Summary
Background
Limited data are available on the interval of disease-free status after endoscopic submucosal dissection (ESD) for early gastric cancer and precancer lesion in Taiwan. In this long-term (2–105 months) follow-up study, we analyzed the risk factors that affect the local recurrence and noncurative resection (non-CR) of these lesions.
Methods
We retrospectively studied 65 consecutive treatment-naïve patients with 69 EGC lesions who were selected to be treated by ESD. A total of 56 lesions (48 CR lesions and 8 non-CR lesions) were analyzed for local recurrence after ESD.
Results and Discussion
ESD was curative for gastric epithelial tumors in 51 (73.9%) but not in 18 (26.1%) lesions. Unfortunately, five (8.93%) of these 56 lesions in 53 patients had local recurrence. None of the patients died from gastric cancer-associated diseases during follow-up. In our studies, the cumulative local recurrence rates were 5.3% in the CR group and 56.7% in the non-CR group (p = 0.0091). The disease-free status was high (94.7%) with CR. The risk factors that affect the non-CR were tumor location (p = 0.013), deeper invasion (p < 0.001), undifferentiated histopathology (p < 0.001), and ulcer presence (p = 0.045).
Conclusion
ESD offers good outcome after treatment for EGC. To decrease the risk of local recurrence, preoperative diagnosis of tumor extent and accurate postoperative pathological evaluation are very important.
Keywords
Early gastric cancer ; Endoscopic submucosal dissection ; Long-term outcomes
Introduction
Early gastric cancer (EGC) is defined as a cancer confined to the mucosal or the submucosal (SM) layer, irrespective of lymph node metastases (T1 , any N) [1] . The incidence of EGC varies among different countries. Interestingly, the rate of gastric cancer in Japan is > 50% [2] , whereas the rate is < 5% in the West [3] . The reason for this difference in incidence rate between Japan and the West may be due to diagnostic discrepancy, because mucosal cancer, as defined in Japan, is often diagnosed as dysplasia in the West. However, this situation is gradually resolving after pathologists reached a consensus at a meeting held in Vienna [4] .
According to the guidelines of International and Japanese Gastric Cancer Associations [5] , indications for endoscopic mucosal resection (EMR) are differentiated adenocarcinoma, intramucosal cancer, lesions < 20 mm, and depressed lesions without ulceration or scarring. However, these aforementioned criteria may be too strict to avoid unnecessary surgery. Based on the analysis of the risk of lymph node involvement in EGC, Gotoda et al [6] proposed extended criteria for endoscopic resection in the endoscopic SM dissection (ESD) era, which include the following: mucosal cancers without ulcer, regardless of lesion size; mucosal lesions with ulcer < 30 mm; minute (< 500 μm from the muscularis mucosae) SM invasive cancers < 30 mm. Isomoto et al [7] reported no difference in outcomes between treatments based on the aforementioned guideline and the expanded criteria [7] .
Aims
The ESD technique was first developed in Japan [8] and has become the treatment of choice for endoscopic resection of EGC. In previous long-term follow-up studies, risk factors for local recurrence, such as EGC location, EGC size, complete resection rate, and pathological diagnosis after ESD (poorly differentiated adenocarcinoma and EGC invasion depth) have been discussed. However, only limited data are available on the interval of disease-free status after ESD for EGC in Taiwan [9] . In this long-term follow-up study, we analyzed the risk factors that affect the local recurrence and noncurative resection (non-CR) of lesions during 2–105 months of follow-up after ESD.
Methods
Patients
The patients were enrolled based on the guideline criteria for EMR and expanded criteria for ESD, as described earlier [5] ; [6] . Patients with an EGC who did not fall into these categories were excluded from the study and received gastrectomy with lymph node removal. We retrospectively studied 162 consecutive treatment-naïve patients with gastric neoplasm for whom the Department of Gastroenterology (Chang Gung Memorial Hospital, Chiayi, Taiwan) had recommended ESD treatment between August 2004 and December 2009. After excluding the benign lesions according to the pathological report of specimens, 65 consecutive treatment-naïve patients with 69 EGC lesions were selected. The study protocol was approved by the Clinical Research Committee of the Chang Gung Memorial Hospital. The diagnosis of EGC was confirmed by pathological examination. We only included EGCs with a pathological result confirming high-grade dysplasia, carcinoma in situ , or adenocarcinoma. The clinical data were reviewed, and the following parameters were recorded: sex, age, location, macroscopic appearance, biopsy result, pathological result, endoscopic ultrasonography finding, EGC size, specimen size, procedure time, hospital stay, complication, endoscopic follow-up period, local recurrence status, and survival status.
