Summary
A 27-year-old man with a huge esophageal leiomyoma, 4.5 cm in size, at the distal esophagus suffered from postprandial belching and chest discomfort. Endoscopic submucosal tunnel dissection was implemented to resect his leiomyoma. His symptoms resolved after tumor resection without any sequelae, such as luminal stricture that often occurs after large-size esophageal lesion resection. Endoscopic submucosal tunnel dissection has been reported to be a useful method for the resection of esophageal submucosal tumors; however, such large-size tumors are rare.
Keywords
Endoscopic submucosal dissection ; Endoscopic submucosal tunnel dissection ; Esophageal leiomyoma
Introduction
Endoscopic submucosal dissection (ESD) for early esophageal neoplastic lesions has widely been utilized recently; it is also used to remove esophageal subepithelial lesions, and even to treat achalasia [1] ; [2] ; [3] . Endoscopic submucosal tunnel dissection (ESTD) is an application of ESD for the removal of subepithelial tumors through an artificially created submucosal tunnel without full resection of the mucosal layer [4] ; [5] ; [6] ; [7] . Due to the continuity of the mucosal layer, patients could be free from perforation and obtain rapid recovery after the procedure. Previous reports on ESTD are mostly from China and the reported subepithelial lesions were mostly under 4 cm [4] . Herein, we report a case with an esophageal leiomyoma, 4.5 cm in size, which was removed successfully by ESTD.
Case report
A 27-year-old man received esophagogastroduodenoscopy for his postprandial belching and chest discomfort. A large submucosal tumor located at the esophagocardial junction was noted and diagnosed as leiomyoma through forceps biopsy (Figure 1 A). Subsequent endoscopic ultrasound disclosed that this lesion originated from the superficial muscularis propria layer (Figure 1 B). He received medical treatment including proton-pump inhibitors and prokinetic agents, but his symptoms persisted half a year later. He requested surgery for his esophageal leiomyoma. Preoperative evaluation revealed that excision of this tumor by ESD was feasible. However, given its larger size, the mucosa defect after excision would be over two-thirds of the circumference and might have raised the possibility of postprocedural stenosis. ESTD to retain mucosal layer was decided to perform for this patient.
|
|
|
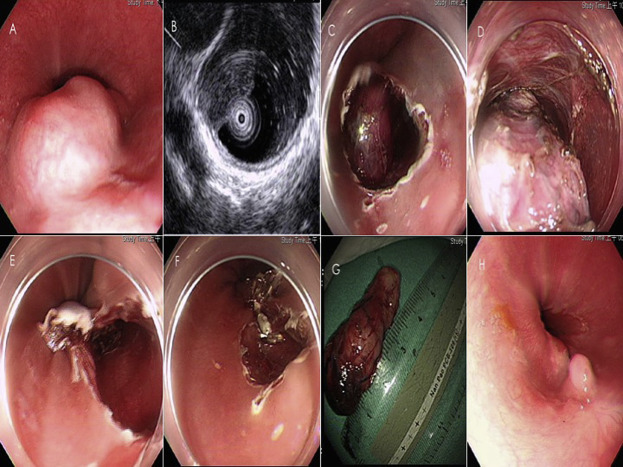
Figure 1. (A) Huge submucosal tumor is noted at a distal segment of the esophagus, close to the esophagocardial junction. (B) Endoscopic ultrasound using high-frequency miniprobe (UM-DP12-25R; Olympus, Tokyo, Japan) discloses that it was originated from the superficial muscularis propria layer of the esophagus. (C) Inlet for endoscopic approach is incised and the tumor is unveiled. (D) Dissection into the submucosal space to excavate the tumor is continued. (E) The tumor is totally excavated out, and (F) the inlet is closed by clips from the distal part. (G) A tumor of up to 4.5 cm in size is noted. (H) Endoscopy follow-up 3 months later reveals a well-healed scar, without luminal stricture. |
The patient was sedated by intravenous midazolam with continuous cardiopulmonary monitoring. The endoscope used was Olympus GIF-Q260J (Olympus, Tokyo, Japan). The electrosurgical unit was Olympus ESG-100 (Olympus). The fluid used for submucosal injection was glycerol, plus 4–5 mL of indigo carmine to identify the submucosal layer easily. We used a dual knife (KD-650-L; Olympus) for incision that was begun 4 cm proximal to the tumor. After a 2 cm incision was made, submucosal dissection was started with the dual knife to create a tunnel (Figure 1 C). When the proximal side of the tumor was exposed, dissection along the tumor continued with both the dual knife and the IT knife (PulseCut slow 30 W; Forced Coag 2 30 W; Figure 1 D). Heavy bleeding, if occurred, was stopped by a Coagrasper (Soft Coag 80 W). After excision of the tumor, the wound was closed securely by endoclips from the distal side (Figures 1 E and 1F). The total procedure time was 100 minutes. This lesion, 4.5 cm in size, was resected en bloc , revealing the free margin pathologically ( Figure 1 G). The patient was instructed to take nothing by mouth, and was administered intravenous proton-pump inhibitor injection every 12 hours for 2 days. Then, he resumed oral intake with a clear liquid diet. The patient did not receive any pre- or postoperative antibiotic treatment. He was discharged uneventfully 5 days later. Follow-up esophagogastroduodenoscopy 3 months later revealed complete healing of the wound without stenosis (Figure 1 H). Belching and chest discomfort also disappeared after the removal of this tumor.
Discussion
ESD is now a feasible, effective, and safe technique for the removal of early esophageal neoplasms. The common complications of ESD for esophageal lesions include perforation, pneumothorax, and postprocedural luminal stricture [1] . Postprocedural stricture occurs more frequently if the lesions involved are over two-thirds of the circumference [8] ; [9] . Injections with steroids or other agents just after ESD are used to prevent postprocedural luminal stricture [10] . If luminal stricture develops, balloon dilatations are frequently used to manage it.
