Summary
Background
The role of pelvic exenteration in locally advanced rectal cancer (LARC) has not been clearly defined. This procedure carries a mortality rate of approximately 10%. The challenges during pelvic surgery are different between men and women. The morbidity in men with LARC who received pelvic exenteration was analyzed.
Methods
Medical records of men with LARC undergoing total pelvic exenteration or supralevator pelvic exenteration from January 1991 to December 2007 were retrospectively reviewed.
Results
A total of 23 cases were included in the analysis. Thirteen patients had primary cancer; 10 had recurrent cancer. Microscopically clear surgical margins were obtained in 14 patients (60.9%). Sixteen patients (69.6%) experienced major or minor postoperative complications. The overall in-hospital mortality rate was 8.7%. Ten patients (43.5%) died within 1 year after surgery. All 10 patients with early mortality experienced refractory complications and repeated surgeries. The longest survival of patients with margin involvement was 25 months. The correlation between involved surgical margins and 1-year mortality was statistically significant (p = 0.001).
Conclusion
Resection margins with tumor involvement after pelvic exenteration is associated with poor prognosis and early mortality in men with locally advanced rectal cancer.
Keywords
LARC;locally advanced rectal cancer;men’s health;pelvic exenteration;rectal cancer;surgical complications
1. Introduction
Total pelvic exenteration was first described by Bruncshwig in 1948.1 Numerous reports have been published in the gynecologic literature that have confirmed the technical feasibility in treating squamous cell carcinomas of the cervix.2 ; 3 The role of pelvic exenteration in locally advanced rectal cancer (LARC) has not been clearly defined. It demands advanced surgical technique, and it is sometimes difficult to obtain clear resection margins.4 Pelvic exenteration carries a mortality rate of about 10%, and one-half of the patients experience significant morbidity.4; 5; 6; 7; 8 ; 9 Even though several reports have concluded that pelvic exenteration is an effective treatment for LARC,5 ; 6 this procedure is still not routinely performed in many institutions.
Most reports regarding the surgical and oncologic outcomes of pelvic exenteration for LARC did not focused on patient sex.1; 4; 5; 6; 7; 8; 9; 10 ; 11 In our experience, the anatomy as well as the surgical challenges of pelvic surgery is different between men and women. The uterus and vagina in women is located between the rectum and the urinary bladder, and buffering from this tissue will delay invasion of the urinary system. In men, aggressive cancers will directly invade the urogenital organs such as the urinary bladder, seminal vesicles, or prostate. In addition, men have a narrower pelvic bone cage, and deeper lower rectum, which increases the difficulty of surgical dissection and obtaining satisfactory resection margins.
Various complications of pelvic exenteration have been reported in the literature. The most common include bowel obstruction, intra-abdominal or intrapelvic abscess formation, enterocutaneous fistula, ureteral fistula or obstruction, bowel perforation, anastomotic leakage, pneumonia, deep venous thrombosis, and renal failure.5; 6; 7 ; 8 These complications are often life threatening, or significantly compromise postoperative quality of life. In our experience, we found that a large percentage of patients experienced serious morbidities without significant benefit. Some patients experienced prolonged, repeated hospitalizations before their death, while other patients had disease control or cure. In this retrospective report, we analyzed the morbidity and outcomes in men with LARC who received pelvic exenteration.
2. Methods
All patients with LARC who underwent total pelvic exenteration (TPE) or supralevator pelvic exenteration (SLE) from January 1991 to December 2007 were identified in the database of Taichung Veterans General Hospital, and their medical records were retrospectively reviewed. Women diagnosed with squamous cell carcinoma, indicating a different treatment policy, were excluded. TPE was defined as the removal of the rectum, urinary bladder, seminal vesicles, prostate, anus, and soft tissue within the endopelvic fascia, and performing fecal and urine diversion. SLE was defined as TPE without removal of the anal sphincter and anus, which allows immediate or delayed restoration of bowel continuity. The past histories, clinical characteristics, pathologic reports, oncological outcomes, complications, morbidities, and postoperative courses were analyzed and summarized. Tumor invasions of radial margins, adjacent organs, or resection lines were recorded. Margin status was defined as: R0 resection, no tumor involvement of the surgical margin; R1 resection, microscopic tumor invasion of the surgical margin; and R2 resection, gross tumor invasion of margin.