ESD technique
ESD was performed after the patients were sedated with midazolam titration. The procedure began with the identification of the lesion and marking a zone 5–10 mm outside the margin of the target lesion with a precut needle knife (CD-1L; Olympus) and electrosurgical coagulation current (forced coagulation, 20 W). The electrosurgical unit ERBE ICC 350 (ERBE Elektromedizin, Tübingen, Germany) was used for this purpose. Glycerol (10%), diluted epinephrine (1:100,000), and diluted indigo carmine were then injected into the SM layer to lift the mucosa. An initial incision was made with a conventional needle knife. Next, a circumferential mucosal incision was made around the lesion using an insulated-tip knife (IT knife) with an electrosurgical current (Endocut mode, effect 3, 80 W). Lesions were completely removed by SM dissection using an IT knife.
Histopathological evaluation
All resected specimens were flattened and fixed in formalin solution. The fixed specimens were sectioned into 2-mm-thick slices and classified by a pathologist according to the Vienna classification [4] . The sizes of the resected specimens were measured at the greatest width of the lesion. The resected margin and depth of tumor invasion were observed carefully and recorded.
Complications
Major bleeding was defined as a hemoglobin decrease of > 2 mg/dL following the procedure. Perforation was diagnosed endoscopically during the procedure as free air on a plain abdominal radiograph.
Patient follow-up
The follow-up program consisted of physical examination, laboratory blood test, endoscopy, and/or ultrasound 2 months, 6 months, and 12 months after the ESD to assess the completeness of the resection in the 1st year; thereafter, it was examined annually to detect local recurrence. Biopsy was repeated for histopathological evaluation when tumor recurrence was suspected. For a recurrent EGC case, the recurrence location was carefully compared with the original location. “Recurrence” was defined as newly developed EGC after initial complete resection at the same location as the original; “metachronous lesion” was defined as newly developed EGC in a different location; and “synchronous lesion” was defined as the presence of two EGC lesions in different locations at the same time.
Statistical evaluation
Statistical analysis was performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA). Patient characteristics were represented as the mean ± standard deviation. Long-term disease-free status data were analyzed by the Kaplan–Meier method with significance based on Wilcoxon (Gehan) test and with median survival time indicated. Comparisons of risk factors that affect complete resection were calculated by the Chi-square (Pearson) test.
Results
The demographic characteristics of the 65 patients with 69 lesions who were scheduled to receive treatment by ESD for gastric epithelial cancers are presented in Table 1 . Our patient cohort included 39 men and 26 women with a mean age of 67.3 years. The majority of the tumors were located in the lower (n = 53) stomach. Most cancers were classified as “flat, elevated superficial cancer, type IIa” (n = 23) and “IIa + IIc” (n = 26) by endoscopic morphological classification. The mean diameter of the tumors was 1.90 cm, whereas that of the resected specimens was 3.24 cm. The depth of the gastric epithelial cancers was evaluated after pathological assessment: 65 early cancers were limited to the mucosal layer, two to the SM superficial layer, and two to the muscularis propria layer.
| Male/female | 39/26 |
| Age (y) | 67.3 ± 12.7 (36–85) |
| Lesion’s location | |
| Upper third/middle third/lower third | 8/8/53 |
| AW/PW/LC/GC | 18/15/28/8 |
| Lesion’s macroscopic appearance | |
| I/IIa/IIb/IIc/IIa + IIc | 12/23/2/6/26 |
| Ulcer (+/–) | 37/32 |
| Lesion size (mm; pre-ESD’s lesion size) | 19.0 ± 11.0 (5–70) |
| Pre-ESD biopsy result | |
| Adenocarcinoma | 18 |
| High-grade dysplasia or carcinoma in situ | 26 |
| Adenomatous polyp | 3 |
| Intestinal metaplasia | 1 |
| Resected specimen size (mm) | 32.4 ± 11.8 (10–72) |
| (post-ESD’s specimen size) | |
| Pathological result | |
| High-grade dysplasia or carcinoma in situ | 49 |
| Adenocarcinoma | 20 |
| Well/poorly differentiated | 6/14 |
| EUS depth (M/SM/NA) | 43/7/19 |
| Pathological depth (M/SM/MP) | 65/2/2 |
| Procedure duration (min) | 85.5 ± 63.4 (12–360) |
| Hospital stay (d) | 4.8 ± 2.8 (1–13) |
Data are expressed as mean ± standard deviation.
