Summary
Background/Objective
Anastomotic insufficiency is associated with increased morbidity and mortality. A collagen fleece that supports anastomosis is effective for preventing anastomosis insufficiency. The objective of this study was to compare between the stability of sutured anastomoses and that of anastomoses sealed with a thrombin/fibrinogen-coated collagen fleece in a rat peritonitis model.
Methods
In 72 male Wistar rats, peritonitis was induced with a specially prepared human fecal solution. Surgery at the rectosigmoid junction was performed 24–36 hours later. The different anastomotic techniques used were circular sutured anastomoses, semicircular sutured anastomosis and closure of the anterior wall with collagen patch, and complete closure with a collagen fleece. Bursting pressure, histology of anastomosis, mRNA expression of collagen types I and III, matrix metalloproteinase-13, and vascular endothelial growth factor (VEGF) were investigated after 24 hours, 72 hours, and 120 hours.
Results
All animals developed peritonitis of comparable severity. There were no differences in bursting pressures between the three suture techniques after 24 hours, 72 hours, or 120 hours. Anastomoses sealed with a collagen fleece appeared to be slightly less stable only at 24 hours, whereas they appeared to be more stable than semisutured or fully sutured anastomoses at 72 hours and 120 hours. Sealing with a collagen fleece was associated with an increase in granulation tissue, higher mRNA levels for collagen types I and III, and higher VEGF compared to sutured anastomoses.
Conclusion
The use of a thrombin/fibrinogen-coated collagen fleece showed similar efficacy to conventional sutures in colorectal anastomoses in the presence of peritonitis inflammation, and may provide additional benefits due to an increase in mature granulation tissue.
Keywords
anastomoses insufficiency;colorectal anastomoses;fibrinogen-coated collagen fleece;sealing
1. Introduction
Anastomotic leakage remains one of the most feared postoperative complications in colorectal surgery. It can result in anastomotic strictures, an increased risk of permanent stoma, recurrence of cancer, and mortality. In order to reduce the rate of anastomotic leakage, several studies have evaluated innovative sealing procedures, mainly using liquid fibrin glue. The overall efficacy data, however, are unconvincing.1; 2 ; 3
A collagen fleece is a tissue sealant patch composed of equine collagen, which is coated with human fibrinogen and human thrombin.4; 5 ; 6 Upon administration to the wound surface, thrombin and fibrinogen form a clot, activate endogenous hemostasis, and seal the patch tightly to the wound surface.7 Hemostasis is achieved usually between 3 minutes and 5 minutes. Efficacy in hemostasis and tissue sealing has been demonstrated in cardiovascular and lung surgery as well as in kidney and liver resection.4; 6; 8; 9; 10; 11; 12 ; 13
Intestinal anastomoses are generally closed by sutures, but these may cause local ischemia at the anastomotic line and subsequent impairment of wound healing.14 Suture-free bowel anastomosis using a thrombin/fibrinogen-coated collagen fleece would possibly circumvent this complication. In fact, colorectal anastomosis using a collagen fleece covered with fibrin glue components has been shown to be technically feasible in rodents5; 15 ; 16 and pigs.17 ; 18 In both models, sealed anastomoses were equally efficient as the sutured ones.
Although these studies used healthy animals, peritonitis is a frequent sequel of colorectal anastomoses and is known to impair reparative collagen synthesis and wound healing.19 ; 20 Peritoneal soilage for 24 hours caused by cecal ligation and puncture was shown to affect anastomotic healing and wound strength in the rat colon for up to 7 days.21
Anastomotic bursting pressure is a well-established and frequently applied parameter used to determine the strength and sufficiency of experimental bowel anastomosis. The anastomotic character can further be determined by the presence of important tissue healing factors such as vascular endothelial growth factor (VEGF), a key mediator of angiogenesis in normal tissues22; collagen type I for forming mature scar tissue23; collagen type III; collagen of granulation tissue23; and matrix metalloproteinase (MMP)-13, a principal enzyme for cleaving fibrillar collagen from the endothelial matrix.24
The aim of this study was to investigate the effect of a thrombin/fibrinogen-coated collagen fleece on the stability and wound healing processes of colorectal anastomoses in rats with induced peritonitis by comparing three groups receiving three different closure techniques (circular sutures, semicircular sutures of the posterior wall and sealing of the anterior wall with a collagen fleece, and a complete sealing).
2. Materials and methods
2.1. Animals
Wistar WU rats (Charles River Laboratories, Sulzfeld, Germany), 12 weeks old, with body weights of 280–417 g were used. The animals were single housed in macrolon cages, were fed a standardized pellet diet, and received tap water ad libitum. The investigation was approved by the Schleswig–Holstein Ministry of Agriculture, Environment, and Rural Areas under license number 35/2007.
2.2. Preparation of a human fecal suspension for peritonitis induction
A rat model with standardized severity of peritonitis was established by injecting a fixed dose of a human fecal suspension into the abdominal cavity. The fecal suspension was prepared according to the procedure described by Lorenz et al25 and Bauhofer et al,26 with minor modifications. Fresh human stools were collected from three healthy adult individuals, and standardized fecal inoculums of each of these samples were pooled. This was mixed with thioglycolate containing 10% barium sulfate in a 1:1 ratio. The suspension was resuspended homogenously in a 1:10 ratio in phosphate-buffered saline containing 10% glycerin and 0.19 mg catalase (from bovine liver) per 100 mL suspension. The mixture was homogenized in a blender for 10 minutes to a macroscopically homogenous suspension and filtered through a sterile gauze. Aliquots of 2 mL were prepared and frozen at –80°C. Quantitative cultural analyses of representative aliquots were performed prior to freezing and after repeated thawing, to demonstrate the stability of dominant viable bacterial communities (e.g., enterobacteria, enterococci, staphylococci, Streptococcus viridans, lactobacilli, Bacteroides/Prevotella spp., clostridia, etc.). The required amount of fecal suspension was thawed immediately prior to the peritonitis surgery.
