Summary
Background/Objective
Hepatocyte transplantation is a promising alternative to liver transplantation in children with liver metabolic disorders and acute liver failure. Currently, it is difficult to assess rapidly hepatocyte function before transplantation. The aim of this study was to investigate whether the uptake and release of indocyanine green (ICG) by hepatocytes could be used.
Methods
Human hepatocytes (106 cells) isolated from unused donor livers were incubated at 37°C for 30 minutes with ICG (0–2 mg/mL) in both cell suspension and on collagen-coated culture plates. Cells were then incubated in medium without ICG for 3 hours with supernatants collected at 1, 2 and 3 hours for measurement of ICG release. Cell viability was determined by trypan blue exclusion, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (mitochondrial dehydrogenase activity) and sulforhodamine B (SRB) assay (cell attachment). HepG2 cells were also used.
Results
ICG was taken up and secreted by hepatocytes with the release reaching a plateau level soon after 1 hour. Concentrations of ICG > 1.0 mg/mL had toxic effects on hepatocytes. Hepatocytes incubated with 1.0 mg/mL ICG had higher mitochondrial dehydrogenase activity compared to 0.5 mg/mL ICG or control cells (0.025 ± 0.0004 OD unit vs. 0.019 ± 0.0008 OD unit or 0.020 ± 0.002 OD unit, p < 0.05). Incubation of HepG2 cells with ICG reduced albumin production (98.9 ± 0.02 ng/mL, 66.6 ± 0.05 ng/mL and 39.1 ± 0.4 ng/mL for control cells, and 0.5 mg/mL and 1.0 mg/mL ICG, respectively), and decreased [3H]-thymidine incorporation in a dose-dependent manner. Addition of taurine (20 mM) to plated hepatocytes gave greater release of ICG and hepatocyte attachment compared to controls, at all ICG concentrations (SRB 1.360 ± 0.083 optical density units vs. 0.908 ± 0.159 optical density units, p = 0.011 at 1.0 mg/mL).
Conclusion
With further refinement, ICG could be used to develop a rapid assay for assessment of the function of isolated human hepatocytes.
keywords
cell transplantation;functional assessment;human hepatocyte transplantation;indocyanine green;taurine
1. Introduction
Hepatocyte transplantation was first introduced into clinical practice in 19921 as a promising alternative to liver transplantation or as a bridging therapy for patients with metabolic diseases and acute liver failure.2 ; 3 Hepatocyte transplantation is a safe and less-invasive procedure for patients with liver disease than whole organ transplantation. Animal studies have clearly proven the efficacy of hepatocyte transplantation, however, this has not translated into clinical practice where there is often limited benefit.4 One of the major reasons for this is the quality of the hepatocytes that have been infused, which are often isolated from livers that have been rejected for transplantation. Currently, trypan blue exclusion is used as a rapid test of cell viability, which determines cell integrity by staining the nuclei of dead cells. This test does not reflect the metabolic function of the hepatocytes, which is important in vivo. Measures of specific synthetic function such as albumin and clotting factor 7 synthesis require cell culture, and are not applicable to determine suitability of hepatocytes for immediate infusion into a patient.
Current commercial assays use technologies that are either nonspecific to hepatocytes (ATP detection; redox activity, membrane integrity) or not available for routine clinical use (P450 assays by HPLC, mass spectrometry). Indocyanine green (ICG) is an organic anion used in hepatobiliary surgery to assess liver reserve before resection, and is specifically eliminated by the liver.5 ICG uptake by hepatocytes assessed by microscopy has been recently used to assess the in vitro function of stem-cell-derived hepatocytes. 6 ; 7 At the cellular level, ICG is taken up by hepatocytes via the transporter organic anion transport protein (OATP)1B1 [OATP2 (rats)/OATP-C (humans)], which is exclusively expressed in the basolateral membrane of hepatocytes, 8; 9 ; 10 as used by bilirubin,11 and then excreted into the bile canaliculi by multidrug resistance protein 2, which requires ATP. 12 ; 13 ICG uptake can also reflect the degree of hepatic triglyceride content in a dose–response relationship.14
In this study we investigated whether quantitative measurement of the uptake and release of ICG by human hepatocytes has potential to develop a rapid test of metabolic function before hepatocyte transplantation.
