine irrigation through the chest tube. Pleural epinephrine irrigation caused a mild increase in the systolic blood pressure of these patients. Eight patients required surgical intervention: two received VATS and six received VATS that was then converted to open-window thoracostomy for the management of hemithorax. Moreover, there were no specific findings (except blood clot accumulation) that presented in every patient during thoracotomy or VATS. Among our patients, three had to delay their operations until the early discontinuation of ECMO within 1 week. Three patients required repeated operations for persistent bleeding. After surgical intervention, oxygen saturation was temporarily increased in all patients.
| Variable | Surviving patients (n = 9) Mean ± SD | Deceased patients (n = 5) Mean ± SD | p |
|---|---|---|---|
| Age (y) | 39.6 ± 20.4 | 39.1 ± 17.9 | 0.947 |
| Male/female | 8/1 | 4/1 | 1.0 |
| Comorbidity | 1/9 | 5/5 | 0.031 |
| Duration of ECMO (h) | 632.7 ± 434.9 | 456.2 ± 464.3 | 0.317 |
| PaO2/FiO2 ratio | 191.7 ± 123.6 | 178.9 ± 73.4 | 0.841 |
| Tidal volume before treatmenta (mL) | 516.0 ± 123.7 | 484.0 ± 91.3 | 0.853 |
| Tidal volume (mL) during hemothorax | 364.6 ± 214.0 | 178.6 ± 160.0 | 0.096 |
| Tidal volume after treatment (mL) | 524.6 ± 106.6 | 527.3 ± 89.9 | 0.540 |
| Blood loss (mL/d) | 1743.3 ± 1251.5 | 2252.0 ± 1380.2 | 0.423 |
| Blood transfusion (mL/d) | 2323.9 ± 1534.2 | 5424.0 ± 3151.4 | 0.039 |
| Complicated pneumothorax | 2/9 | 4/5 | 0.301 |
SD: standard deviation.
a. Treatment included drainage, thoracoscopic surgery, or thoracotomy.
Furthermore, the mean tidal volume and PaO2/FiO2 ratio during the most active bleeding episode tended to be lower in nonsurvivors (p = 0.096 and 0.841, respectively). However, factors such as age (p = 0.947), ECMO circuit duration (p = 0.317), and complicated pneumothorax (p = 0.301) did not statistically or significantly correlate with mortality.
4. Discussion
ECMO is a common technique used to sustain life during recovery from acute cardiopulmonary failure. Typically, anticoagulation is used from the initiation of the ECMO circuit through the cessation of ECMO support. Hemorrhage is an important complication of ECMO with a reported incidence of 12.2%.5 Hemothorax is associated with an 80–100% risk of mortality when conventionally treated,8 such as by adjusting the heparin dosage or the drainage of pleural hemorrhage.9 Some of these complications are related to the primary disease and some are related to systemic anticoagulation. In addition, a pre-ECMO history of chest needle aspiration is also important because complications such as hemothorax or hemopericardium can arise once ECMO is initiated,9 as occurred in one of our patients. Chest radiography remains the mainstay of the diagnosis of hemithorax,10 but computed tomography and sonography provide better imaging of the surrounding structures when considering surgical intervention.