2.1. Statistical analyses
The survival was calculated using the Kaplan-Meier method. Statistical significance was calculated with Fisher’s exact test. Analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (SPSS Inc., Chicago, IL, USA).
3. Results
During the period from January 1991 to December 2007, 27 patients diagnosed with LARC who received TPE or SLE were identified. One patient was excluded due to a pathologic diagnosis of squamous cell carcinoma. One woman was not included in the analysis. Two patients were excluded because there was only fibrotic and necrotic tissue without evidence of tumor recurrence in the TPE surgical specimen. Thus, a total of 23 men were eligible and included in the analysis. The mean age at pelvic exenteration was 57.6 years (range, 36–82 years). Thirteen patients had primary cancer, and 10 patients had recurrent cancer. Eight patients with recurrent disease had experienced chemotherapy and radiotherapy before or after their previous surgery. The average length of hospital stay (LOS) was 24.0 days (range, 6–85 days). The mean LOS of complicated and uncomplicated cases was 32.0 and 16.8 days, respectively. Three patients underwent combined procedures including cholecystectomy, small bowel resection, liver resection for metastasis, or removal of the coccyx in the same operation. Fourteen patients accepted intravenous or oral chemotherapy after pelvic exenteration. Nine patients accepted postoperative radiotherapy.
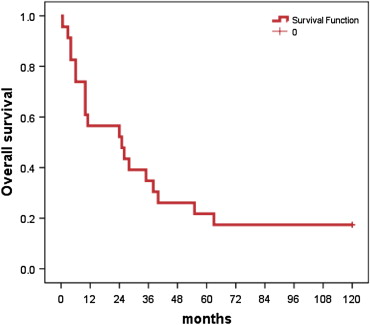
The oncologic characteristics of the patients and types of surgery are summarized in Table 1. Eighteen patients underwent TPE and five patients underwent SLE. The methods of urine diversion included ileal conduit (78.3%), bilateral ureterostomy (8.7%), and ureteroureterostomy with a single opening (13.0%). Tumor invasion to levater ani muscle or perianal skin was the indicator for performing TPE. There was no significant difference in the pattern of organ invasion between patients who underwent TPE and SLE. The oncologic outcomes and patient survival are shown in Table 2. The majority of patients (87.0%) were diagnosed with moderately differentiated adenocarcinoma after pathologic examination of the surgical specimen. Microscopically clear surgical margins were obtained in only 14 patients (60.9%). Eight patients with R1 or R2 resections and two patients with R0 resections, including a patient with poorly differentiated adenocarcinoma, died within 1 year after surgery. The Kaplan-Meier survival curve is shown in Fig. 1. Interestingly, none of the patients who survived the first year died within the second year. One patient died of brain metastasis 63 months after the surgery. Four patients (17.4%) were disease-free after 10 years.
| Number of patients (N = 23), % | |
|---|---|
| Tumor | |
| Primary | 13 (56.5) |
| Recurrent | 10 (43.5) |
| Procedure | |
| TPE | 18 (78.3) |
| SLE | 5 (21.7) |
| Tumor invasion | |
| Urinary bladder | 14 (60.9) |
| Prostate | 8 (34.8) |
| Seminal vesicle | 6 (26.1) |
| Pelvic wall | 9 (39.1) |
| Ureter | 2 (8.7) |
| Jejunum | 1 (4.3) |
| Coccyx bone | 1 (4.3) |
| Urine diversion | |
| Ileal conduit | 18 (78.3) |
| Ureterostomy | 2 (8.7) |
| Ureteroureterostomy | 3 (13.0) |
SLE = supralevator pelvic exenteration; TPE = total pelvic exenteration.