2. Materials and methods
2.1. Isolation and culture of human hepatocytes
Hepatocytes were isolated from unused donor liver tissue using a modified collagenase perfusion technique.15 Donor data and liver characteristics are shown in Table 1. Viability was assessed by 0.4% trypan blue exclusion test. Cell suspensions contained 106 cells. Collagen-coated culture plates were seeded with fresh or defrosted cells, which were then incubated in Williams’ medium E, supplemented as previously described.16 HepG2 cells (human hepatocellular carcinoma cell line) were cultured in RPMI1640/10% fetal calf serum overnight before use. Cell suspensions were used for experiments with ICG immediately. Cells were cultured overnight to determine the effects of ICG treatment on hepatocyte functions.
| Liver | Age (yr) | Sex | Cause of death | Status at retrieval | Cold ischemia (h) | Warm ischemia (min) | Viability (%) |
|---|---|---|---|---|---|---|---|
| 1 | 31 | F | ICH | Cadaveric | 10 | — | 70.0 |
| 2 | 57 | M | ICH | NHBD fatty liver | 12 | 15 | 79.8 |
| 3 | 52 | M | ICH | NHBD | 13 | 20 | 52.1 |
| 4 | 26 | M | Cardiac arrest | NHBD | 12.5 | 22 | 76.0 |
| 5 | 76 | M | ICH | Cadaveric | 10 | — | 78.6 |
| 6 | 60 | F | Heart attack | NHBD | 12 | 19 | 39.7 |
| 7 | 28 | M | Cardiac arrest | Cadaveric | 11.5 | — | 67.0 |
F = female; ICH = intracranial hemorrhage; M = male; NHBD = non-heart-beating donor.
2.2. ICG treatment
ICG dry powder 5 mg (Cardiogreen; Sigma–Aldrich, Gillingham, Dorset, UK) was dissolved in 1 mL solvent [100 μL dimethyl sulfoxide (DMSO), CryoSure-DMSO; WAK-Chemie Medical GmbH, Steinbach, Germany] and culture medium was added to obtain a stock of 5 mg/mL. The solution was shaken for 2 minutes to ensure that the powder was completely dissolved. The experimental solutions used were different concentrations of ICG (0.25 mg/mL, 0.5 mg/mL, 1.0 mg/mL and 2.0 mg/mL) and in further experiments at 0.5 mg/mL and 1.0 mg/mL. Cells were incubated with ICG as a suspension or plated for 30 minutes at 37°C in 95% O2/5% CO2. Cells were washed with phosphate-buffered saline (PBS) and centrifuged to obtain the pellets if in suspension and then reincubated in medium alone to determine ICG release. Supernatants were collected after 1 hour, 2 hours and 3 hours for measurement of ICG concentration against a standard curve using a DYNEX Technologies MRX microplate reader, (supplied by Prior Laboratory Supplies Ltd., Forest Row, East Sussex, UK), at optical density 820 nm (OD820). Plates were reincubated overnight, and cell function analyzed using the following assays.
2.3. Cell attachment: sulforhodamine (SRB) assay
To determine cell numbers on culture plates, the SRB assay was performed as described previously.17 In each well of the 96-well plate, 50 μL ice-cold 50% trichloroacetic acid solution was gently layered on top of the medium overlaying the cells. The plates were then incubated for 1 hour at 4°C. Wells were rinsed five times with water and the cells stained with 0.4% SRB solution (100 μL stain/well) for 15 minutes at room temperature. SRB staining solution was poured off, wells were rinsed five times with 1% acetic acid to remove unbound dye, and left to air dry. The bound SRB dye was then solubilized by adding unbuffered Tris-base solution (200 μL/well), and plates were placed on a plate shaker for 1 hour at room temperature. Plates were then read at OD564, using a microplate reader.
2.4. Mitochondrial activity: modified 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay
The assay was as described previously.18 Briefly, 20 μL MTT solution was added to 200 μL medium in each well of the 96-well plate, and the plate incubated at 37°C for 4 hours. The medium was then removed by aspiration, 120 μL isopropanol/HCl added per well, the plate shaken for 15 minutes, and absorbance at OD630 measured.