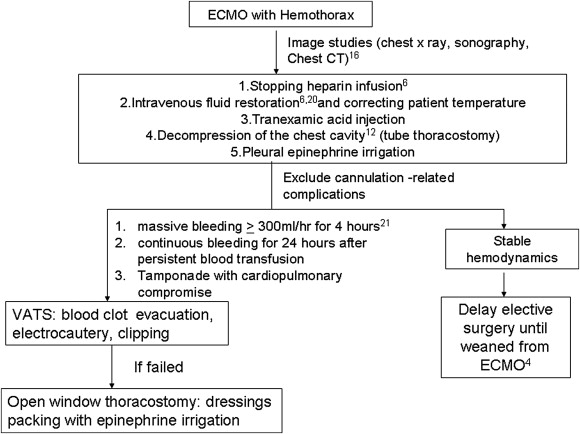
Because ECMO patients are critically ill and frequently have coexisting conditions, half of our patients were diagnosed with multiple organ failure. Without a doubt, it is very difficult to identify the factors associated with survival in this small cohort of patients with diverse diagnoses. However, blood transfusion and comorbidity were determined to be factors related to survival outcome in this study. Increased blood transfusion demand results in the greater deterioration of the patients physiological condition. When confronting this troublesome situation, adjusting the heparin dosage is the only treatment mentioned in the literature,6 ; 11 and the results are discouraging. Based on our experiences of treating patients with hemothorax-complicated ECMO, we propose a therapeutic algorithm for the treatment of these critically ill patients (Fig. 2). Treatment of the underlying disease and adjusting the anticoagulant therapy are mandatory when hemothorax occurs. Major surgical intervention must be postponed, if possible, until near the termination of ECMO therapy or until the patient can be rapidly weaned off ECMO because bleeding tendency contributes to postoperatively persistent hemothorax.12 Furthermore, these patients are often so critically ill that they might be unsuitable for surgery. Although our experiences suggest that surgical intervention while on ECMO is technically feasible, three of our patients postponed hemothorax evacuation until the ECMO circuit was discontinued. Drainage of these hemorrhage results in the improvement of the patients clinical status and is possibly essential to the patients survival. Drainage of hemothorax allows for the apposition of the visceral and parietal pleura, which aids hemostasis.13 Clamping of the chest tube does not decrease hemorrhage or hypotension, but it does negatively affect gas exchange, decrease PaO2, and is associated with significant hypercapnia. Therefore, chest tube clamping is not recommended for the treatment of massive hemothorax.13 Recombinant activated factor VII (rFVIIa) is a drug with proven efficacy for counteracting intractable hemorrhage in various scenarios. Its use in patients on ECMO, however, is limited by the increased risk of thrombotic events.14 ; 15 Moreover, neither a reduction in chest tube output nor blood transfusion requirements reached a level of statistical significance when using rFVIIa to treat ECMO-related hemothorax.16
|
|
|
Figure 2. Management algorithm for ECMO-related massive hemothorax based on indications, hemodynamics, and the quantity of blood loss. |
Massive hemothorax and continual bleeding with unstable hemodynamics are indications for further surgical intervention,17 especially if conventional treatment has failed. VATS plays an important role in the diagnosis and treatment of certain thoracic injuries.18 For the treatment of retained hemothorax, VATS is the favored alternative to thoracotomy for evacuation and adequate hemothorax drainage. However, to avoid prolonged surgery and to control intractable hemothorax, conversion to open-window thoracostomy due to soaked dressings is another alternative method for treating critically ill patients. We also developed the use of pleural epinephrine irrigation, which stimulates vascular contractions and further improves hemostasis. Before definitive surgical treatment for hemothorax, we recommend that pleural lavaged streams of epinephrine be used first. However, some hemothoraces (e.g., in patients with ruptured great vessels, organ rupture, or massive postoperative hemothorax) require indispensably immediate and definitive surgical intervention. Therefore, pleural epinephrine irrigation should not be continuously used if bleeding does not stop or decrease.
Though the case number is limited and the survival rate (64%) needs to be improved in this study, it is the largest series available on this serious complication (Table 3). Based on our experiences and the findings of previous reports,6; 11; 19; 20 ; 21 we recommend an algorithm-guided approach to effectively determine whether surgical exploration is necessary or whether nonoperative treatment is sufficient for ECMO patients with massive hemothorax. In conclusion, ECMO-related massive hemothorax usually occurs unilaterally and presents as a life-threatening condition. With intensive treatment, nearly two-thirds of the patients were saved. The significant risk factor for mortality was the presence of a comorbidity such as sepsis, diabetic mellitus, or immunocompromised status. Further research is required to optimize patient selection, determine the optimal treatment strategies, and establish the important predictors of clinical outcome.
| Hemothorax no. | Surviving | Treatment | |
|---|---|---|---|
| Wagner 199020 | 2 | 1 | Thoracotomy |
| Nagaraj 19929,a | 6 | 0 | 5 tube thoracostomy;1 thoracotomy |
| Lidegran 200512 | 4 | 2 | 1 conservative; 3 thoracotomy |
| Marasco 20076 | 4 | 1 | Adjust anticoagulant dosage |
| Huang et al. | 14 | 9 | Multimodality strategy |
a. This study examined neonates.
Acknowledgments
Written consent for publication was obtained from either the patient or a relative. There are no funding sources related to this study.
