Summary
Organ transplant recipients under immunosuppressive therapy have a highly increased risk of acquiring unusual opportunistic infections. Diagnosis of the etiology of infection may be difficult in clinical manifestations, which need further histological and biological investigations. We recently treated a male renal transplant recipient with a cutaneous phaeohyphomycosis due to Alternaria species. The diagnosis was based on microscopy and culture of the skin lesions. Treatment with oral itraconazole for 5 weeks was ineffective, then clinical improvement was achieved by combination of amphotericin B wet-packing and systemic antifungal therapy with oral voriconazole. Alternaria species are ubiquitous plant-inhabiting saprobes, which are increasingly associated with opportunistic phaeohyphomycosis in immunocompromised individuals. To the best of our knowledge, this is the second case report noting sporotrichoid pattern as the manifestation of cutaneous alternariosis. In this context, we reviewed recent renal-transplant-related cutaneous alternariosis reported in the English-language literature during 1995 to 2011 to summarize its clinical features and outcomes, and to guide clinicians in the care of kidney transplant patients with cutaneous alternariosis.
Keywords
cutaneous alternariosis;organ transplant recipient;phaeohyphomycosis
1. Introduction
Survival following organ transplantation has significantly improved during the past decade. One of the reasons is that rejection rates have dramatically declined due to the development of immunosuppressants. However, organ transplant recipients under immunosuppressive therapy have a highly increased risk of acquiring unusual opportunistic infections such as phaeohyphomycosis.1 Phaeohyphomycosis is a heterogeneous group of diseases caused by hyphomycetous fungi that develop in host tissue in the form of dark-walled melanized septate mycelial elements, which are ubiquitous in nature, but rarely cause human disease.2 ; 3 The majority of cases of phaeohyphomycosis due to Alternaria species have been encountered in immunocompromised individuals, 4 ; 5 and some authors have suggested that it may be an emerging opportunistic infection in transplant recipients.1
2. Case report
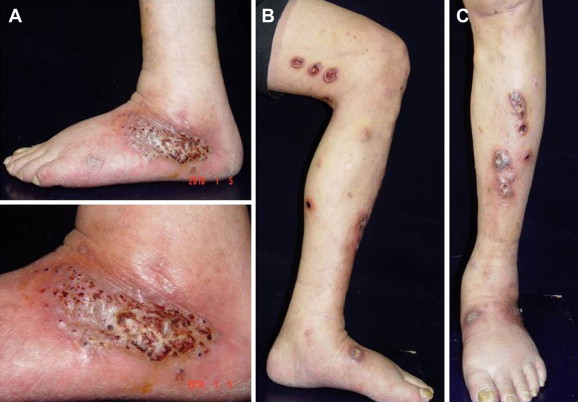
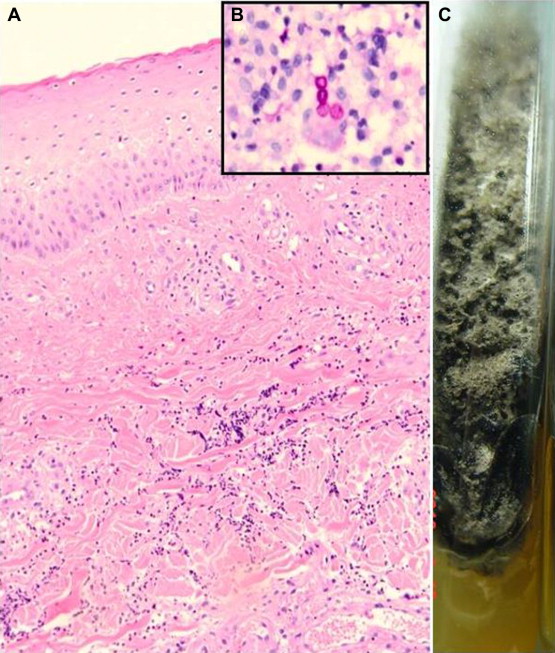
A 61-year-old male retired laborer had been transplanted with a cadaver kidney because of end-stage renal disease secondary to diabetic nephropathy. There was acute rejection 3 months after transplantation, and the patient was taking the following long-term immunosuppressive drugs: tacrolimus (1 mg twice a day orally), mycophenolate mofetil (500 mg triple a day orally), and methylprednisolone (8 mg once a day orally). After the episode of acute rejection, the serum creatinine level was relative stable, around 1.0–1.5 mg/dL. One year after transplantation, he developed a verrucous plaque on the left lateral ankle. The patient did not recall any local trauma occurring since transplantation. Three months later, he underwent excision of the verruca with microabscess. The pathological finding was pseudoepitheliomatous hyperplasia with hyperkeratosis and foci of neutrophilic aggregates, compatible with verruca with inflammation. No special staining for microorganisms was carried out. Two months