| Number of patients (%) or survival | |
|---|---|
| Tumor cell type | |
| MDA | 20 (87.0) |
| MA | 1 (4.3) |
| LS | 1 (4.3) |
| PDA | 1 (4.3) |
| Resection margin | |
| R0 | 14 (60.9) |
| R1 | 3 (13.0) |
| R2 | 6 (26.1) |
| DFS after R0 resection | |
| Range | 3–48 mo |
| Mean | 19.6 mo |
| Median | 20.0 mo |
| Overall survival | All | R0 resections |
|---|---|---|
| Median | 25.0 mo | 39.0 mo |
| 1 y | 13 (56.5) | 12 (85.7) |
| 2 y | 13 (56.5) | 12 (85.7) |
| 3 y | 8 (34.8) | 8 (57.1) |
| 5 y | 5 (21.7) | 5 (35.7) |
| 10 y | 4 (17.4) | 4 (28.6) |
DFS = disease-free survival; LS = leiomyosarcoma; MA = mucinous adenocarcinoma; MDA = moderately differentiated adenocarcinoma; PDA = poorly differentiated adenocarcinoma; R0 resection = no tumor involvement of the surgical margin; R1 resection = microscopic tumor invasion of the surgical margin; R2 resection = gross tumor invasion of margin.
|
|
|
Figure 1. Kaplan-Meier survival curve. |
The postoperative courses of the patients were quite diverse. Each patient experienced different combinations of surgical complications, disease progression, tumor relapse, and adverse effects of chemotherapy. The postoperative complications are summarized in Table 3. Sixteen patients (69.6%) experienced major or minor postoperative complications. The most common complications were bowel obstruction (17.4%), wound infection (17.4%), and pelvic abscess (17.4%). Two patients (8.7%) experienced small bowel perforation induced by obstruction or strangulation. Nine patients (39.1%), including all three patients who received a ureteroureterostomy, experienced urinary system complications. The overall in-hospital mortality rate was 8.7%.
| Number of patients (%) | |
|---|---|
| Urinary diversion complications | |
| Urine leakage | 2 (8.7) |
| Hydronephrosis | 2 (8.7) |
| Renal stone | 2 (8.7) |
| Urinary tract infection | 2 (8.7) |
| Pyelonephritis | 1 (4.3) |
| GI complications | |
| Upper GI bleeding | 2 (8.7) |
| Enterocutaneous fistula | 1 (4.3) |
| Bowel obstruction | 4 (17.4) |
| Bowel perforation | 2 (8.7) |
| Wound complications | |
| Infection | 4 (17.4) |
| Dehiscence | 1 (4.3) |
| Pelvic abscess | 4 (17.4) |
| Respiratory failure | 1 (4.3) |
| Hepatic failure | 1 (4.3) |
| Sepsis | 2 (8.7) |
| With complicationsa | 16/23 (69.6) |
| Perioperative mortalityb | 2/23 (8.7) |
GI = gastrointestinal.
a. Patients experienced at least one postoperative complication.
b. Patients died without discharge from hospital after pelvic exenteration.
The postoperative courses and overall survival are summarized in Table 4. One patient was lost to follow-up in the 26th month postoperatively; at that time, he was in stable condition without disease recurrence. Another patient was lost to follow-up 25 months after surgery; he was in the terminal stage of disease and reportedly died. Four patients were disease-free after 10 years of follow-up, while 10 patients (43.5%) died within 1 year after surgery. The shortest survival was 18 days after the surgery, and death was due to postoperative complications. Another patient had no chance to be discharged from the hospital until he died 83 days after surgery due to repeated bowel obstructions, leakages, and abscess formation. No patient died within the second postoperative year.