2.5. [3H]-thymidine incorporation into HepG2 cells
The effect of the ICG (0 mg/mL, 0.5 mg/mL and 1 mg/mL) on DNA synthesis of HepG2 cells was assessed using [3H]-thymidine incorporation assay. The medium in each well was replaced with an equal volume (200 μL) of fresh medium containing [3H]-thymidine (0.5 μCi/well, Amersham International, Amersham, Bucks, UK). The plates were incubated overnight. The cells were then harvested onto glass fiber membranes using a cell harvester (FilterMate; Packard Instruments, Pangbourne, Berks, UK). The filters were dried and the radioactivity counted (MATRIX 9600 Plate Counter; Packard Instruments) to determine the incorporation of radioactivity into the cells.
2.6. Albumin synthesis
Concentration of albumin in culture media was determined by enzyme-linked immunosorbent assay (ELISA) kit (Bethyl Laboratories, Montgomery, TX, USA) using a sheep antihuman albumin antibody. The assay was done according to the manufacturer’s instructions. The absorbance was read at 450 nm.
2.7. Cell viability: staining with fluorescein diacetate (FDA)/ethidium bromide (EtBr)
A stock solution of FDA (Sigma–Aldrich) was prepared by dissolving 5 mg/mL in DMSO. The FDA working solution was freshly prepared by adding 0.01 mL stock to 5 mL EtBr (Sigma–Aldrich) stock solution prepared by dissolving 10 mg/mL in PBS. Cells in supernatants were collected by washing with 0.5 mL PBS at room temperature and centrifugation for 50 × g at 4°C for 4 minutes. Cells were resuspended with 0.2 mL FDA/EtBr solution and incubated for 6 minutes at room temperature. Cells were then collected again by removing the staining solution, washing twice with PBS, and centrifugation. Stained cells were resuspended with two drops of antifading reagent and placed onto a microscope slide with a coverslip. The cells were observed under a fluorescent microscope (filter set 09 ZEISS, excitation = 450–490 nm, emission = 520 nm), whereas nuclei were stained red (excitation = 506 nm, emission = 610 nm) at 100–400× magnification.
2.8. Taurine treatment of hepatocytes
Fresh human hepatocytes were cultured overnight with taurine (Sigma–Aldrich) at 20 mM, and cells were incubated with ICG and tested as above.
2.9. Statistical analysis
Statistical analysis of the results was carried out using Student’s t test and Pearson correlation test. A value of p < 0.05 was considered significant.
3. Results
3.1. ICG uptake and release by hepatocytes and HepG2 cells
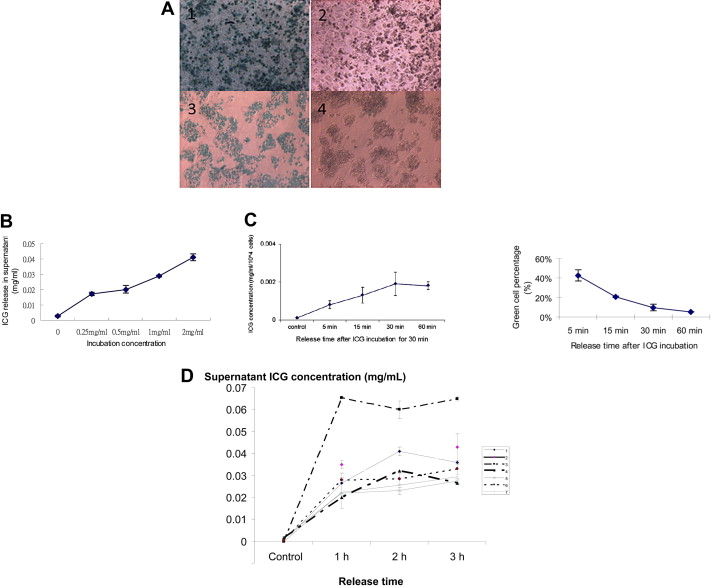
Human hepatocytes and HepG2 cells took up ICG with cells turning green after incubation for 30 minutes. After 3 hours, the ICG was released by the cells (Fig. 1A). The release of ICG by human hepatocytes (Fig. 1B) and HepG2 cells (Fig. 1C) was related to the initial ICG concentration during uptake from both the loss of green color of the cells and appearance of ICG detected microscopically in the culture medium. There was a significant correlation between the ICG release and the viability of human hepatocytes measured by trypan blue uptake (r = 0.85, p = 0.008). The pattern of ICG release in human hepatocytes showed a rapid release, reaching a plateau level soon after 1 hour (Fig. 1D). This effect was seen with cells both in suspension and in culture, but tended to be more rapid in culture.