References
- 1 R.H. Bartlett, D.W. Roloff, J.R. Custer, J.G. Younger, R.B. Hirschl; Extracorporeal life support: the University of Michigan experience; JAMA, 283 (2000), pp. 904–908
- 2 D.J. Schuerer, N.S. Kolovos, K.V. Boyd, C.M. Coopersmith; Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement; Chest, 134 (2008), pp. 179–184
- 3 S.C. Huang, E.T. Wu, Y.S. Chen, et al.; Extracorporeal membrane oxygenation rescue for cardiopulmonary resuscitation in pediatric patients; Crit Care Med, 36 (2008), pp. 1607–1613
- 4 D. Cruz, R. Bellomo, J.A. Kellum, M. de Cal, C. Ronco; The future of extracorporeal support; Crit Care Med, 36 (2008), pp. S243–S252
- 5 C. Masalunga, M. Cruz, B. Porter, S. Roseff, B. Chui, E. Mainali; Increased hemolysis from saline pre-washing RBCs or centrifugal pumps in neonatal ECMO; J Perinatol, 27 (2007), pp. 380–384
- 6 S.F. Marasco, A. Preovolos, K. Lim, R.F. Salamonsen; Thoracotomy in adults while on ECMO is associated with uncontrollable bleeding; Perfusion, 22 (2007), pp. 23–26
- 7 G.W. Gross, C.H. Dougherty; Pleural hemorrhage in neonates on extracorporeal membrane oxygenation and after repair of congenital diaphragmatic hernia: imaging findings; AJR Am J Roentgenol, 164 (1995), pp. 951–955
- 8 J.B. Zwischenberger, T.T. Nguyen, J.R. Upp Jr., et al.; Complications of neonatal extracorporeal membrane oxygenation: collective experience from the Extracorporeal Life Support Organization; J Thorac Cardiovasc Surg, 107 (1994), pp. 838–848 discussion 48–49
- 9 H.S. Nagaraj, K.A. Mitchell, M.E. Fallat, D.B. Groff, L.N. Cook; Surgical complications and procedures in neonates on extracorporeal membrane oxygenation; J Pediatr Surg, 27 (1992), pp. 1106–1109 discussion 9–10
- 10 M.K. Lidegran, H.G. Ringertz, B.P. Frenckner, V.B. Linden; Chest and abdominal CT during extracorporeal membrane oxygenation: clinical benefits in diagnosis and treatment; Acad Radiol, 12 (2005), pp. 276–285
- 11 F. Bakhtiary, H. Keller, S. Dogan, et al.; Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock: clinical experiences in 45 adult patients; J Thorac Cardiovasc Surg, 135 (2008), pp. 382–388
- 12 J.B. Atkinson, H. Kitagawa, B. Humphries; Major surgical intervention during extracorporeal membrane oxygenation; J Pediatr Surg, 27 (1992), pp. 1197–1198
- 13 J. Ali, W. Qi; Effectiveness of chest tube clamping in massive hemothorax; J Trauma, 38 (1995), pp. 59–62 discussion 63
- 14 R.P. Chalwin, R. Tiruvoipati, G.J. Peek; Fatal thrombosis with activated factor VII in a paediatric patient on extracorporeal membrane oxygenation; Eur J Cardiothorac Surg, 34 (2008), pp. 685–686
- 15 M. Swaminathan, A.D. Shaw, R.A. Greenfield, K.P. Grichnik; Fatal thrombosis after factor VII administration during extracorporeal membrane oxygenation; J Cardiothorac Vasc Anesth, 22 (2008), pp. 259–260
- 16 A. Veldman, C. Neuhaeuser, H. Akintuerk, et al.; rFVIIa in the treatment of persistent hemorrhage in pediatric patients on ECMO following surgery for congenital heart disease; Paediatr Anaesth, 17 (2007), pp. 1176–1181
- 17 R.C. Jacoby, F.D. Battistella; Hemothorax; Semin Respir Crit Care Med, 22 (2001), pp. 627–630
- 18 J. Freixinet, F. Rodriguez de Castro, S. Quevedo, et al.; Traumatic hemothorax treated by video-assisted thoracoscopic surgery; Arch Bronconeumol, 31 (1995), pp. 424–425
- 19 R.P. Chalwin, J.L. Moran, P.L. Graham; The role of extracorporeal membrane oxygenation for treatment of the adult respiratory distress syndrome: review and quantitative analysis; Anaesth Intensive Care, 36 (2008), pp. 152–161
- 20 P.K. Wagner, M. Knoch, C. Sangmeister, E. Muller, H. Lennartz, M. Rothmund; Extracorporeal gas exchange in adult respiratory distress syndrome: associated morbidity and its surgical treatment; Br J Surg, 77 (1990), pp. 1395–1398
- 21 A. Combes, P. Leprince, C.E. Luyt, et al.; Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock; Crit Care Med, 36 (2008), pp. 1404–1411
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?