2.3. Exploratory study for establishing the peritonitis model
An exploratory study was conducted in 12 animals to determine the appropriate dose of a human fecal sample needed to be injected into the abdominal cavity to create a clinically relevant peritonitis with a mortality rate below 5%. After induction of anesthesia with ketamine (80 mg/kg i.p.) and xylazine (3 mg/kg i.p.), a 3 cm midline laparotomy was performed, and an aliquot of the fecal suspension was instilled into the abdominal cavity at different doses. Thereafter, the abdomen was closed and animals were monitored for up to 4 days. Cefuroxime (10 mg/kg i.v., prior to and after surgery) and metronidazole (3.5 mg/kg i.v., prior to and after surgery) were used to control the systemic spread of the infection in the initial phase of peritonitic reaction. Butorphanol (1 mg/kg s.c.) was used for postsurgery analgesia.
2.4. Experimental groups in main study
A total of 72 male Wistar rats were used for the main study. Peritonitis was induced 24 hours prior to anastomosis surgery. Animals were assigned to three experimental groups according to the different techniques used for anastomosis surgery [circular suture (A), semicircular suture of the posterior intestinal wall and sealing of the anterior intestinal wall with a thrombin/fibrinogen-coated collagen fleece (B), and complete sealing with a collagen fleece (C)]. Each of these experimental groups was further divided into three subgroups, of which the rats were sacrificed 24 hours, 72 hours, or 120 hours after performing the anastomosis. At the time of sacrifice, the anastomotic bursting pressure was measured and tissues were collected for further analysis.
2.5. Surgical procedures
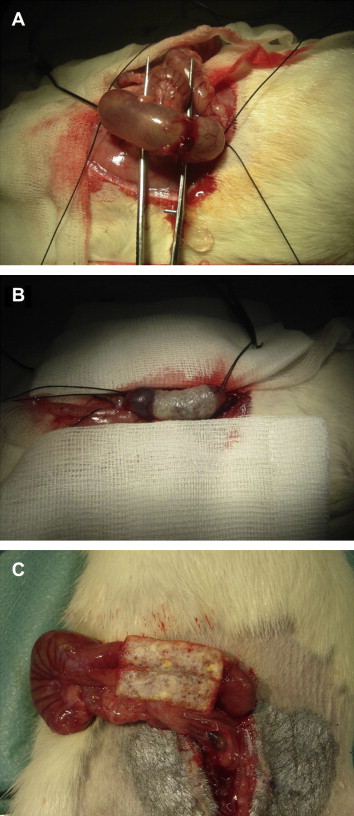
Twenty-four hours to 30 hours after inducing peritonitis, laparotomy was performed under general anesthesia as described above, carefully exposing the distal colon at the transition to the rectum. Single-layer, end-to-end anastomoses were performed using circular sutures (5-0 Prolene, Ethicon, Hamburg, Germany; Group A, 24 animals), semicircular sutures of the posterior intestinal wall using 5-0 Prolene and sealing the anterior wall with a 2 × 1 cm2 TachoSil patch (Nycomed GmbH, Konstanz, Germany; Group B, 22 animals), or completely sealing the anastomosis with a 2 × 2 cm2 collagen fleece (Group C, 18 animals). The collagen patches (which are available at a size of 9.5 × 4.8 cm2) were cut to the desired size, soaked in an isotonic saline solution, and, under slight pressure, were wrapped carefully around the anastomosis (Fig. 1). The abdomen was closed, and animals were positioned under a heating lamp, monitored until recovery from anesthesia, and then returned to the cage. Postoperative analgesia was achieved with butorphanol (1 mg/kg s.c.). Postoperative antibiosis was carried out with intravenous administration of cefuroxime (10 mg/kg) and metronidazole (3.5 mg/kg). For volume substitution, Ringers solution was used (8–10 mL/animal s.c.). To avoid postoperative ileus and enable adequate energy intake during the first 48 hours after inducing peritonitis, the animals were offered sugared water (20 g sugar/100 mL tap water) instead of conventional pellet diet. From Day 2 onward, the animals additionally received chopped white bread.
|
|
|
Figure 1. Photographs of three different types of colorectal anastomosis performed in a rat peritonitis model: (A) circular suture, (B) semicircular suture of the posterior intestinal wall and sealing of the anterior intestinal wall with a 1 × 1 cm2 patch of thrombin/fibrinogen-coated collagen, and (C) complete sealing with a 2 × 2 cm2 patch of collagen fleece. |
2.6. Clinical monitoring
The animals were monitored on a daily basis for behavior and appearance. Body weights were measured daily with a precision scale. Prior to peritonitis induction, on the day of anastomosis surgery, and on the day of bursting pressure measurement/necropsy, blood samples were taken from the retro-orbital plexus and monitored for white blood cell count, red blood cell count, differential blood cell count, hemoglobin content, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelet count, total protein, albumin, fibrinogen, and haptoglobin.
2.7. Macroscopic evaluation
During anastomosis surgery and at the time of bursting pressure measurement/necropsy, the abdominal cavity was examined visually in order to evaluate the site of anastomosis and the degree of peritonitis.
2.8. Sacrifice and necropsy
The animals were killed with diethyl ether 24 hours, 72 hours, and 120 hours after anastomosis surgery. Immediately thereafter, the abdominal cavity was opened and inspected visually, and the bursting pressure was measured. Afterward, a general macroscopic evaluation was conducted and samples were collected for histology and molecular investigations.
2.9. Bursting pressure
The anastomotic bursting pressure was measured in situ in all groups at the time of sacrifice by an investigator unaware of the group assignment. The intestine was ligated approximately 2 cm proximal to the anastomosis. A blunt cannula was placed into the lumen, approximately 1.5 cm distal to the anastomosis, and the intestine was ligated posterior to the cannula. The cannula was connected to an infusion pump and a pressure transducer connected to a Hewlett Packard intensive care monitor for data acquisition. The ligated part was filled with saline solution at an infusion rate of 100 mL/h. The bursting pressure was defined as the pressure at which the infused saline emerged out of the sutured anastomosis and the measured pressure started to drop. The maximum pressure value registered by the pressure transducer and the Hewlett Packard intensive care monitor was registered as the bursting pressure.