after surgery, multiple newly formed tender nodules and ulcers on the left lower extremity were noted. There was no fever, cough, or manifestations of systemic illness. On examination, a well-defined, 9-cm diameter, dark-red erythematous plaque with rice-grain-sized black papules and crusts were present on the lateral aspect of the left ankle (Fig. 1A). Multiple sporotrichoid erythematous to violaceous nodules and ulcers on the left lower extremity were also found (Fig. 1B and C). To evaluate this process further, an incisional biopsy was performed on the left thigh, which showed marked neutrophilic infiltrates primarily in the reticular dermis and dermosubcutaneous junction, without caseous necrosis or microabscess formation (Fig. 2A). No bacteria were identified by Gram’s stain. Grocott’s Methenamine Silver and periodic acid-Schiff stain demonstrated a few spores within the infiltrate in the reticular dermis (Fig. 2B). The diagnosis was suppurative dermatitis with deep fungal infection. Culture of the biopsy specimen yielded Alternaria species ( Fig. 2C). To exclude the possibility of a systemic infection, chest X-ray was performed, with negative results. The final diagnosis was cutaneous alternariosis.
|
|
|
Figure 1. (A) One crusted verrucous plaque on the external aspect of the right ankle; (B and C) skin lesions were multiple nodules with ulceration over the left lower extremity in a sporotrichoid pattern. |
|
|
|
Figure 2. (A) There were marked neutrophilic infiltrates primarily in the reticular dermis and dermosubcutaneous junction without caseous necrosis or microabscess formation (hematoxylin and eosin, 100×). (B) Periodic acid-Schiff stain showed a few spores within the infiltrate in the reticular dermis (400×). (C) Macroscopic appearance of cultured Alternaria Spp.: typical dark-grey fungal colonies with wooly texture grown in Sabouraud’s dextrose agar. |
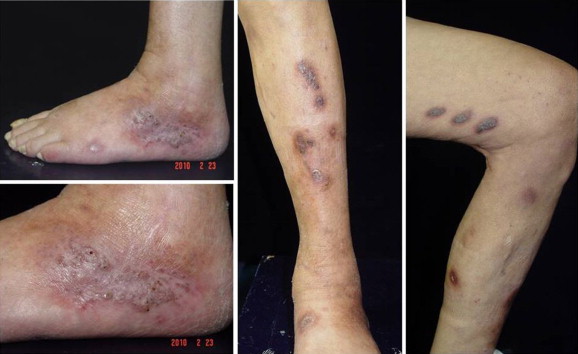
The size and number of the lesions made local excision technically difficult, thus, the patient was started on itraconazole orally at a dose of 400 mg daily. Despite treatment for 5 weeks, the lesions progressed to deeper ulcers with enlarging perimeter and more pain. Itraconazole therapy was discontinued, and treatment was commenced with oral voriconazole (400 mg per day) with daily topical wet-packing with amphotericin B (1 mg/mL, total 10 mg) for 11 days was, in association with close follow-up of tacrolimus serum levels. The serum trough level of tacrolimus was around 10 ng/mL before voriconazole use and was elevated to 29 ng/mL on day 4 of voriconazole therapy. The tacrolimus dose was adjusted from 2 mg to 1 mg daily to maintain the therapeutic level. Renal function remained stable after the adjustment. Dramatic improvement was noted a few days later and the patient received regular treatment and follow-up at our outpatient department. Two months after treatment with voriconazole, we observed almost-resolved lesions with residual scars (Fig. 3). Unfortunately, during the third month of voriconazole treatment, the patient was admitted because of pneumonia and the serum trough level of tacrolimus was 36 ng/mL. Eventually, the patient died from bacterial sepsis with multiorgan failure. At the time of death, the patient had residual scars without active lesions on his left leg.