AW = anterior wall; EGC = early gastric cancer; ESD = endoscopic submucosal dissection; GC = greater curvature; LC = lesser curvature; M = mucosa; MP = muscularis propria; NA = not available; PW = posterior wall; SM = submucosa.
a. Endoscopic ultrasonography (EUS) was not performed in every case because the EGC’s depth can be predicted only by EGD in some cases.
Three cases with initial diagnosis (pre-ESD biopsy result) as adenomatous polyp turned to be cases of severe dysplasia (n = 2) and carcinoma in situ (n = 1) after ESD. One case with initial diagnosis as intestinal metaplasia turned out to be carcinoma in situ . According to the final pathological classification, the majority of EGCs resected were high-grade dysplasias (n = 49) or adenocarcinomas (n = 20).
The mean operation time was 85.5 minutes (range, 12–360 minutes) and the mean length of stay was 4.8 days (range, 1–13 days). The complications after ESD are presented in Table 2 .
| Complications | 11 (15.9) |
| Failure due to severe fibrosis of SM | 5 (7.2) |
| Perforation due to severe fibrosis of SM | 2 a |
| MP layer involvement | 3 a |
| Failure due to endoscopic view obscured by bleeding | 1 (1.4) |
| Put on hold due to suspected perforation, repeat ESD 1 mo later | 1 (1.4) |
| Major bleeding (hemoglobin decrease > 2 mg/dL) | 4 (5.8) |
| Recurrent bleeding requiring another EGD for hemostasis | 3 a |
Data are presented as n (%).
ESD = endoscopic submucosal dissection; MP = muscularis propria; SM = submucosa; EGD = esohago-gastro-duodenoscopy.
a. Cases included.
In the intention-to-treat group (69 lesions), the procedure had to be changed to operative treatment due to suspected SM invasion (because of severe fibrosis of the submucosa) in five (7.25%) cases; obscured endoscopic view in one (1.45%) case with massive bleeding (Table 2 ). Four (5.80%) patients had major bleeding post-ESD and three patients even needed a second intervention after the ESD procedure. Two (2.90%) patients had perforation during ESD; one patient underwent closure by hemoclip and the other received emergency surgery. The cancers in these two cases were 1.0- and 7.0-cm intramucosal lesions. The perforations were located high in the body and had SM fibrosis changes.
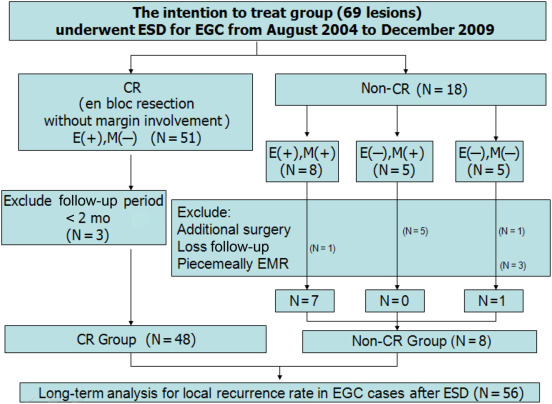
“Curative treatment” was defined as a one-piece resection with at least 1-mm cancer-free margins, including lateral and vertical margins, no lymphovascular involvement, and cancer depth limited to the SM superficial layer (< 500 μm to the muscular muscle layer) after pathological assessment. For gastric epithelial cancers, 51 (73.9%) patients had curative treatment with ESD and 18 (26.1%) patients had noncurative treatment with ESD (Fig. 1 ). Of the 18 noncurative lesions, eight received en bloc resection along the margin of the cancer and five had deep-margin involvement. These eight patients were advised to undergo additional surgery. One of these was lost to follow-up and the remaining seven refused surgery because of comorbidity with major systemic diseases, such as congestive heart failure and chronic renal insufficiency.