2.10. Histology
After the in situ measurement of the bursting pressure, the colon was removed. Tissue specimens of the anastomosis (half of the anastomosis) and normal colon were dissected and fixed by immersion in 8% (w/w) buffered formalin. The formalin-fixed tissue was embedded in paraffin wax and sectioned. The sections were stained with hematoxylin and eosin or Picrosirius red (Roti Histo-Kit, Sheffield, UK), and evaluated under a light microscope. Findings were recorded according to their character and severity.
2.11. mRNA-expression of collagen types I and III, VEGF, and MMP-13
Additionally, the other half of the anastomosis as well as the normal colon was preserved in liquid nitrogen for further investigations on mRNA expression.
Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) analyses for collagen types I and III, VEGF, and MMP-13 were performed by a LightCycler system using the LightCycler software 3.5 (Roche Diagnostics, Mannheim, Germany). Primers were designed using the LightCycler Probe Design Software 2.0 (Roche), each amplifying an approximately 150 base pair product. Primers were manufactured by TIB Molbiol (Berlin, Germany) with the following sequence: collagen type I—sense: 5′-TACTGCAACATGGAGACAGG-3′, antisense: 5′-CGCTGTTCTTGCAGTGATAG-3′; collagen type III—sense: 5′-CTGAAATTCTGCCACCCTGA-3′, antisense: 5′-GCATCTGTCCACCAGTGCT-3′; VEGF—sense: 5′-ACCAAAGCCAGCACATAGGAG-3′, antisense: 5′-ACGCTCCAGGATTTAAACCGG-3′; MMP-13—sense: 5′-CGAGCATCCATCCCCGAGAC-3′, antisense: 5′-GTCCTCAAAGTGAACCGCAG-3′; and hydroxymethylbilane synthase (HMBS)—sense: 5′-AGCCAAGGACCAGGATATCT-3′, antisense: 5′-AGTCAGGTACAGTTGCCCAT-3′.
HMBS was included as a housekeeping gene to control the variations in mRNA amounts. Relative amounts of mRNA were calculated using the Relative Quantification Software (Roche). The LightCycler runs were performed in duplicates. The principle of real-time RT-PCR has previously been described in detail.27 ; 28 PCR was performed using the LightCycler Faststart DNA SYBR Green I-Kit (Roche) according to the manufacturers instructions. A volume of 5 μL of diluted cDNA (1:5 in PCR-grade water), 0.5 μL primers (10 pmol/μL), and 0.8 μL MgCl2 (25mM) were used in a 10 μL final reaction mixture. After a 15-minute incubation at 95°C for activation of the polymerase, each of the 45 cycles consisted of 15 seconds of denaturation at 95°C and amplification of primers for 30 seconds at 64°C. The melting curve analysis was performed in one cycle of 95°C for 1 second and 65°C for 10 seconds, each with a temperature transition rate of 20°C/s and then ramping to 95°C at 0.1°C/s.
2.12. Statistical analysis
For the statistical evaluation of bursting pressure, individual data were described using location parameters (mean and median) and scale parameters (standard deviation, and minimum and maximum values). Statistical analysis was carried out by the SAS GLM PROC (SAS Institute Inc., Cary, NC, USA) using the following class variables: treat [0 (suture), 1 (suture and collagen patch), and 2 (complete collagen patch sealing)], time [1 (24 hours), 2 (72 hours), and 3 (120 hours)], and concentration.
Relative amounts of mRNA were compared across the three intervention groups at three different time points using the Kruskall–Wallis and Dunns multiple comparison tests.
3. Results
3.1. Peritonitis
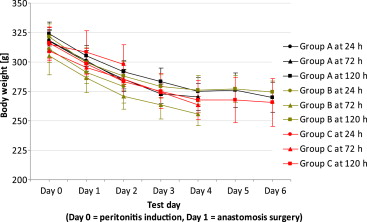
In some rats of all groups, intra-abdominal instillation of the human fecal sample was followed by clinical signs of peritonitis such as chromodacryorrhea, hunched position, piloerection, or soft feces/diarrhea. These findings were related to the stress of the anesthesia, sepsis, and abdominal surgery. Over time, the animals recovered and feces normalized. No differences were observed in behavior and appearance between groups. Body weights decreased significantly following the induction of peritonitis, but started to recover from 4 days after induction (Fig. 2). The course of the body weight did not differ between groups.
|
|
|
Figure 2. Pre- and postoperative bodyweights of all groups, after induction of peritonitis, measured daily up to 6 days after surgery. The course of the body weight did not differ significantly between groups. |
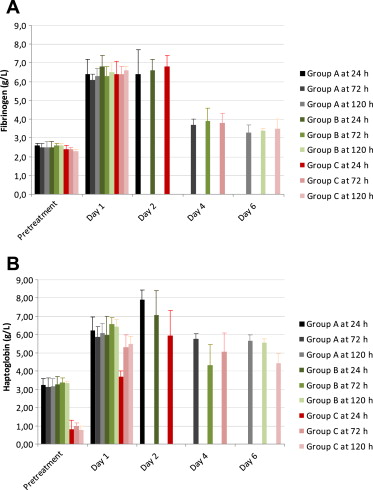
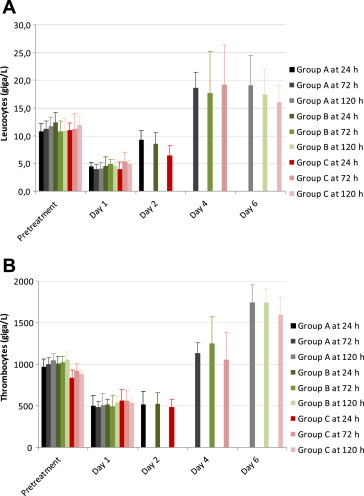
Monitoring of hematologic and clinical chemistry parameters after intra-abdominal instillation of the human fecal sample revealed an acute systemic inflammatory response in all animals. Serum levels of the acute-phase reactants, fibrinogen and haptoglobin (Fig. 3), were increased on Days 1 and 2 after peritonitis induction and returned to normal thereafter. On Day 1, leukocyte and platelet counts were decreased with a marked left shift in the differential counts, whereas on Study Days 2–6 leukocyte and platelet counts were increased (Fig. 4), again associated with a relative increase in polymorphonuclear granulocytes and a relative decrease in lymphocytes. No relevant changes in red blood cell count, total protein, and albumin were detected at any time point.