|
|
|
Figure 3. Near resolution of ulcers had occurred after 8 weeks of treatment with voriconazole. |
3. Literature review and discussion
Alternaria has worldwide distribution and is a large and complex genus that encompasses hundreds of species, but only eight have been involved in human or animal infections, 6 with many species being common saprophytes in soil and air. It can cause several different types of human infections, such as paranasal sinusitis, ocular infections, cutaneous and subcutaneous infections, and, more rarely, granulomatous pulmonary disease, and disseminated disease.7 However, skin is the most frequent site of infection with around 200 cases of cutaneous alternariosis being reported to date.7 Although Alternaria infects mainly immunocompromised hosts, infections in immunocompetent hosts have also been reported. 5 The incidence of cutaneous alternariosis in transplant recipients is 1 in 1000 in the United States.8 Including the present patient, a totality of 34 kidney transplant patients with cutaneous alternariosis have been reported in the English-language literature since 1995 8; 9; 10; 11; 12; 13; 14; 15; 16; 17; 18; 19; 20; 21; 22; 23; 24; 25; 26; 27; 28; 29 ; 30: 25 men and nine women, aged 36–73 years (mean: 55 years). Most of the patients were following different immunosuppressive treatment regimens after transplantation (Table 1). The incubation gap (from the date of last transplant to lesion formation) was uneven, with a range of 2 months to 8 years. Most of the lesions (73%) happened within 1 year after the date of final transplantation. This might have been due to the relatively high dose of immunosuppressants during the first year after transplantation. The mean duration from rash formation to diagnosis of Alternaria infection was 3.7 months. In the present case, the solitary verrucous lesion on the left lateral ankle was diagnosed as verruca with inflammation initially, and progressed to multiple sporotrichoid nodules and ulcers 2 months later. Delayed diagnosis made local excision technically difficult because of the presentation of multiple lesions. Therefore, diagnosis of uncharacteristic inflammatory skin lesions in transplant recipients must include an appropriate search for infection, especially fungi. Further special stains or fungal culture could give clues to fungal infection. Sites of cutaneous alternariosis are summarized in Table 2. All lesions were located on exposed areas, except for one on the buttock. Most of the cases had no trauma history with only five (14.7%) cases having a local trauma history. Only one patient (Case 9) might have had systemic involvement. This patient presented with newly formed pulmonary infiltrate as well as microscopically confirmed lesions on three noncontiguous body sites, which strongly suggests that the disease was disseminated, although fungi could not be detected in the bronchoalveolar lavage fluid samples. This tendency for Alternaria infections to remain locally invasive without dissemination may be related to its active growth in vitro at 20 °C, with inhibited growth at 37 °C. 31 The spectrum of clinical presentations is broad. The case presented here appeared remarkable for the sporotrichoid distribution in the skin lesions. Such a distribution is mainly seen in sporotrichosis caused by Sporothrix schenckii, but has also been described in mycobacterial infections and leishmaniasis. To the best of our knowledge, the clinical picture was first documented with cutaneous alternariosis in 2001 32 and this is only the second report since then.
| Characteristic | No. of patients |
|---|---|
| Sex | |
| Male | 25 |
| Female | 9 |
| Under immunosuppressive treatment, n = 33 | 32 |
| Incubation gap (mo),an = 26 | |
| 0–6 | 9 |
| 7–12 | 10 |
| 13–24 | 3 |
| >24 | 4 |
| Duration of diagnosis(mo),bn = 26 | |
| 0 | 3 |
| 1–3 | 13 |
| 4–6 | 7 |
| >6 | 3 |
| History of trauma, n = 34 | 5 |
| Systemic involvement | 1 |
| Pathogen, n = 34 | |
| NI | 17 |
| Alternaria alternata | 11 |
| Alternaria tenuissima | 3 |
| Alternaria infectoria | 2 |
| Alternaria chartarum | 1 |
| Prognosis, n = 34 | |
| Eventually cured | 26 |
| Died of diseasec | 1 |
| DOD | 4 |
| Lost to follow-up | 3 |
| Initial treatment of choice (cured/no. of cases), n = 31 | |
| Antifungal drug | 3/19 |
| Operation | 1/3 |
| Combination therapy | 5/6 |
| Topical therapy | 3/3 |
| Eventually cured by, n = 26 | |
| Operation | 1 |
| Antifungal drug | 6 |
| Combination therapy | 16 |
| Topical therapy | 3 |
| Relapse during follow-up, n = 20 | 2 |
DOD = died of other disease; NI = not identified to species level.
a. From last transplant to date of lesion formation.
b. From rash formation to diagnosis of Alternaria infection.
c. No autopsy.
| Lesion no. | No. of patients |
|---|---|
| Solitary | 21 |
| Multiple | 13 |
| Multifocal (>2 sites) | 9 |
| Body site | |
| Face | 1 |
| Trunk | 1a |
| Upper extremities | 7 |
| Lower extremities | 29 |
Number of patients was more than 34, because each location of a lesion was included if multiple body sites were affected.
a. Buttock.