| Date of surgery | OS (mo) | Age | Invaded organs | Margins | Cause of death | Postoperative course summary |
|---|---|---|---|---|---|---|
| 2004.06 | 0.6 | 82 | B, Ur | R2 | C | Expired 18 d after surgery due to complications |
| 2003.04 | 2.8 | 70 | J, B, PW | R2 | C | Prolonged hospital stay for 83 d before death |
| 1992.09 | 4 | 63 | B, PW | R2 | C, PD | Died of PD and complications at 4 mo |
| 2003.09 | 4 | 61 | P, SV | R2 | C, PD | Died of PD and complications at 4 mo |
| 1994.09 | 6 | 45 | P, LNs | R2 | C, PD | Died of PD and complications at 6 mo |
| 2007.01 | 6 | 60 | P | R1 | C | Died of complications of surgery and chemotherapy |
| 2005.03 | 10 | 38 | B, P, SV | R2 | PD | Repeated resection for recurrent tumor until death |
| 2003.09 | 10 | 47 | B, SV | R0 | C | Refractory bowel obstruction and malnutrition until death |
| 2006.05 | 10 | 34 | B, SV, J, Ur | R1 | PD | Pre-existing lung metastasis, died of PD |
| 2001.12 | 11 | 67 | B | R0 | C | Prolonged hospital stay for complications before death |
| 1995.04 | >25 | 63 | B | R0 | Lost | Lost to follow-up at 25 mo, terminal stage |
| 2003.01 | 25 | 71 | B, P, SV | R1 | PD | Recurrence at 11 mo, died of disease |
| 2007.05 | >26 | 65 | B, SV, P | R0 | Lost | Multiple complications, lost to follow-up in stable condition |
| 1993.07 | 28 | 67 | PW | R0 | PD | 20 mo of DFS after liver metastasis resection |
| 1992.12 | 35 | 39 | P, CB | R0 | PD | CB removed, achieved 30 mo of DFS |
| 1993.05 | 38 | 40 | B | R0 | PD | DFS 22 mo before local recurrence |
| 1991.01 | 40 | 66 | B, P | R0 | PD | Local recurrence at 3 mo, but lived for 40 mo |
| 1995.07 | 55 | 69 | B | R0 | PD | DFS 43 mo before lung metastasis |
| 2004.06 | 63 | 50 | B | R0 | PD | DFS 14 mo before lung metastasis |
| 1994.09 | >120 | 40 | B | R0 | No recurrence | |
| 1998.07 | >120 | 75 | B | R0 | No recurrence | |
| 1991.11 | >120 | 57 | B, PW | R0 | No recurrence | |
| 1992.11 | >120 | 57 | B, P | R0 | No recurrence |
B = urinary bladder; C = complications; CB = coccyx bone; DFS = disease-free survival; J = jejunum; LNs = lymph nodes; OS = overall survival; P = prostate; PD = progressive disease; PW = pelvic wall; SV = seminal vesicle; Ur = ureter.
Seven patients (30.4%) died within 6 months after surgery due to surgical complications with or without cancer progression. One patient died 10 months after surgery due to repeated local recurrence and reoperations. One patient with lung metastases that was diagnosed preoperatively died of disease progression 10 months postoperatively. All 10 patients with early mortality (defined as survival <1 year) experienced refractory complications, prolonged hospital stay, and repeated salvage surgeries in their postoperative courses. All mortalities that occurred more than 2 years after surgery were due to disease relapse rather than surgical complications. Every patient with R2 resection margins died within 10 months, and 83.3% of them died within 6 months. The longest survival of patients with involved margins (R1 or R2) was 25 months; however, eight of them (88.9%) died within 10 months. Fourteen patients had free surgical margins (R0 resection). In this group, two patients died of complications within 1 year. Eight of them died due to disease relapse between 28 and 63 months postoperatively. Four patients were disease-free after 10 years. The 2-year survival of R0 patients was 85.7%. One patient who underwent combined hepatectomy for liver metastasis had a disease-free survival (DFS) of 20 months and overall survival (OS) of 28 months. One patient who underwent combined coccyx resection due to tumor invasion had a DFS of 30 months and OS of 38 months.