|
|
|
Figure 3. Serum levels of acute-phase reactants (fibrinogen (A) and haptoglobin (B)) in all groups, prior to and after induction of peritonitis, measured daily up to 6 days after surgery. The course of the serum levels did not differ significantly between groups. |
|
|
|
Figure 4. Leucocyte and thrombocyte counts in all groups, prior to and after induction of peritonitis, measured daily up to 6 days after surgery. The course of the cell counts did not differ significantly between groups. |
At 24–36 hours after peritonitis induction, variable degrees of an inflammatory exudate were found in the abdominal cavity of all rats. Adhesions between different abdominal structures, whitish-yellow spots and nodules on various organs (correlating to bacterial abscesses), and enlarged mesenteric lymph nodes were seen in a large number of animals. The severity of macroscopic changes decreased over time, indicating a healing process.
No deaths related to peritonitis or anastomosis occurred in any of the groups.
3.2. Macroscopic and microscopic evaluation of anastomosis
At autopsy, one animal of Group B showed signs of an ileus. In another animal of this group, the diameter of the colon caudal to the anastomosis was less than the cranial to the anastomosis, but no further signs of obstruction or ileus were noted.
Evidence of anastomosis insufficiency (feces within the abdominal cavity) was found in two animals of Group C 72 hours after performing the anastomosis. Two additional animals of this group showed a microleakage between the intestinal mucosa and the collagen patch in the microscopic examination. However, no feces in the abdominal cavity or any other sign of leakage was observed in these animals.
3.3. Bursting pressure
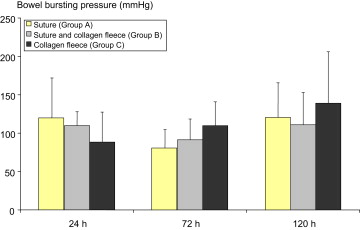
Bursting pressure measurement was not feasible in the two animals from Group C showing anastomosis insufficiency. For all other animals, no statistically significant difference between treatment groups was observed for the anastomosis bursting pressure, regardless of the time of investigation (Fig. 5). At 24 hours after anastomosis surgery, the mean bursting pressure of Group C was slightly lower compared to the other two groups, whereas it was slightly higher at 72 hours and 120 hours.
|
|
|
Figure 5. Bursting pressures of three different types of colorectal anastomoses, measured at 24 hours, 72 hours, and 120 hours after surgery. Data presented are mean values and standard deviation (6–8 animals per group). Differences are not significant. |
3.4. Histology
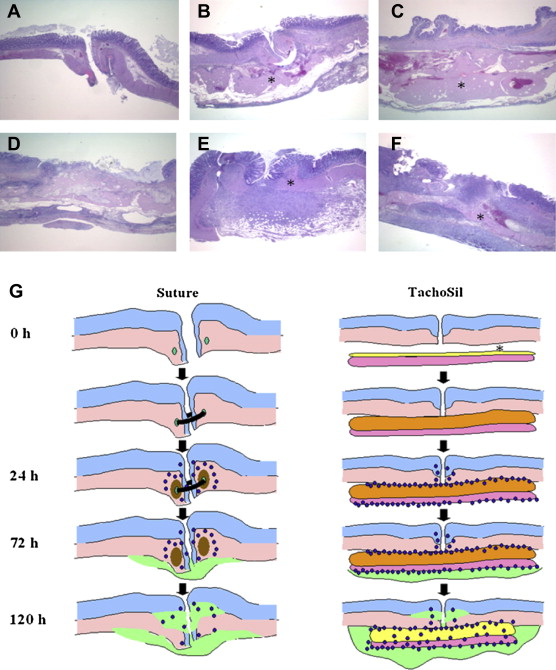
At 24 hours after surgery, the anastomosis was characterized by invagination of the colonic wall and mild edema in the tunica submucosa. In groups with sutures, these were detectable in the outer tunica muscularis, associated with mild necrosis, and surrounded by moderate amounts of neutrophils (Fig. 6A). The tunica adventitia was expanded slightly by neutrophils and bacteria. In groups with collagen patches, there was an extensive accumulation of erythrocytes and fibrin along the tunica serosa. This fibrin clot was surrounded by large amounts of neutrophils (fibrinolysis) and merged into dispersed layers of compacted hyalinized collagen, which are compatible with the equine collagen fibers of the thrombin/fibrinogen-coated collagen patch (Fig. 6C). Small colonies of bacteria were observed between the anastomotic site and the fibrin clot, and scattered bacteria within the fibrin and the collagen fibers. Anastomotic sites covered with a collagen matrix were characterized mainly by discontinuation of the tunica muscularis and some edema therein.