Alternaria can be isolated from normal human skin or as a laboratory contaminant, therefore, its involvement in human infection must be verified by histological evidence of its presence in tissue. All 34 cases had received skin biopsy and 23 of them were described in the text, which are reviewed here. Fungal elements were detected in each of the biopsy specimens, thus leading toward the diagnosis of deep fungal infection. For further identification, mycological culture is required. All 34 fungal cultures form biopsy specimens yielded Alternaria species, except one that remained negative, from a patient who was diagnosed by real-time polymerase chain reaction. Half of them were identified to species level. Alternaria alternata, Alternaria tenuissima and Alternaria infectoria were the species most often implicated.
The course of cutaneous alternariosis in this group was very capricious and treatment was not definitively standardized. The treatments and outcomes of the reported cases are summarized in Table 3.4; 9; 10; 11; 12; 13; 14; 15; 16; 17; 18; 19; 20; 21; 22; 23; 24; 25; 26; 27; 28; 29 ; 30 Three cases were lost to follow-up during the treatment and excluded from the therapeutic analysis. Successful treatment rate was 83.8%. Most of the cases (61.5%) were cured by combination therapy with surgery and systemic antifungal drugs. Six cases (23%) were cured by systemic antifungal drugs alone. In four (15.3%) cases, infection was resolved by local treatment such as thermotherapy, excisional surgery, and daily occlusive application of ketoconazole. Only one patient (Case 25) might have died from Alternaria infection but no autopsy was performed. Four cases (12.9%), including the present case, died of other disease during treatment, between 7 days and 12 years (time of diagnosis). The most recalcitrant case, Case 30, underwent excision combined with several antifungal drugs such as itraconazole, voriconazole and caspofungin during the 12-year treatment and eventually died of post-transplant lymphoproliferative disorder. Despite the relatively high cure rate (83.8%), most of the therapeutic plans had been changed during the treatment course. Surgical intervention was used as an adjunct to oral therapy for some patients. Systemic antifungal medication had to be replaced by others for various reasons (e.g., poor response and adverse drug reaction).
| Case no. | Age/ Sex | Immune suppression | Incubation gap (mo) | Duration (mo) | Lesion/location | Systemic involvement | Trauma Hx | Pathology/presence of fungal elements (special stain) | Culture | Course and treatment | Duration of follow-up, outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61/M | Tac, MMF, steroid | 12 | 5 | Nodules, ulcers/ Left lower extremity | N | N | Neutrophilic infiltrate/Y (PAS, GMS) | NI | Itra 200 mg for 5 wk → progression, change to Vori 400 mg for 3 mo + amphB wet packing for 11 d, almost healed | DOD | |
| 2 | 50/F | CsA, AZA, steroid | 12 | 2 | One nodule/ Left leg | N | Y | Granulomatous inflammatory/ Y (PAS) | NI. | Daily occlusive local application of Keto for 2 mo → resolved | 18 mo f/u: no lesion | 9 |
| 3 | 55/M | CsA, steroid | ND | 4 | Two nodules/ right leg | N | Y | Hyperplasic epidermis, giant-cell epithelioid granulomas with microabscess/ Y (H&E, FMS, GMS) | A. tenuissima | Ex → recurrence 1 mo later, Ex → recurrence 2 mo later, Ex + Itra 100 mg for 2 mo → resolved | 12 mo f/u: no lesion | 10 |
| 4 | 70/M | CsA, AZA, steroid | 2 | 4 | Ulcers/ right leg, nodules/ right arm | N | N | Pseudoepitheliomatous hyperplasia with dermal abscess/Y (H&E, GMS) | A. alternata | Itra 400 mg for 2.5 mo → resolved | ND | 11 |