|
|
|
Figure 1. A flow diagram of treatment of the 69 early gastric cancer (EGC) lesions in this study. Patients without curative resection also received argon plasma coagulation to destroy the residual tumor. CR = curative resection; E(+) = en bloc resection; E(−) = no en bloc resection; EMR = endoscopic mucosal resection; M(+) = specimen with margin involvement; M(−) = specimen without margin involvement. |
Patients without CR further received argon plasma coagulation (APC) to destroy the residual tumor. The long-term follow-up analysis was divided into the following two subgroups: CR group and non-CR group. The characteristics of the two groups are presented in Table 3 ; Table 4 . Finally, data on 56 patients were collected for local recurrence rate analysis, as shown in Fig. 1 .
| 48 lesions (46 patients) after curative resection (CR group) |
|---|
| Synchronous (1 patient, N = 1) |
| Because of the different locations of the 2 lesions occurring at the same time in the same patient, we excluded the 2nd lesion as treatment was shifted to EMR due to difficulty in dissecting the lesion owing to its location. |
| Metachronous (1 patient, N = 2) |
| The lesions received ESD because they had different locations and occurred at different times in the same patient. |
| Local recurrence (2 patients, N = 3) |
| 1 patient received additional ESD at the 20th mo. Thereafter, no recurrence during 23 months’ follow-up period (N = 2, the lesion after ESD was a new one). |
| 1 patient received additional EMR due to severe SM fibrosis at the 9th mo (N = 1, because we excluded the second lesion as treatment was shifted to EMR). |
| 42 patients, N = 42 |
| 42 patients with one lesion, without synchronous, metachronous, or local recurrence. |
CR = curative resection; EMR = endoscopic mucosal resection; ESD = endoscopic submucosal dissection; SM = submucosal.
| 8 lesions (7 patients) after noncurative resection (non-CR group) |
|---|
| E(−), M(−) (1 patient, N = 1) |
| Because of severe fibrosis, APC was applied to destroy the residual tumor; no recurrence during 91 mo follow-up period. |
| E(+), M(+) (6 patients, N = 7) |
| Synchronous (1 patient, N = 2) |
| Because the 2 lesions occurred at different locations at the same time in the same patient, APC was applied to destroy the suspected residual tumor; no recurrence during 17 mo follow-up period. |
| Local recurrence (3 patients, N = 3) |
| 1 received surgery after 3 mo follow-up; EGD revealed recurrence. |
| 1 patient was lost to follow-up after recurrence at the 31st mo. |
| 1 received additional ESD (twice) at the 38th mo and 64th mo recurrence; no recurrence during 3 mo of follow-up until the time of writing. |
| Two patients, N = 2 |
| 2 patients with 1 lesion, without synchronous, metachronous, or local recurrence. |
APC = argon plasma coagulation; CR = curative resection; E(−) = no en-bloc resection; EGC = early gastric cancer; ESD = endoscopic submucosal dissection; M(−) = specimen without margin involvement.
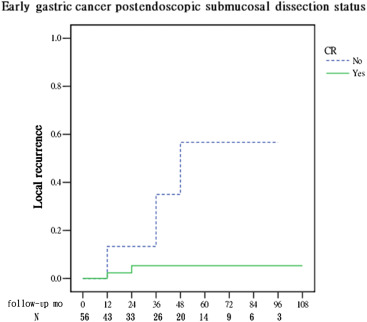
The cumulative local recurrence rates were 5.3% in the CR group and 56.7% in the non-CR group (p = 0.0091; Fig. 2 ). The disease-free status was high (94.7%) with CR. Furthermore, in the CR group (48 patients), the risk factor affecting the disease-free status was analyzed and no significant factor was identified (data not shown in the figure).
|
|
|
Figure 2. Comparison of local recurrence rate in early gastric cancer lesions that underwent endoscopic submucosal dissection [56 lesions; categorized by whether or not they received curative resection(CR)]. The cumulative local recurrence rates were 5.3% in the CR group and 56.7% in the non-CR group in the 84th month [calculated by the Wilcoxon (Gehan) statistical method, p = 0.009]. |
Among the patients with local recurrence (2/48 in the CR group and 3/8 in the noncurative group), one underwent surgery, one was lost to follow-up, and three underwent repeat ESD; of these three patients, one received additional ESD when recurrence was noted at the 38th month and 64th month. No recurrence was noted at the 3-month follow-up period and all were free of recurrence at the time of this writing, as shown in Table 4 . None of the patients died of gastric cancer-associated diseases during the follow-up period.