|
|
|
Figure 6. Histology of colorectal anastomoses treated with (A, D) circular sutures, (B, E) semicircular sutures and collagen patches, (C, F) and collagen fleeces only, at (A–C) 24 hours after surgery and (D–F) 120 hours after surgery. Hematoxylin and eosin stain was used. (G) A schematic drawing of the wound healing process occurring in a sutured anastomosis compared to a collagen fleece-glued anastomosis. In sutured anastomoses, leukocytes (blue dots) were observed after 24 hours, associated with local tissue necrosis (brown area) and followed by ingrowth of granulation tissue (green area). In collagen fleece-glued anastomoses, a large fibrin clot is formed between the anastomosis and the collagen patch (orange area), which becomes infiltrated by leukocytes (blue dots) and surrounded by granulation tissue (green area). ∗ TachoSil patch. |
At 72 hours after surgery, there were mild necrosis of the tunica muscularis and marked neutrophilic inflammation in the tunica submucosa, muscularis, and adventitia, mixed with some eosinophils, macrophages, and lymphocytes. A mild to marked growth of granulation tissue was observed within the tunica adventitia, extending into the tunica muscularis. Even with a collagen patch, there was still an extensive accumulation of fibrin expanding along the tunica serosa, and the fibrin clot was surrounded by very large amounts of neutrophils (fibrinolysis) and merged into dispersed layers of compacted hyalinized collagen. Large amounts of neutrophils infiltrated collagen fibers, covered by a large amount of granulation tissue. Small colonies of bacteria were found within the fibrin clot and scattered bacteria within the collagen fibers. In the group treated with a coated collagen matrix only, the anastomosis was characterized by discontinuation of the tunica muscularis, with marked neutrophilic inflammation in the tunica adventitia, extending into the tunica muscularis.
At 120 hours after surgery, moderate neutrophilic inflammation was reported within the tunica muscularis and tunica adventitia. Prominent granulation tissue was formed within the tunica adventitia, extending into the tunica muscularis (Fig. 6D). With a collagen patch, an extensive fibrin clot covered the tunica adventitia and merged into dispersed collagen fibers that were infiltrated heavily by neutrophils and surrounded by granulation tissue (Fig. 6F). In the group treated with a collagen fleece only, the anastomosis was characterized by discontinuation of the tunica muscularis, with granulation tissue formation within the tunica adventitia, extending into the tunica muscularis.
3.5. Collagen types I and III mRNA levels
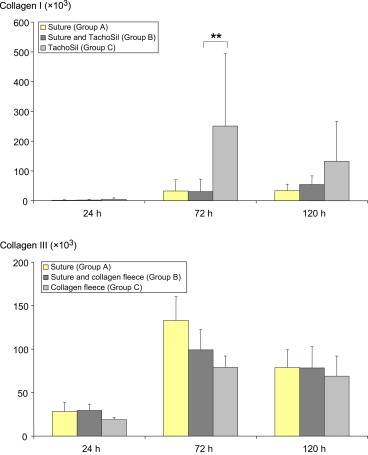
At 24 hours after surgery, no differences were observed in the mRNA levels of collagen types I and III between the three intervention groups (Fig. 7). At 72 hours, mean mRNA levels of collagen I were statistically different across groups (p = 0.0101), with higher mean levels in Group C compared to that in Groups A and B (p < 0.01 for Group C vs. Group B). A higher mean level of collagen type I mRNA was also found in Group C at 120 hours after surgery, although with no statistical significance. For collagen type III mRNA levels at 72 hours and 120 hours after surgery, no significant difference across groups was detected ( Fig. 7).
|
|
|
Figure 7. Levels of mRNA (in arbitrary units) for collagen types I and III in anastomosis tissue (6–8 animals per group). Data presented are mean levels and standard deviation. Levels were normalized to mRNA expression of the housekeeping gene HMBS. Differences are not significant. ∗∗ p < 0.01. HMBS = hydroxymethylbilane synthase. |
3.6. VEGF mRNA levels
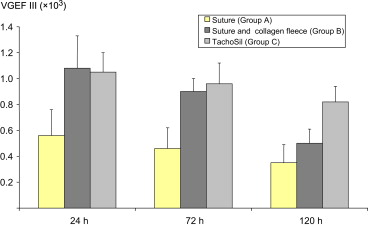
No statistically significant difference was found across groups for VEGF mRNA levels at any time point (Fig. 8). However, a trend toward higher mean levels was observed in both semicircular/sealed anastomoses (Group B) and TachoSil-treated anastomoses (Group C), compared to the sutured counterparts (Group A).
|
|
|
Figure 8. Levels of mRNA (in arbitrary units) for cVEGF in anastomosis tissue (6–8 animals per group). Data presented are mean levels and standard deviation. Levels were normalized to mRNA expression of the housekeeping gene HMBS. Differences are not significant. cVEGF = vascular endothelial growth factor C; HMBS = hydroxymethylbilane synthase. |
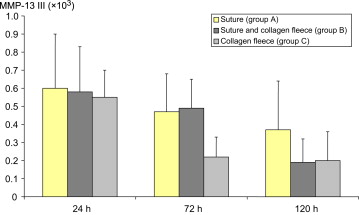
3.7. MMP-13 mRNA levels
The mRNA levels of MMP-13 did not show any significant differences between groups (Fig. 9). They appeared to decline over time, with a more rapid decline in the case of the anastomoses treated with a collagen fleece only (Group C).