| 5 | 58/ F | CsA, AZA, steroid | 39 | 6 | Nodules/right leg | N | N | Pseudoepitheliomatous hyperplasia, granulomatous infiltrate/Y (H&E, PAS) | A. tenuissima | Terb 250 mg for 2 mo + electrosurgical destruction → resolved | 9 mo f/u: no lesion | 12 |
| 6 | 52/ F | CsA, steroid | 60 | ND | One plaque/ right hand | N | N | Granulomatous inflammatory/Y (PAS, GMS) | NI | Itra 100 mg for 1 mo → no effect | DOD | 13 |
| 7 | 63/ F | CsA, AZA, steroid | 11 | 1 | Nodules/nasal septum, left leg, right knee | N | N | Granulomatous inflammatory/Y (PAS) | A. chartarum | Itra 200 mg for 3 mo + decreased AZA and DC steroid → resolved | 12 mo f/u: no lesion | 14 |
| 8 | 44/M | ND | 4 | 2 | One nodule/ left foot | N | N | ND/ND | NI | Itra 400 mg for 2 mo → no response, Ex + amphB for 1.5 mo → resolved | ND | 15 |
| 9 | 60/M | Tac, steroid | 5 | 0 | Nodules/ right thumb, feet | Y (lung) | N | Dermal microabscess, giant cells/Y (H&E, FMS) | NI | Itra 400 mg for 0.73 mo + serial Ex → progression, liposomal amph B for 1 mo → resolved | 20 mo f/u: no lesion | 16 |
| 10 | 68/ F | Tac, steroid | 8 | 2 | One nodule/ right knee | N | N | Granuloma/Y (PAS, GMS) | A. alternata | Ex + Itra 200 mg for 1 mo → resolved | 10 mo f/u: no lesion | 17 |
| 11 | 58/M | CsA, AZA, steroid | 48 | 0 | Multiple papules/both forearms | N | N | Microabscess, granuloma/Y (H&E, GMS) | NI | Itra 200 mg for 6 mo → progress, 400 mg for 4 mo → new lesions on left leg, liposomal amphB 750 mg for 1.5 mo → resolved | 36 mo f/u: no lesion | 18 |
| 12 | 55/M | Tac, MMF, steroid | 4 | 0 | Nodules/both legs | N | N | Granulomatous inflammation/Y (H&E, PAS, GMS) | NI | Ex → resolved | 5 mo f/u: no lesion | 19 |
| 13 | 65/M | Tac, MMF | 4 | 4 | Ulcers, nodules/both legs | N | N | Granulomatous inflammation/Y (H&E) | A. alternata | Terb 200 mg for 1 mo → no response, Itra 200 mg for 6 mo, resolved | ND | 20 |
| 14 | 54/ F | Tac, MMF | 8 | ND | Two nodules/leg | N | N | ND/Y (H&E) | A. alternata | Ex + Itra for unknown period → resolved | 20 mo f/u: no lesion | 20 |
| 15 | 58/M | CsA, AZA, steroid | 5 | 0.75 | One nodule/ left thigh | N | Y | Granuloma Y (GMS) | NI | Ex + Itra 400 mg for 3 mo → resolved | 84 mo f/u: no lesion | 21 |
| 16 | 41/M | Tac, AZA, steroid | 5 | 1 | One nodule/ left knee | N | Y | Granulomatous inflammation with intradermal microabscess/Y(H&E, GMS) | A. alternata | Itra 400 mg for 3 mo → relapse on left knee 15 mo later, Itra 400 mg for 6 mo + Ex in the 5th mo → resolved | 60 mo f/u: no lesion | 21 |
| 17 | 65/M | AZA, steroid | ND | 1 | One ulcer/left leg | N | N | ND/ND | NI | Liposomal AmphB 3 mg/kg/d for 7 d → persistent | DOD | 4 |
| 18 | 67/M | CsA, AZA, steroid | ND | 18 | One plaque/ right thigh | N | Y | ND/ND | NI | Fluc 100 mg for 7 mo + Fluc intralesional injection → improvement | Loss f/u | 4 |
| 19 | 53/M | Nil | ND | 1 | One plaque/ left buttock | N | N | ND/ND | NI | Itra 200 mg 3 mo → loss f/u | Loss f/u | 4 |
| 20 | 55/M | MMF, Tac, steroid | ND | 4 | One plaque/ right knee | N | N | ND/ND | A. alternata | Thermotherapy → resolved | 12 mo f/u: no lesion | 4 |
| 21 | 55/M | MMF, Tac, steroid | 2 | 4 | One plaque/ right knee | N | N | Chronic and acute inflammatory infiltrate/Y (GMS) | A. alternata | Thermotherapy for 12 mo → resolved | 24 mo f/u: no lesion | 22 |
| 22 | 28/M | Tac, AZA, steroid | 6.5 | 0.5 | One nodule/ right knee | N | N | florid granuloma/Y (GMS) | A. tenuissima | Itra 400 mg for 0.5 mo, Itra 200 mg for 1 mo + Ex on the 1st month → resolved | 36 mo f/u: no lesion | 23 |