|
|
|
Figure 1. Characteristics of ICG uptake and release by human hepatocytes and HepG2 cells in vitro. (A) Uptake and release of ICG: after ICG incubation (A1 and A3) and 3 hours release of ICG (A2 and A4). A1 and A2: human hepatocytes; A3 and A4: HepG2 cells. (B) ICG release into the supernatant by human hepatocytes increased with ICG concentration used for incubation. (C) HepG2 cells in plates release ICG with time as shown as decrease in the percentage of green cells. (D) Release patterns of ICG by human hepatocytes from different donors. ICG = indocyanine green. |
3.2. Effect of incubation with ICG on hepatocyte function
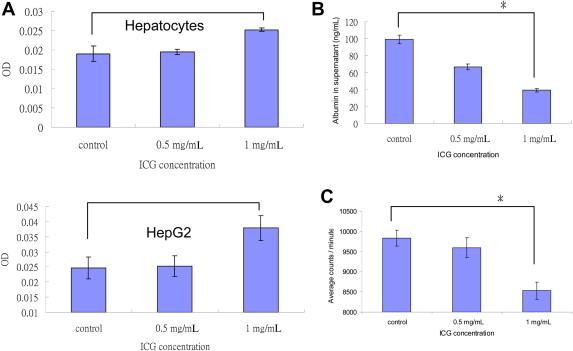
The effects of increasing concentrations of ICG on hepatocyte function were determined. With the MTT assay, hepatocytes incubated with 1.0 mg/mL ICG had higher mitochondrial dehydrogenase activity compared to 0.5 mg/mL ICG or controls (0.025 ± 0.0004 OD unit vs. 0.019 ± 0.0008 or 0.020 ± 0.002, p < 0.05 for hepatocytes; 0.038 ± 0.004 vs. 0.025 ± 0.003 or 0.025 ± 0.004, p < 0.05 for HepG2 cells) (Fig. 2A).
|
|
|
Figure 2. Functional disturbances of human hepatocytes and HepG2 cells following ICG treatment. (*p < 0.05). (A) MTT assay showed increased mitochondrial function in hepatocytes (top) and HepG2 cells (bottom) incubated with 1.0 mg/mL ICG. (B) Albumin synthesized by HepG2 cells decreased in a dose-response relationship as increased ICG incubation concentration (n = 2). (C) Incubation with 1.0 mg/mL ICG decreased HepG2 cell proliferative activity (n = 2). ICG = indocyanine green; MTT = (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
Incubation of HepG2 cells with ICG reduced albumin production (98.9 ± 0.02 ng/mL, 66.6 ± 0.05 ng/mL and 39.1 ± 0.4 ng/mL for controls and 0.5 mg/mL and 1.0 mg/mL ICG, respectively) and decreased [3H]-thymidine incorporation in a dose-dependent manner (Fig. 2B and 2C).