|
|
|
Figure 9. Levels of mRNA for MMP-13 in anastomosis tissue (6–8 animals per group). Data presented are mean levels and standard deviation. Levels were normalized to mRNA expression of the housekeeping gene HMBS. Differences are not significant. HMBS = hydroxymethylbilane synthase; MMP = matrix metalloproteinase. |
4. Discussion
Clinical anastomotic leakage is the most serious complication in colorectal surgery, as it contributes considerably to postoperative morbidity and mortality.29; 30; 31 ; 32 Accounting for a third of all complications, leakage at the anastomotic site is not only the decisive mortality factor in surgery of rectal cancer,29 ; 31 but also associated with a higher local recurrence rate and lower long-term survival.33 ; 34 Risk factors for anastomotic leakage are smoking,31 chronic heart disease,31 diabetes,35 and obesity.36
Although much progress has been made in colorectal surgery, about 16% of the patients have anastomotic leakage with clinical signs.15 In light of morbidity following the second stage of a Hartmann procedure (reversal of colostomy), longer hospital stay, and permanent stoma rate, primary anastomosis is the method of choice in colonic injury surgery. Intra-abdominal peritonitis, however, is considered a contraindication for primary anastomoses, because the healing process may be impaired by the infection in the peritoneum.19 ; 20 Twenty-four hours of peritoneal soilage caused by cecal ligation and puncture was shown to affect anastomotic healing and wound strength adversely for up to 7 days.21 The debate whether or not primary anastomosis can be recommended in this situation is ongoing.37; 38; 39 ; 40
Primary anastomoses are performed conventionally with sutures, despite these causing local ischemia and impairing wound healing.14 The benefit of additive materials, such as fibrin sealants, to cover anastomoses has been debated for several years.41 Still this management has not yet become a standard in colorectal surgery. A thrombin/fibrinogen-coated collagen fleece is a ready-to-use hemostat and is used in an increasing number of surgical disciplines such as cardiovascular and lung surgery, as well as kidney or liver resections.4; 6; 8; 9; 10; 11; 12 ; 13 It is a collagen sponge covered with a layer of human fibrinogen and human thrombin. Upon administration to the wound surface, thrombin and fibrinogen form a clot, activate endogenous hemostasis, and seal the patch to the wound surface tightly.7
Successful anastomotic healing is defined by the ability of the previously injured bowel to withstand tensile forces.5 If sufficient tissue strength cannot be restored, the anastomosis may burst upon a challenge with intraluminal pressure. Bursting pressures of the colorectal anastomoses in our study showed no differences between the groups. This indicates a comparable stability of sutured and sealed anastomoses.
In previous studies using healthy animals, the use of a thrombin/fibrinogen-coated collagen fleece has been shown to be as efficient as conventional sutures in sealing colorectal anastomoses.15 ; 17 In the present study, we now confirm the equal stability of sealed and sutured anastomoses under the condition of peritonitis. In this clinical situation, mainly caused by bowel perforation, the rates of complications such as impaired wound healing or infection are increased. In the present study, animals of all groups developed peritonitis and sepsis to a similar degree, showing clinical signs of impaired general condition and abdominal pain, systemic changes of clinical chemistry parameters for inflammation, as well as signs of local inflammation in the abdominal cavity at the time of necropsy. The severity of peritonitis declined with time, reflecting a normal healing process. The healing process did not differ between groups. Bursting pressures were equal across groups, with no statistically significant difference between sutured anastomoses and those that were sealed with a collagen fleece only. Histology and quantitative mRNA levels of collagen type I suggest that anastomoses sealed only with a collagen matrix show a higher rate of mature granulation tissue compared to sutured anastomoses. VEGF mRNA levels indicate that a collagen patch increases angiogenesis, which may be an important factor for rapid maturation of granulation tissue.
Several factors play an important role in the process of anastomotic healing: apart from the experience of the surgeon, adequate tissue perfusion and oxygen delivery, and tension-free construction of the suture are necessary.24 ; 42 However, collagen synthesis and its potential disturbance are assumed to account for the delayed appearance of dehiscence between Day 5 and Day 7 after surgery.43 Collagen is a vital extracellular matrix protein determining mechanical tissue stability during wound healing. Collagen I is responsible for the formation of mature scar tissue, whereas collagen III is found mainly during the early phases of wound healing.44 Collagen degradation is usually a result of the synergistic action of several MMPs, a family of zinc-dependent endopeptidases that can degrade most of the components of the extracellular matrix. MMP-13 is the principal matrix enzyme cleaving particularly interstitial collagen types I and III.24 ; 44 In animal studies, MMP levels in the tissue at the suture line increased during the first 3 days after anastomosis, which coincided with a decreased collagen concentration in the colonic wall.45 In uncomplicated healing, MMPs are restricted to the region of the suture line, implicating an impact of these enzymes on physiological tissue breakdown and remodeling.46 In our study, we observed a tendency for lower MMP-13 mRNA levels in the anastomoses sealed with a thrombin/fibrinogen-coated collagen fleece only at later time points, which may indicate a more stable wound healing process associated with this technique. Animal studies have shown a significant reduction in the collagen concentration at the site of the anastomosis during the early postoperative period.45 It has also been demonstrated that collagen concentration decreased significantly during the anastomotic healing process, not only directly at the suture, but also several centimeters proximal.47 Furthermore, in ischemic48 or infectious bowels, a further decrease was seen in collagen levels around the anastomoses, underlining the importance of collagen for a successful healing process. Collagen accounts for the mechanical strength as well as elasticity of wounds and scars. We found an increase in granulation tissue and a significantly higher collagen type I mRNA concentration in the collagen fleece group, indicating that the granulation tissue in this group was more mature compared to that in groups with sutured anastomoses.
Overall, the use of a coated collagen matrix showed a good sealing efficacy. Nevertheless, two animals of Group C (complete sealing with a collagen fleece) showed a completely insufficient anastomosis at necropsy. Under conditions of peritonitis and sepsis, the technique chosen for anastomosis surgery has only a minor influence on the stability and integrity of the anastomosis, but the use of a coated collagen fleece may accelerate wound healing. This finding would implicate that, in the case of an anastomotic insufficiency, sealing of the anastomosis with a collagen fleece may influence and stimulate the matrix to form a scar tissue and thus promote healing, because sutures alone cannot stabilize the surgical wound. Also, the degree of vascularization in the healing tissue may be of major importance. VEGF concentration is a sign of increased angiogenesis, and was increased in both groups where a collagen fleece was used to perform the anastomosis.
In summary, our results show that colorectal anastomoses sealed with a thrombin/fibrinogen-coated collagen fleece are of similar efficacy to conventional suturing, even under conditions of a clinically manifested peritonitis. There were no significant differences in bowel bursting pressures between the anastomosis types, whereas histology and mRNA levels for collagen type I and VEGF may indicate a greater amount of granulation tissue in collagen fleece-sealed anastomoses. Whether this translates into a clinical superiority of additionally sealed anastomoses over sutured anastomoses remains to be verified in further nonclinical and randomized clinical studies.