| 23 | 52/M | CsA, steroid | ND | ND | One nodule/ right forearm | N | N | Granulomatous inflammation/Y(PAS, GMS) | A. alternata | Itra 100 mg for 1 mo → improvement | Loss f/u | 24 |
| 24 | 58/M | Tac, MMF, steroid | 12 | 3 | Nodules/both legs | N | N | Hyperkeratosis, pseudoepitheliomatous hyperplasia, microabscess, granulomatous infiltrate/ Y (H&E, PAS, GMS) | NI | Itra 400 mg for 1 mo → change to cryotherapy due to nephrotoxic effect of Tac interaction → relapse on right arm → Terb 250 mg for 3 mo → new lesion, change to Flu 100 mg for 4 mo → resolved → relapse on right leg 24 mo late, excision → resolved | 24 mo f/u: no lesion | 25 |
| 25 | 61/M | CsA, Pred | 96 | 2 | One plaque/ right knee | N | N | Dermal abscess/Y (H&E) | A. alternata | liposomal amphB for 0.75 mo → change to Itra 400 mg for 10 d → DC due to hepatotoxicity → 2 mo later, patient died of unknown reason with pyodermatitis on right limb s/p liposomal AmphB for 0.75 mo | Died of disease (no autopsy) | 26 |
| 26 | 47/ F | Tac, steroid | ND | 3 | One nodule/ left ankle | N | N | Hyperplasic epidermis with a granulomatous reaction/Y (H&E) | A. alternata | Vori 150 mg for 10 d → toxicity of Tac, change to Itra 400 mg for 5 mo → resolved | ND | 27 |
| 27 | 68/M | Tac, MMF, steroid | ND | 2.5 | One nodule/ right foot | N | N | Granulomatous inflammation/Y (PAS) | A. infectoria | Itra 400 mg for 1 wk, 200 mg for 3 wk, 100 mg for 1 mo → resolved | ND | 28 |
| 28 | 36/M | MMF, Tac, steroid | 24 | ND | Papules/left leg, left forearm | N | N | ND/ND | NI | Itra for 4 mo → no improvement, Ex + Itra for 4 mo → resolved | 72 mo f/u: no lesion | 8 |
| 29 | 41/M | MMF, Tac, steroid | 12 | ND | One nodule/ left leg, | N | N | ND/ND | NI | Ex → recurrence with multiple lesions on left leg, Ex + Itra for 24 mo → resolved | 72 mo f/u: no lesion | 8 |
| 30 | 51/M | MMF, Tac, steroid | 7 | ND | Nodules/both lower extremities | N | N | ND/ND | NI | Itra for 4 mo → no improvement, Ex + Itra → new lesions on upper extremities, trunk and ear → change to vori, DC due to photosensitivity → Itra: adequate dose for 4 y, progression 4 y later → added caspofungin for 2 mo, keep Itra for 48 mo → persistent infection | DOD | 8 |
| 31 | 40/M | MMF, Tac, steroid | 22 | 9 | One nodule/ right leg | N | N | ND/ND | NI | Ex + Itra for 3 mo → resolved | 48 mo f/u: no lesion | 8 |
| 32 | 60/ F | MMF, Tac, steroid | 10 | ND | One plaque/ right foot | N | N | ND/ND | NI | Nodule while receiving prophylactic Itra, excisional + Vori for 5 d, DC due to hepatotoxicity, Itra for 4.5 mo → resolved | 36 mo f/u: no lesion | 8 |
| 33 | 73/M | Tac, MMF, steroid | 24 | 12 | One plaque/ right hand | N | N | Granuloma, necrosis/Y (PAS) | Culture (–), RT-PCR:A. infectoria | Itra 200 mg for 6 mo → progression, Ex + Itra for 6 mo → resolved | ND | 29 |
| 34 | 51/ F | Tac, MMF, steroid | 3 | ND | One nodulopustule/right knee | N | N | ND/ND | A. alternata | Ex + Vori 400 mg for 3 mo → resolved | 6 mo f/u: no lesion | 30 |
amphB = amphotericin B; AZA = azathioprine; CsA = cyclosporine; DC = discontinue; DOD = died of other disease; Ex = excision; F = female; Fluc = fluconazole; FMS = Fontana-Masson stain; f/u = follow-up; GMS = Grocotts methenamine silver stain; H&E = hematoxylin and eosin; Hx = history; Itra = intraconazole; Keto = ketoconazole; M = male; MMF = mycophenolate mofetil; N = no; ND = no data; NI = not identified to species level; PAS = periodic acid-Schiff; Tac = tacrolimus; Terb = terbinafine; Vori = voriconazole; Y = yes.