The risk factors that affect the non-CR are tumor location (p = 0.013), deeper invasion (p < 0.001), undifferentiated histopathology (p < 0.001), and ulcer presence (p = 0.045), as shown in Table 5 .
| Risk factor | p |
|---|---|
| Location: PW | 0.204 |
| Location: low | 0.013 |
| Ulcer | 0.045 |
| Poorly differentiated adenocarcinoma | <0.001 |
| Invasion depth (mucosal or submucosal) | <0.001 |
| Tumor size (≥ 2 cm) | 0.055 |
| Skill mature (Case ≥ 20th ) | 0.180 |
PW = posterior wall.
a. Comparisons of risk factors were calculated by the Chi-square (Pearson) statistical method.
Discussion
EGC has a good prognosis with long-term survival rates as high as 95% after gastrectomy with removal of lymph nodes [10] . Follow-up studies are required to confirm whether endoscopic treatment can be an alternative treatment choice for EGC and whether its efficacy is similar to that of conventional surgery. In a previous study, Chang et al [9] reported a short-term outcome of ESD for gastric epithelial tumor in Taiwan. Here, we have presented the long-term follow-up outcome results.
EMR can achieve complete resection in the case of some EGCs; however, local recurrence occasionally occurs [11] . In a conventional EMR, incomplete and piecemeal resections are the main reasons for local recurrences, which often occur in lesions > 2 cm, depressed lesions, and those in difficult-to-access locations [12] . In addition, piecemeal resection of the lesions makes it difficult for a pathologist to diagnose and confirm the staging. En bloc resection of ESD provides much higher complete resection rates than piecemeal resections of EMR. In the event of SM invasion, muscularis propria invasion, or severe fibrosis in SM that cannot always be predicted prior to ESD, the initial plan to perform ESD is usually shifted to EMR.
In this study, the en bloc and CR rates were 85.5% and 73.9%, respectively, which are relatively lower than those of other study [13] . The reason may be due to the small case numbers in our study, which can overweigh the failure part.
In the intention-to-treat group (69 lesions), we included the ESD failure and incomplete resection cases because of the shift to the EMR method. In addition, we also included patients who refused further operative treatment to find out the incidence of the complication (Table 2 ) and evaluate whether the risk factors affect CR or not (Table 5 ).
In our daily practice, we found that some patients do not prefer further surgical procedure. The coagulation mode was applied to stop the easy bleeding tendency while SM invasion occurred or APC was applied to destroy the residual tumor site for the non-CR cases. However, due to the burn effect of coagulation, the margin of the pathological specimens could not be well evaluated. In this study, the cumulative local recurrence rates were 5.3% in the CR group and 56.7% in the non-CR group (p = 0.0091; Fig. 2 ). Therefore, we should always emphasize the importance of regular follow-up, especially for noncurative cases.
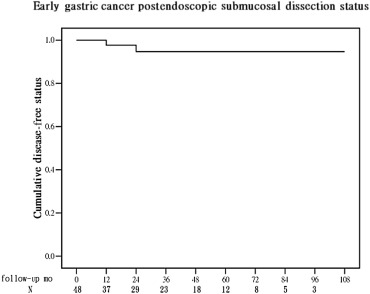
The disease-free status was high (94.7%) with CR, as shown in Fig. 3 . After excluding the factors affecting non-CR lesions in the CR group (n = 48 lesions), no other risk factor was significant in the disease-free status analysis.
|
|
|
Figure 3. The cumulative disease-free status of early gastric cancer lesions (n = 48) that underwent curative endoscopic submucosal dissection. The disease-free status was 94.7%. |
In a previous study, Takenaka et al [14] reported that tumor size (> 30 mm) and tumor location (upper 3rd) were significant predictors of local recurrence after ESD. In this study, for the intention-to-treat group (69 lesions), the non-CR lesions significantly increased local recurrence risk. The risk factors for non-CR are tumor location (p = 0.013) and tumor size (≥ 2 cm; p = 0.055, Table 5 )
The rate of lymph node metastasis from EGC was reported to be 0.4% in differentiated mucosal cancers and 4.2% in undifferentiated mucosal cancers [15] . This difference is an important criterion to select patients for ESD. In addition, undifferentiated EGC is more likely to invade the SM layer, even for tumors that are small. Undifferentiated EGCs are often distributed in a discontinuous pattern. This also makes it difficult to define a definite margin of a lesion prior to ESD. In our study, the non-CR significantly increased local recurrence risk, and was significantly higher for lesions having deeper invasion (p < 0.001) and those having an undifferentiated histopathology (p < 0.001; Table 5 ).