The correlations between clinical factors and 1-year mortality are presented in Table 5. The 1-year mortality was relatively higher for those with recurrent disease (60.0%); however, it was without statistical significance. Elderly patients (>60 years old) did not have a higher 1-year mortality rate (46.2%). Tumor involvement of the resection margins was a significant negative factor for 1-year survival (p = 0.001). The occurrence of surgical complications was not associated with an increase in 1-year mortality.
| Alive | Death | p | |
|---|---|---|---|
| Margin | |||
| Positive | 1 (7.7%) | 8 (80%) | 0.001 |
| Negative | 12 (92.3%) | 2 (20%) | |
| Surgery method | |||
| SLE | 4 (30.8%) | 1 (10%) | 0.251 |
| TPE | 9 (69.2%) | 9 (90%) | |
| Primary tumor | |||
| Yes | 9 (69.2%) | 4 (40%) | 0.164 |
| No | 4 (30.8%) | 6 (60%) | |
| Age > 60 y | |||
| Yes | 7 (53.8%) | 6 (60%) | 0.552 |
| No | 6 (46.2%) | 4 (40%) | |
| Complications | |||
| Yes | 8 (61.5%) | 8 (80%) | 0.313 |
| No | 5 (38.5%) | 2 (20%) | |
TPE = total pelvic exenteration; SLE = supralevator pelvic exenteration.
4. Discussion
Pelvic exenteration is a challenging surgical procedure, and it has been used to treat LARC with different surgical variations and combined with various modalities of therapy.5; 6; 7; 8; 9; 10; 11; 12; 13; 14; 15 ; 16 Because of the limited indications of pelvic exenteration and high morbidity, large scale studies are very rare. Yeung et al. reported 50 cases of TPE for LARC with curative or palliative intent in 1993.8 In those 50 cases, there were 71 total complications, the in-hospital death rate was 14%, and the overall 5-year survival was 6%. Alternatively, Gannon et al reported 72 cases of pelvic exenteration for primary LARC.5 In this study, complications occurred in 43% of the cases, and the 5-year DFS was 52%. The authors concluded that an aggressive surgical approach provides most patients 5-year DFS, and selected patients with recurrent LARC will also benefit from pelvic exenteration. Bannura et al studied 30 women with primary LARC who underwent posterior pelvic exenteration (PPE), and concluded that PPE prolonged operative time and increased postoperative complications showed a trend toward poor prognosis with respect to recurrence and survival.10 Vermaas et al treated 35 consecutive patients with primary or recurrent LARC with preoperative external beam radiation therapy, with or without intraoperative radiotherapy, combined with TPE and achieved an 88% 5-year local control rate and a 52% 5-year OS for primary cases.6 Ike et al studied 71 patients with primary T3 or T4 rectal cancer who underwent curative TPE in Japan.12 The authors reported a 66.2% complication rate and 51.4% 5-year survival. Yamada et al reported 20 cases of LARC who underwent TPE with sacral resection in 2002, and they concluded that pelvic exenteration and sacral resection for primary or recurrent rectal cancer are tolerable procedures with a low mortality rate.15 Most published papers have focused on survival and complication rates with different treatment strategies. The morbidity of the patients with postoperative complications had not been systemically reported. As shown in Table 4, patients with complications had prolonged hospitalizations, and most of these patients spent their residual lives in the hospital. Considering the balance between costs and benefits, we believe that there should be more restricted indications of pelvic exenteration for LARC.
The cases analyzed in this report occurred over a range of 17 years. Surgical instruments, chemotherapies, and radiotherapies have evolved during this time interval. At our institution, pelvic exenteration for LARC had never been combined with IORT or local flaps. Radiotherapy was reported to be beneficial for local tumor control without improvement on overall survival for resectable rectal cancer.17 ; 18 Wiid reported in 2002 that preoperative radiotherapy had a negative effect on pelvic exenteration.7 In our series, patients did not accept preoperative chemoradiotherapy due to various reasons, including severe pain, tumor obstruction, or previous radiotherapy. Nine of our patients accepted postoperative radiotherapy because they had better performance status and less surgical complications. Fourteen patients accepted different combinations (including 5-fluorouracil, capecitabine, tegafur, irinotecan, or oxaliplatin) and intervals of chemotherapies after pelvic exenteration. The impact of chemotherapy in this study was inaccessible due to complicated treatments and small number of patients. In addition, postoperative chemotherapy or radiotherapy could only be started after patients recovered from the surgery. Patients with postoperative morbidities had less chance to gain benefit from adjuvant chemoradiotherapy. In our study, pre-existing selection bias had obscured the impact of chemoradiotherapy on patient outcomes. Surgical complications—rather than chemotherapy or radiotherapy—is the key factor for patient survival in this study. The pelvic anatomy and organs surrounding the rectum are different in males and females. The patterns of local cancer invasion and surgical technical difficulties are also different. As a tertiary center of veterans, most of our patients are men. The man-to-woman ratio who underwent pelvic exenteration was 26:1, and, by excluding women, this analysis does not contain biases that may be due to the anatomic differences between the sexes.