Twenty cases were followed up for 6 months to 6 years (mean: 23 months). Relapse occurred in two cases (10%) after 1.5 and 2 years. In our case the infection was refractory to itraconazole. Voriconazole proved an effective alternative, resulting in disappearance of the lesions. The patient’s death did not permit proper follow-up of the cutaneous lesions, so we cannot know if there would have been a relapse after drug withdrawal.
In summary, diagnosis of uncharacteristic inflammatory skin lesions in transplant recipients must include an appropriate search for infection, especially fungi. Further special stains or fungal culture could give clues to fungal infection. In the present case, the solitary verrucous lesion on the left lateral ankle was diagnosed as verruca with inflammation initially, and progressed to multiple sporotrichoid nodules and ulcers 2 months later. Delayed diagnosis made combination therapy technically difficult because of the presentation of multiple lesions. According to the reviewed cases, we suggest that excisional surgery, when possible, combined with systemic antifungal therapy are the choice of treatment in kidney transplant recipients with cutaneous alternariosis. Within the systemic antifungal regimens, itraconazole is generally most effective for treatment of alternariosis. Besides close monitoring of adverse effects, drug serum level and adjusting the dosage of immunosuppressants are recommended during and after systemic antifungal therapy. Relapse may occur even after prolonged treatment and long-term clinical follow-up seems advisable.
References
- 1 R. Ben-Ami, R.E. Lewis, I.I. Raad, D.P. Kontoyiannis; Phaeohyphomycosis in a tertiary care cancer center; Clin Infect Dis, 48 (2009), pp. 1033–1041
- 2 L. Ajello, L.K. Georg, R.T. Steigbigel; A case of phaeohyphomycosis caused by a new species of Phialphora; Mycologia, 66 (1974), pp. 490–498
- 3 M.R. McGinnis; Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology; J Am Acad Dermatol, 8 (1983), pp. 1–16
- 4 M. Gilaberte, R. Bartralot, J.M. Torres, et al.; Cutaneous alternariosis in transplant recipients: clinicopathologic review of 9 cases; J Am Acad Dermatol, 52 (2005), pp. 653–659
- 5 K.E. Lyke, N.S. Miller, L. Towne, W.G. Merz; A case of cutaneous ulcerative alternariosis: rare association with diabetes mellitus and unusual failure of itraconazole treatment; Clin Infect Dis, 32 (2001), pp. 1178–1187
- 6 M.L. Romero, A.H. Siddiqui; Diagnosis: cutaneous alternariosis; Clin Infect Dis, 30 (2000), pp. 174–175
- 7 F.J. Pastor, J. Guarro; Alternaria infections: laboratory diagnosis and relevant clinical features; Clin Microbiol Infect, 14 (2008), pp. 734–746
- 8 R.D. Boyce, P.J. Deziel, C.C. Otley, et al.; Phaeohyphomycosis due to Alternaria species in transplant recipients; Transpl Infect Dis, 12 (2010), pp. 242–250
- 9 P.A. Bécherel, O. Chosidow, C. Francés; Cutaneous alternariosis after renal transplantation; Ann Intern Med, 1 (122) (1995), p. 71
- 10 C. Romano, L. Valenti, C. Miracco, et al.; Two cases of cutaneous phaeohyphomycosis by Alternaria alternata and Alternaria tenuissima; Mycopathologia, 137 (1997), pp. 65–74
- 11 K.M. Acland, R.J. Hay, R. Groves; Cutaneous infection with Alternaria alternata complicating immunosuppression: successful treatment with itraconazole; Br J Dermatol, 138 (1998), pp. 354–356
- 12 G.F. Altomare, V. Capella, V. Boneschi, M.A. Viviani; Effectiveness of terbinafine in cutaneous alternariosis; Br J Dermatol, 142 (2000), pp. 840–841