Acknowledgments
This study was supported by funding from Nycomed GmbH, Germany.
References
- 1 I. Kanellos, D. Kazantzidou, I. Evangelou, K. Galovatsea, T. Zaraboukas, I. Dadoukis; Healing of colonic anastomoses after immediate and delayed administration of 5-fluorouracil plus folinic acid; Eur Surg Res., 30 (1998), pp. 312–317
- 2 A.C. van der Ham, W.J. Kort, I.M. Weijma, H.F. van den Ingh, J. Jeekel; Effect of fibrin sealant on the healing colonic anastomosis in the rat; Br J Surg., 78 (1991), pp. 49–53
- 3 A.C. van der Ham, W.J. Kort, I.M. Weijma, H.F. van den Ingh, H. Jeekel; Effect of antibiotics in fibrin sealant on healing colonic anastomoses in the rat; Br J Surg., 79 (1992), pp. 525–528
- 4 U. Anegg, J. Lindenmann, V. Matzi, J. Smolle, A. Maier, F. Smolle-Juttner; Efficiency of fleece-bound sealing (TachoSil®) of air leaks in lung surgery: a prospective randomised trial; Eur J Cardiothorac Surg., 31 (2007), pp. 198–202
- 5 D. Pantelis, A. Beissel, P. Kahl, S. Wehner, T.O. Vilz, J.C. Kalff; The effect of sealing with a fixed combination of collagen matrix-bound coagulation factors on the healing of colonic anastomoses in experimental high-risk mice models; Langenbecks Arch Surg, 395 (2010), pp. 1039–1048
- 6 S. Siemer, S. Lahme, S. Altziebler, et al.; Efficacy and safety of TachoSil® as haemostatic treatment versus standard suturing in kidney tumour resection: a randomised prospective study; Eur Urol., 52 (2007), pp. 1156–1163
- 7 A. Rickenbacher, S. Breitenstein, M. Lesurtel, A. Frilling; Efficacy of TachoSil® a fibrin-based haemostat in different fields of surgery—a systematic review; Expert Opin Biol Ther., 9 (2009), pp. 897–907
- 8 A. Frilling, G.A. Stavrou, H.J. Mischinger, et al.; Effectiveness of a new carrier-bound fibrin sealant versus argon beamer as haemostatic agent during liver resection: a randomised prospective trial; Langenbecks Arch Surg, 390 (2005), pp. 114–120
- 9 G. Lang, A. Csekeo, G. Stamatis, et al.; Efficacy and safety of topical application of human fibrinogen/thrombin-coated collagen patch (TachoComb) for treatment of air leakage after standard lobectomy; Eur J Cardiothorac Surg., 25 (2004), pp. 160–166
- 10 F. Maisano, H.K. Kjaergard, R. Bauernschmitt, et al.; TachoSil® surgical patch versus conventional haemostatic fleece material for control of bleeding in cardiovascular surgery: a randomised controlled trial; Eur J Cardiothorac Surg., 36 (2009), pp. 708–714
- 11 J. Briceno, A. Naranjo, R. Ciria, et al.; A prospective study of the efficacy of clinical application of a new carrier-bound fibrin sealant after liver resection; Arch Surg., 145 (2010), pp. 482–488
- 12 L. Fischer, C.M. Seiler, C.E. Broelsch, et al.; Hemostatic efficacy of TachoSil® in liver resection compared with argon beam coagulator treatment: an open, randomized, prospective, multicenter, parallel-group trial; Surgery., 149 (2010), pp. 48–55
- 13 G.M. Marta, F. Facciolo, L. Ladegaard, et al.; Efficacy and safety of TachoSil®((R)) versus standard treatment of air leakage after pulmonary lobectomy; Eur J Cardiothorac Surg., 38 (2010), pp. 683–689
- 14 H. Hogstrom, U. Haglund, B. Zederfeldt; Suture technique and early breaking strength of intestinal anastomoses and laparotomy wounds; Acta Chir Scand., 151 (1985), pp. 441–443
- 15 H. Rieger, M. Kruschewski, F. Khalilullah, H.J. Buhr, U. Pohlen; Bursting pressure, vascular integrity and collagen content of sutured and glued colorectal anastomoses in the rat model; E. Faist (Ed.), 7th World Congress on Trauma, Shock, Inflammation and Sepsis (2007), pp. 125–130 Pianoro, Italy
- 16 S.K. Ozel, A. Kazez, N. Akpolat; Does a fibrin–collagen patch support early anastomotic healing in the colon? An experimental study; Tech Coloproctol., 10 (2006), pp. 233–236
- 17 M. Stumpf, K. Junge, R. Rosch, C. Krones, U. Klinge, V. Schumpelick; Suture-free small bowel anastomoses using collagen fleece covered with fibrin glue in pigs; J Invest Surg., 22 (2009), pp. 138–147
- 18 T. Nordentoft, J. Römer, M. Sorensen; Sealing of gastrointestinal anastomoses with a fibrin glue-coated collagen patch: a safety study; J Invest Surg., 20 (2007), pp. 363–369
- 19 G.M. Ahrendt, U.S. Tantry, A. Barbul; Intra-abdominal sepsis impairs colonic reparative collagen synthesis; Am J Surg., 171 (1996), pp. 102–107
- 20 W. Vaneerdeweg, J.M. Hendriks, P.R. Lauwers, M. Leven, E.J. Eyskens; Effect of gentamicin-containing sponges on the healing of colonic anastomoses in a rat model of peritonitis; Eur J Surg., 166 (2000), pp. 959–962
- 21 M.M. Reijnen, B.M. de Man, T. Hendriks, V.A. Postma, J.F. Meis; van GH. Hyaluronic acid-based agents do not affect anastomotic strength in the rat colon, in either the presence or absence of bacterial peritonitis; Br J Surg., 87 (2000), pp. 1222–1228