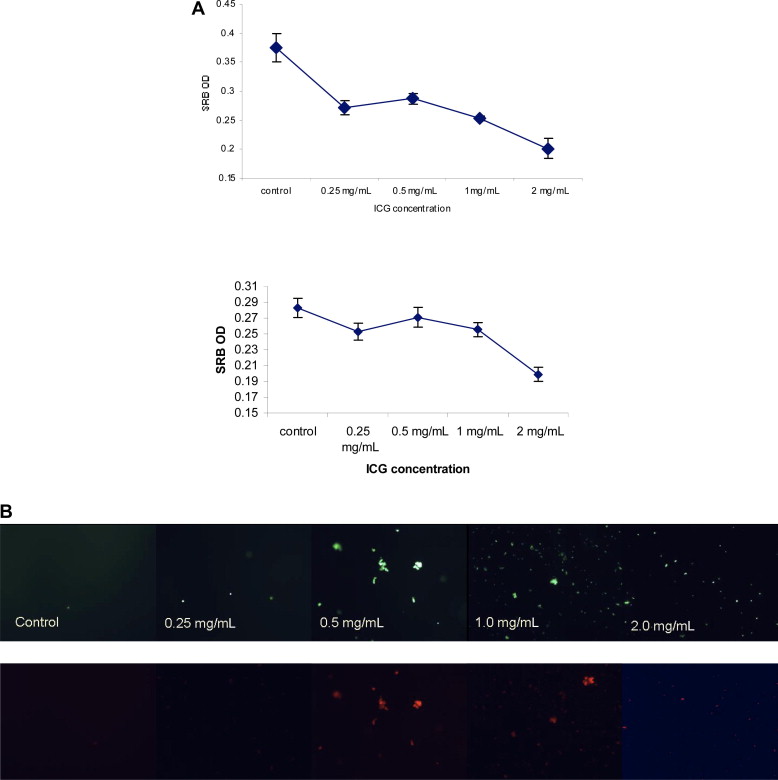
Cells had lower attachment when tested 6 hours after incubation with increasing concentrations of ICG (Fig. 3A). However, if the plates were reincubated overnight, the cells reattached. To investigate these further, cells in the supernatants were collected after incubation with ICG (0–2 mg/mL) and stained with FDA/EtBr. Greater numbers of viable cells were detached at higher ICG concentrations (0.5 mg/mL and 1 mg/mL) than at 0.25 mg/mL ICG and in the controls (Fig. 3B).
|
|
|
Figure 3. Human hepatocytes and HepG2 cells detach when releasing ICG. (A) Detachment of human hepatocytes (top) and HepG2 cells (bottom) on incubation with increasing ICG concentration. (B) Cell viability staining with fluorescein diacetate/ethidium bromide showed that more viable cells detached when incubated with higher concentrations of ICG. ICG = indocyanine green. |
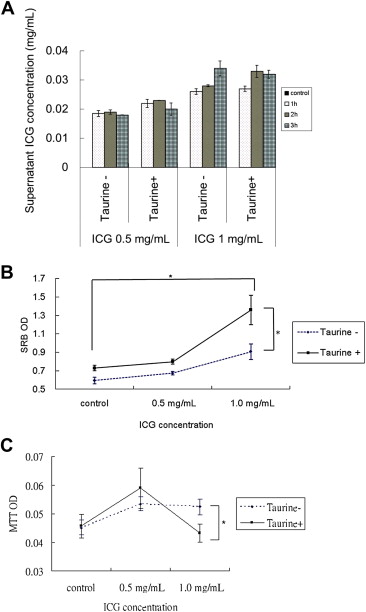
3.3. Effect of taurine on hepatocyte transport of ICG
Pretreatment of fresh human hepatocytes with taurine in culture overnight gave greater amounts of ICG release and the pattern of ICG release was maintained with high ICG concentrations (Fig. 4A). Compared with controls, taurine also resulted in a higher degree of cell attachment, and enhanced reattachment overnight following ICG incubation (Fig. 4B). Pretreatment with taurine prevented the stimulatory effect on the MTT assay at 1.0 mg/mL ICG, but not at 0.5 mg/mL (Fig. 4C).