- 13 C. Baykal, R. Kazancioğlu, N. Büyükbabani, et al.; Simultaneous cutaneous and ungual alternariosis in a renal transplant recipient; Br J Dermatol, 143 (2000), pp. 910–912
- 14 S. Magina, C. Lisboa, P. Santos, et al.; Cutaneous alternariosis by Alternaria chartarum in a renal transplanted patient; Br J Dermatol, 142 (2000), pp. 1261–1262
- 15 M.L. Romero, A.H. Siddiqui; Photo quiz. Cutaneous alternariosis; Clin Infect Dis, 30 (13) (2000), pp. 174–175
- 16 T. Halaby, H. Boots, A. Vermeulen, et al.; Phaeohyphomycosis caused by Alternaria infectoria in a renal transplant recipient; J Clin Microbiol, 39 (2001), pp. 1952–1955
- 17 P. Mayser, M. Nilles, G.S. de Hoog; Case report. Cutaneous phaeohyphomycosis due to Alternaria alternata; Mycoses, 45 (2002), pp. 338–340
- 18 E. Merino, J. Bañuls, V. Boix, et al.; Relapsing cutaneous alternariosis in a kidney transplant recipient cured with liposomal amphotericin B; Eur J Clin Microbiol Infect Dis, 22 (2003), pp. 51–53
- 19 J.O. Kim, G.H. Kim, B.C. Kim, K.S. Lee; Cutaneous alternariosis in a renal transplant recipient; Int J Dermatol, 42 (2003), pp. 630–631
- 20 M. Yehia, M. Thomas, H. Pilmore, W. Van Der Merwe, I. Dittmer; Subcutaneous black fungus (phaeohyphomycosis) infection in renal transplant recipients: three cases; Transplantation, 77 (2004), pp. 140–142
- 21 A. Kazory, D. Ducloux, G. Reboux, et al.; Cutaneous Alternaria infection in renal transplant recipients: a report of two cases with an unusual mode of transmission; Transpl Infect Dis, 6 (2004), pp. 46–49
- 22 J.M. Torres-Rodríguez, M.P. González, J.M. Corominas, R.M. Pujol; Successful thermotherapy for a subcutaneous infection due to Alternaria alternata in a renal transplant recipient; Arch Dermatol, 141 (2005), pp. 1171–1173
- 23 H. Robertshaw, E. Higgins; Cutaneous infection with Alternaria tenuissima in an immunocompromised patient; Br J Dermatol, 153 (2005), pp. 1047–1049
- 24 C. Romano, L. Vanzi, D. Massi, E.M. Difonzo; Subcutaneous alternariosis; Mycoses, 48 (2005), pp. 408–412
- 25 M. Ara, C. Aspiroz, P. Zaballos, et al.; Relapse of cutaneous Alternaria infectoria in a renal transplant recipient after 2 years; Acta Derm Venereol, 86 (2006), pp. 154–155
- 26 C. Farina, E. Gotti, A. Parma, L. Naldi, A. Goglio; Pheohyphomycotic soft tissue disease caused by Alternaria alternata in a kidney transplant patient: a case report and literature review; Transplant Proc, 39 (2007), pp. 1655–1659
- 27 M.N. Singh, S. Andrew, D. Fitzgerald; Solitary cutaneous nodule in an immunocompromised patient; Arch Dermatol, 143 (2007), pp. 1583–1588
- 28 J. Brasch, J.O. Busch, G.S. de Hoog; Cutaneous phaeohyphomycosis caused by Alternaria infectoria; Acta Derm Venereol, 88 (2008), pp. 160–161
- 29 S. Segner, F. Jouret, J.F. Durant, L. Marot, N. Kanaan; Cutaneous infection by Alternaria infectoria in a renal transplant patient; Transpl Infect Dis, 11 (2009), pp. 330–332
- 30 S.E. Vermeire, H. de Jonge, K. Lagrou, D.R. Kuypers; Cutaneous phaeohyphomycosis in renal allograft recipients: report of 2 cases and review of the literature; Diagn Microbiol Infect Dis, 68 (2010), pp. 177–180
- 31 V.A. Morrison, D.J. Weisdorf; Alternaria: a sinonasal pathogen of immunocompromised hosts; Clin Infect Dis, 16 (1993), pp. 265–270
- 32 R. Gerdsen, M. Uerlich, G.S. Hoog De, T. Bieber, R. Horri; Sporotrichoid phaeohyphomycosis due to Alternaria infectoria; Br J Dermatol, 145 (2001), pp. 484–486
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?