- 22 N. Ferrara; VEGF as a therapeutic target in cancer; Oncology, 69 (Suppl. 3) (2005), pp. 11–16
- 23 P. Brasken; Healing of experimental colon anastomosis; Eur J Surg Suppl., 566 (1991), pp. 1–51
- 24 M. Stumpf, W. Cao, U. Klinge, B. Klosterhalfen, R. Kasperk, V. Schumpelick; Collagen distribution and expression of matrix metalloproteinases 1 and 13 in patients with anastomotic leakage after large-bowel surgery; Langenbecks Arch Surg., 386 (2002), pp. 502–506
- 25 W. Lorenz, K.P. Reimund, F. Weitzel, et al.; Granulocyte colony-stimulating factor prophylaxis before operation protects against lethal consequences of postoperative peritonitis; Surgery, 116 (1994), pp. 925–934
- 26 A. Bauhofer, A. Torossian, W. Lorenz, et al.; Dependence of positive effects of granulocyte colony-stimulating factor on the antibiotic regimen: evaluation in rats with polymicrobial peritonitis; World J Surg., 28 (2004), pp. 834–844
- 27 C.T. Wittwer, M.G. Herrmann, A.A. Moss, R.P. Rasmussen; Continuous fluorescence monitoring of rapid cycle DNA amplification; Biotechniques, 22 (1997), pp. 130–138
- 28 C.T. Wittwer, K.M. Ririe, R.V. Andrew, D.A. David, R.A. Gundry, U.J. Balis; The LightCycler: a microvolume multisample fluorimeter with rapid temperature control; Biotechniques, 22 (1997), pp. 176–181
- 29 A. Agnifili, M. Schietroma, A. Carloni, et al.; The value of omentoplasty in protecting colorectal anastomosis from leakage. A prospective randomized study in 126 patients; Hepatogastroenterology., 51 (2004), pp. 1694–1697
- 30 M.A. Boccola, J. Lin, W.M. Rozen, Y.H. Ho; Reducing anastomotic leakage in oncologic colorectal surgery: an evidence-based review; Anticancer Res., 30 (2010), pp. 601–607
- 31 M. Kruschewski, H. Rieger, U. Pohlen, H.G. Hotz, H.J. Buhr; Risk factors for clinical anastomotic leakage and postoperative mortality in elective surgery for rectal cancer; Int J Colorectal Dis, 22 (2007), pp. 919–927
- 32 J.R. Tuson, W.G. Everett; A retrospective study of colostomies, leaks and strictures after colorectal anastomosis; Int J Colorectal Dis., 5 (1990), pp. 44–48
- 33 G. Branagan, D. Finnis; Prognosis after anastomotic leakage in colorectal surgery; Dis Colon Rectum., 48 (2005), pp. 1021–1026
- 34 K.G. Walker, S.W. Bell, M.J. Rickard, et al.; Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer; Ann Surg., 240 (2004), pp. 255–259
- 35 A. Vignali, V.W. Fazio, I.C. Lavery, et al.; Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients; J Am Coll Surg., 185 (1997), pp. 105–113
- 36 E. Rullier, C. Laurent, J.L. Garrelon, P. Michel, J. Saric, M. Parneix; Risk factors for anastomotic leakage after resection of rectal cancer; Br J Surg., 85 (1998), pp. 355–358
- 37 N. Regenet, P. Pessaux, S. Hennekinne, et al.; Primary anastomosis after intraoperative colonic lavage vs. Hartmanns procedure in generalized peritonitis complicating diverticular disease of the colon; Int J Colorectal Dis., 18 (2003), pp. 503–507
- 38 B.J. Miller; Colonic injury: does colostomy still have a place?; Injury., 32 (2001), pp. 433–434
- 39 I. de Hingh, B.M. de Man, R.M. Lomme, H. van Goor, T. Hendriks; Colonic anastomotic strength and matrix metalloproteinase activity in an experimental model of bacterial peritonitis; Br J Surg., 90 (2003), pp. 981–988
- 40 W. Vaneerdeweg; Is Hartmann still alive?; Acta Chir Belg., 109 (2009), pp. 167–170
- 41 G.B. Agus, A.V. Bono, E. Mira, et al.; Hemostatic efficacy and safety of TachoComb in surgery. Ready to use and rapid hemostatic agent; Int Surg., 81 (1996), pp. 316–319
- 42 A. Vignali, L. Gianotti, M. Braga, G. Radaelli, L. Malvezzi, V. Di Carlo; Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak; Dis Colon Rectum., 43 (2000), pp. 76–82
- 43 P. Brasken, S. Renvall, M. Sandberg; Fibronectin and collagen gene expression in healing experimental colonic anastomoses; Br J Surg., 78 (1991), pp. 1048–1052
- 44 M. Vaalamo, L. Mattila, N. Johansson, et al.; Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds; J Invest Dermatol., 109 (1997), pp. 96–101
- 45 N.L. Chowcat, F.J. Savage, M.R. Lewin, P.B. Boulos; Direct measurement of collagenase in colonic anastomosis; Br J Surg., 77 (1990), pp. 1284–1287
- 46 F.J. Savage, D.L. Lacombe, P.B. Boulos, R.M. Hembry; Role of matrix metalloproteinases in healing of colonic anastomosis; Dis Colon Rectum., 40 (1997), pp. 962–970
- 47 F.L. Hesp, T. Hendriks, E.J. Lubbers, H.H. deBoer; Wound healing in the intestinal wall. A comparison between experimental ileal and colonic anastomoses; Dis Colon Rectum., 27 (1984), pp. 99–104
- 48 A.C. van der Ham, W.J. Kort, I.M. Weijma, H.F. van der Ingh, H. Jeekel; Healing of ischemic colonic anastomosis: fibrin sealant does not improve wound healing; Dis Colon Rectum., 35 (1992), pp. 884–891
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?