In conclusion, ESD offers a good outcome after EGC treatment [16] ; [17] . To decrease the risk of local recurrence, preoperative diagnosis of the tumor extent (e.g., SM invasion and tumor margin positivity) and accurate postoperative pathological evaluation (e.g., resection margin) and close follow-up are very important.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
No financial support was received for this study.
References
- [1] Japanese Gastric Cancer Association; Japanese classification of gastric carcinoma—2nd English edition—response assessment of chemotherapy and radiotherapy for gastric carcinoma: clinical criteria; Gastric Cancer, 4 (2001), pp. 1–8
- [2] S.M. Shimizu, M. Tada, K. Kawai; Early gastric cancer: its surveillance and natural course; Endoscopy, 27 (1995), pp. 27–31
- [3] K.C. Ballantyne, D.L. Morris, J.A. Jones, R.H. Gregson, J.D. Hardcastle; Accuracy of identification of early gastric cancer; Br J Surg, 74 (1987), pp. 618–619
- [4] R.J. Schlemper, R.H. Riddell, Y. Kato, F. Borchard, H.S. Cooper, S.M. Dawsey, et al.; The Vienna classification of gastrointestinal epithelial neoplasia; Gut, 47 (2000), pp. 251–255
- [5] Japanese Gastric Cancer Association; Japanese classification of gastric carcinoma—2nd English edition; Gastric Cancer, 1 (1998), pp. 10–24
- [6] T. Gotoda, A. Yanagisawa, M. Sasako, H. Ono, Y. Nakanishi, T. Shimoda, et al.; Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers; Gastric Cancer, 3 (2000), pp. 219–225
- [7] H. Isomoto, S. Shikuwa, N. Yamaguchi, E. Fukuda, K. Ikeda, H. Nishiyama, et al.; Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study; Gut, 58 (2009), pp. 331–336
- [8] H. Ono, H. Kondo, T. Gotoda, K. Shirao, H. Yamaguchi, D. Saito, et al.; Endoscopic mucosal resection for treatment of early gastric cancer; Gut, 48 (2001), pp. 225–229
- [9] C.C. Chang, I.L. Lee, P.J. Chen, H.P. Wang, M.C. Hou, C.T. Lee, et al.; Endoscopic submucosal dissection for gastric epithelial tumors: a multicenter study in Taiwan; J Formos Med Assoc, 108 (2009), pp. 38–44
- [10] T. Kojima, A. Parra-Blanco, H. Takahashi, R. Fujita; Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature; Gastrointest Endosc, 48 (1998), pp. 550–554 discussion 554–5
- [11] J.C. Park, S.K. Lee, J.H. Seo, Y.J. Kim, H. Chung, S.K. Shin, et al.; Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience; Surg Endosc, 24 (2010), pp. 2842–2849
- [12] H. Makuuchi, Y. Kise, H. Shimada, O. Chino, H. Tanaka; Endoscopic mucosal resection for early gastric cancer; Semin Surg Oncol, 17 (1999), pp. 108–116
- [13] M.K. Choi, G.H. Kim, Y. Park do, G.A. Song, D.U. Kim, D.Y. Ryu, et al.; Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience; Surg Endosc, 27 (2013), pp. 4250–4258
- [14] R. Takenaka, Y. Kawahara, H. Okada, K. Hori, M. Inoue, S. Kawano, et al.; Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection; Gastrointest Endosc, 68 (2008), pp. 887–894
- [15] N. Abe, T. Watanabe, M. Sugiyama, O. Yanagida, T. Masaki, T. Mori, et al.; Endoscopic treatment or surgery for undifferentiated early gastric cancer?; Am J Surg, 188 (2004), pp. 181–184
- [16] O. Goto, M. Fujishiro, S. Kodashima, S. Ono, M. Omata; Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria; Endoscopy, 41 (2009), pp. 118–122
- [17] J.H. Lee, S.J. Hong, J.Y. Jang, S.E. Kim, S.Y. Seol; Outcome after endoscopic submucosal dissection for early gastric cancer in Korea; World J Gastroenterol, 17 (2011), pp. 3591–3595
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?