The 1-year mortality rate in our series was 43.5% (Table 5). The tumor-involved surgical margin is a statistically significant factor (p = 0.001). However, several subgroups of patients do have a lower percentage of 1-year mortality, including patients who underwent SLE (20.0%), primary disease (30.8%), and patients who experienced no surgical complications (28.6%). These factors were reported to have better outcomes in many reports. 4; 5 ; 6 Considering the small total patient number in this study, the value of these factors in predicting surgical outcomes should not be underestimated. Recurrent disease had twice the risk of 1-year mortality compared with primary disease (60.0% vs. 30.8%). Although this factor did not reach statistical significance (p = 0.164), we still suggest that surgeons should be more conservative in performing pelvic exenteration for recurrent cases.
In patients with R0 resection margins, 85.7% were alive after 2 years. The 5-year survival was 35.7% in this curative resection group. In addition, four of the patients (28.6%) were disease-free after 10 years. These data are comparable with those of other published reports.5; 6; 7; 8; 9; 10 ; 11 Curative resection alone cured (defined as disease-free status after 10 years) more than one-fourth of our patients. However, all patients with R2 margins died within 1 year due to complications, with or without disease progression. In fact, eight of the nine patients with R1 or R2 margins were dead within 10 months. The survival of patients was significantly influenced by the margin status in our patients.
Pelvic exenteration has been used as palliative surgery by some authors.8 ; 11 In our study, two of the patients with surgical complications remained in the hospital until their death. All of the six patients with R2 resection margins died within 10 months. In addition, 83.3% of them had repeated complications and disease progression before their death within 6 months postoperatively. With only two exceptions, all mortalities that occurred within 1 year after surgery were related to complications. One patient had pre-existing lung metastasis, and the other patient had poorly differentiated adenocarcinoma and underwent three additional palliative debulking surgeries before he died. These 10 patients with early mortality gained nothing from the surgery, except for suffering. Interestingly, all patients who survived the first year lived at least 2 years. All of the seven confirmed mortalities (two patients were lost to follow-up and excluded) occurred more than 2 years after surgery and were related to disease relapse rather than surgical complications. At least four patients (perhaps 5 because 1 patient was lost to follow-up in the disease-free status) enjoyed 10 years of disease-free survival. Based on these data, the outcomes of the patients seem to be distributed into two groups, with some patients dying early due to complications and some experiencing good disease control for many years.
Our explanation of this phenomenon is that pelvic exenteration is a group of complicated surgeries that includes organ resections, urinary diversion, multiple bowel or urinary tract anastomosis, and stool diversion. The removal of all tissues within the endopelvic fascia also creates a large dead space that is prone to abscess formation. In addition, local radiotherapy, when administered, can interfere tissue healing. These factors, as well as the advanced cancer, lead to a high rate of complications with pelvic exenteration. In our experience, the morbidity of pelvic exenteration is much more complicated and devastating than that of low-anterior resection for rectal cancer. One site of anastomosis leakage often induces the breakdown of surrounding tissues and creates additional leakage. In our series, complications after pelvic exenteration often led to early mortality. In contrast, patients who survived from potential surgical complications had an OS of at least 2 years and a cure rate of 28.6%.
Considering the early mortality rate of the patients in this study, the role of pelvic exenteration for LARC with palliative intent is not justified. A debulking surgery for an unresectable tumor will inevitably result in a positive surgical margin, which is associated with a high early mortality rate. The only patient in our study with pre-existing lung metastasis died 10 months after pelvic exenteration due to disease progression. According to these data, it is reasonable to suggest that pelvic exenteration with palliative intent for LARC should not be performed. In addition, pelvic exenteration should not be performed for LARC if preoperative imaging studies suggest a high probability of positive resection margins.