Recently, ESD has also been applied to treat esophageal diseases. Peroral endoscopic myotomy, for example, is a novel method for managing achalasia [2] ; [3] . In this technique, the mucosal layer is incised without being removed and the submucosal layer is dissected to obtain space for cutting off the inner muscular layer. After the myotomy procedure, the artificially created inlet is closed by clipping and the continuity of the mucosal layer is restored. Therefore, with this technique, patients experience not only symptom relief, but also rapid recovery, compared with laparoscopic myotomy [11] .
ESTD is a method with a similar perspective for removal of subepithelial tumors. In patients with esophageal subepithelial tumors, the mucosal layer is clinicopathologically normal and it is not necessary to resect it for study. ESTD was initiated by some Chinese endoscopists [4] ; [5] . Because the creation of submucosal tunnel needs adequate distance from targeted lesion and stable scope manipulation is also necessary [4] ; [5] ; [7] . Common complications included perforation, subcutaneous emphysema, and pneumothorax, and all could be managed conservatively [4] ; [5] ; [7] . Lesions originating either from superficial or from deep muscularis propria could be resected by ESTD. Gastrointestinal stromal tumors and leiomyoma were the two most common types of tumors being resected. However, close surveillance is necessary for gastrointestinal stromal tumors given their malignant potential. Until now, the largest reported lesion was under 4 cm [4] . Some authors claimed that the larger size of a lesion would be an obstacle for maintaining the manipulation tunnel space [5] . In our opinion, a transparent hood is useful for overcoming this difficulty. ESTD also shows comparable outcome to the conventional ESD [12] .
One of the problems is the procedure time. ESTD excavates a ball-like tumor out from the submucosal space. Both horizontal and vertical dissections are necessary. Therefore, the dissection actions are theoretically more than that of ESD, which requires only horizontal dissection. Although a previous report revealed a shorter procedure time for ESTD than for ESD, this study did not delineate the learning curve effect of ESD. In this case, we also found that the IT knife, rather than the dual knife, might be more suitable for multidirectional dissection in ESTD, although this needs more validated evidence. For smaller esophageal submucosal lesions, our experiences disclosed that ESD was faster as well as effective.
We think that ESTD should be reserved for patients with larger submucosal tumors to prevent postprocedural complications such as luminal stricture or with lesions arising from the muscularis propria layer to avoid free perforation.
Conflicts of interest
All authors declare that there are no conflicts of interests regarding this work.
References
- [1] S. Ono, M. Fujishiro, K. Koike; Endoscopic submucosal dissection for superficial esophageal neoplasms; World J Gastrointest Endosc, 4 (2012), pp. 162–166
- [2] L.L. Swanstrom, A. Kurian, C.M. Dunst, A. Sharata, N. Bhayani, E. Rieder; Long-term outcomes of an endoscopic myotomy for achalasia: the POEM procedure; Ann Surg, 256 (2012), pp. 659–667
- [3] H. Inoue, H. Minami, Y. Kobayashi, Y. Sato, M. Kaga, M. Suzuki, et al.; Peroral endoscopic myotomy (POEM) for esophageal achalasia; Endoscopy, 42 (2010), pp. 265–271
- [4] W. Gong, Y. Xiong, F. Zhi, S. Liu, A. Wang, B. Jiang; Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors; Endoscopy, 44 (2012), pp. 231–235
- [5] M.D. Xu, M.Y. Cai, P.H. Zhou, X.Y. Qin, Y.S. Zhong, W.F. Chen, et al.; Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos); Gastrointest Endosc, 75 (2012), pp. 195–199
- [6] M. Cai, J. Chen, P. Zhou, L. Yao; The rise of tunnel endoscopic surgery: a case report and literature review; Case Rep Gastrointest Med, 2012 (2012), p. 847640
- [7] L.P. Ye, Y. Zhang, X.L. Mao, L.H. Zhu, X.B. Zhou, S.Q. He, et al.; Submucosal tunnelling endoscopic resection for the treatment of esophageal submucosal tumours originating from the muscularis propria layer: an analysis of 15 cases; Dig Liver Dis, 45 (2013), pp. 119–123
- [8] S. Ono, M. Fujishiro, K. Niimi, O. Goto, S. Kodashima, N. Yamamichi, et al.; Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms; Endoscopy, 41 (2009), pp. 661–665
- [9] S. Ono, M. Fujishiro, K. Niimi, O. Goto, S. Kodashima, N. Yamamichi, et al.; Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms; Gastrointest Endosc, 70 (2009), pp. 860–866
- [10] W.J. Lee, H.Y. Jung, H. Kim do, J.H. Lee, K.D. Choi, H.J. Song, et al.; Intralesional steroid injection to prevent stricture after near-circumferential endoscopic submucosal dissection for superficial esophageal cancer; Clin Endosc, 46 (2013), pp. 643–646
- [11] N.H. Bhayani, A.A. Kurian, C.M. Dunst, A.M. Sharata, E. Rieder, L.L. Swanstrom; A comparative study on comprehensive, objective outcomes of laparoscopic Heller myotomy with per-oral endoscopic myotomy (POEM) for achalasia; Ann Surg, 259 (2014), pp. 1098–1103
- [12] L. Wang, W. Ren, Z. Zhang, J. Yu, Y. Li, Y. Song; Retrospective study of endoscopic submucosal tunnel dissection (ESTD) for surgical resection of esophageal leiomyoma; Surg Endosc, 27 (2013), pp. 4259–4266
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?