References
- 1 A. Brunschwig; Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy; Cancer, 1 (1948), pp. 177–183
- 2 S.L. Curry, W.A. Nahhas, A.E. Jahshan, C.W. Whitney, R. Mortel; Pelvic exenteration: a 7-year experience; Gynecol Oncol, 11 (1981), pp. 119–123
- 3 G.W. Morley, S.M. Lindenauer; Pelvic exenterative therapy for gynecologic malignancy: an analysis of 70 cases; Cancer, 38 (1976), pp. 581–586
- 4 H.J. Wanebo, P. Antoniuk, R.J. Koness, et al.; Pelvic resection of recurrent rectal cancer: technical considerations and outcomes; Dis Colon Rectum, 42 (1999), pp. 1438–1448
- 5 C.J. Gannon, J.S. Zager, G.J. Chang, et al.; Pelvic exenteration affords safe and durable treatment for locally advanced rectal carcinoma; Ann Surg Oncol, 14 (2007), pp. 1870–1877
- 6 M. Vermaas, F.T. Ferenschild, C. Verhoef, et al.; Total pelvic exenteration for primary locally advanced and locally recurrent rectal cancer; Eur J Surg Oncol, 33 (2007), pp. 452–458
- 7 J.N. Wiig, J.P. Poulsen, S. Larsen, M. Braendengen, H. Waehre, K.E. Giercksky; Total pelvic exenteration with preoperative irradiation for advanced primary and recurrent rectal cancer; Eur J Surg, 168 (2002), pp. 42–48
- 8 R.S. Yeung, F.L. Moffat, R.E. Falk; Pelvic exenteration for recurrent and extensive primary colorectal adenocarcinoma; Cancer, 72 (1993), pp. 1853–1858
- 9 W.L. Law, K.W. Chu, H.K. Choi; Total pelvic exenteration for locally advanced rectal cancer; J Am Coll Surg, 190 (2000), pp. 78–83
- 10 G.C. Bannura, A.E. Barrera, M.A. Cumsille, et al.; Posterior pelvic exenteration for primary rectal cancer; Colorectal Dis, 8 (2006), pp. 309–313
- 11 A.H. Poletto, A. Lopes, A.L. Carvalho, et al.; Pelvic exenteration and sphincter preservation: an analysis of 96 cases; J Surg Oncol, 86 (2004), pp. 122–127
- 12 H. Ike, H. Shimada, S. Yamaguchi, Y. Ichikawa, S. Fujii, S. Ohki; Outcome of total pelvic exenteration for primary rectal cancer; Dis Colon Rectum, 46 (2003), pp. 474–480
- 13 S. Ishiguro, T. Akasu, S. Fujita, S. Yamamoto, M. Kusters, Y. Moriya; Pelvic exenteration for clinical T4 rectal cancer: oncologic outcome in 93 patients at a single institution over a 30-year period; Surgery, 145 (2009), pp. 189–195
- 14 G.B. Melton, P.B. Paty, P.J. Boland, et al.; Sacral resection for recurrent rectal cancer: analysis of morbidity and treatment results; Dis Colon Rectum, 49 (2006), pp. 1099–1107
- 15 K. Yamada, T. Ishizawa, K. Niwa, Y. Chuman, T. Aikou; Pelvic exenteration and sacral resection for locally advanced primary and recurrent rectal cancer; Dis Colon Rectum, 45 (2002), pp. 1078–1084
- 16 K.M. Boyle, P.M. Sagar, A.G. Chalmers, D. Sebag-Montefiore, A. Cairns, I. Eardley; Surgery for locally recurrent rectal cancer; Dis Colon Rectum, 48 (2005), pp. 929–937
- 17 S. Rolf, B. Heinz, H. Werner, et al.; Preoperative versus postoperative chemoradiotherapy for rectal cancer; N Engl J Med, 351 (2004), pp. 1731–1740
- 18 D. Sebag-Montefiore, R.J. Stephens, R. Steele, et al.; Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomized trial; Lancet, 373 (2009), pp. 811–820
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?