|
|
|
Figure 4. Effect of pretreatment with taurine on human hepatocytes (*p < 0.05). (A) Human hepatocytes released more ICG when pretreated with taurine. When incubated with 1.0 mg/mL ICG, the pattern of ICG release was maintained in taurine-pretreated hepatocytes. (B) Pretreatment with taurine increased hepatocyte attachment. Following incubation with ICG for 30 minutes, plates were reincubated overnight to study cell attachment. Cells pretreated with taurine were attached better with 1.0 mg/mL ICG. (C) Taurine-pretreated hepatocytes had lower MTT activity on incubation with 1.0 mg/mL than with 0.5 mg/mL ICG. ICG = indocyanine green; MTT = (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
4. Discussion
The results of these experiments show that there is a distinct pattern of uptake and release of ICG by human hepatocytes that can be quantitated using readily available laboratory equipment. Hepatocytes take up ICG in 30 minutes and then excrete the unchanged dye in 1–2 hours. The specific uptake of ICG by intact hepatocytes is followed by excretion via the ATP-dependant multidrug resistance protein 2 transporter, which is the rate-limiting step,12 thus being a dual measure of cell metabolic function. Cells with impaired function will have reduced amounts of ATP which will limit ICG excretion. There was a characteristic ICG release curve for hepatocytes that showed a peak in the second hour of incubation. In this preliminary study, only a relatively small number of cell batches were used. Further experiments are needed to define the ICG pattern for cells isolated from fatty livers, which are likely to have impaired function19 and those from non-heart-beating donors exposed to warm ischemia.20
There was a correlation of ICG excretion with cell viability by trypan blue exclusion, suggesting that trypan blue exclusion is related to cell function in the range of cell viabilities studied. However, ICG is specific to hepatocytes and should reveal more about cellular function than just membrane integrity. Ideally, any measure of hepatocyte function should be correlated to engraftment and function of hepatocytes after transplantation.
HepG2 cells, although they are transformed cells, had a similar release pattern of ICG to normal hepatocytes. They are thus a useful tool for developing the conditions for an ICG test, having high viability as “best quality” hepatocytes are not often available. However, HepG2 cells may not predict metabolism in adult human liver cells, because their expression of drug-metabolizing enzymes is different.21 Incubation of human hepatocytes and HepG2 cells at concentrations > 0.5 mg/mL had effects on cell metabolism including stimulation of mitochondrial dehydrogenase activity and inhibition of albumin synthesis and proliferation of HepG2 cells. Other studies in retinal cells have shown high concentrations of ICG to be toxic with a proapoptotic effect.22 The concentration used to test hepatocytes in vitro should be 0.5 mg/mL to avoid these effects.
In the cell attachment experiments using the SRB assay, we found that cells incubated with ICG had lower attachment during ICG release but recovered to give better attachment the next day. It is interesting to speculate whether this is in someway related to location of the ICG uptake OATP transporter on the basolateral membrane, which attaches to the collagen coating on the culture plates.23 Whereas, the excretion of ICG is via the transporter in the canalicular membrane of the hepatocyte.
Taurine has been shown to be involved in membrane stabilization19 and can act as an antioxidant,24 and it was used to modulate cell function to see the effects on ICG transport. Hepatocytes pretreated with taurine appeared to better tolerate better higher concentrations (1.0 mg/mL) of ICG with increased cell attachment. Thus ICG disposition could detect a protective effect on cell function.
In a recent study, Donato et al25 rapidly assessed the cellular P450 enzyme function of human hepatocytes within 1 hour by HPLC–tandem mass spectrometry. However, these assays require sophisticated equipment and are not available in everyday clinical use. They also determined urea synthesis, which is a good specific marker of hepatocyte metabolic function, although this did not correlate with cell viability. It is likely that a panel of rapid assays will give the most useful data.
In conclusion, in vitro hepatocyte function can be assessed by the ICG release pattern within 2 hours. Further refinement of this assay, particularly in reducing the time taken, should lead to a test of hepatocyte function to help assess the quality of isolated human hepatocytes for transplantation.
Acknowledgments
Dr. Chen-Maw Ho supported by the National Taiwan University Hospital under grant MG259.
References
- 1 M. Mito, M. Kusano, Y. Kawaura; Hepatocyte transplantation in man; Transplant Proc, 24 (1992), pp. 3052–3053
- 2 J. Puppi, A. Dhawan; Human hepatocyte transplantation overview; Methods Mol Biol, 481 (2009), pp. 1–16
- 3 A. Nussler, S. Konig, M. Ott, et al.; Present status and perspectives of cell-based therapies for liver diseases; J Hepatol, 45 (2006), pp. 144–159
- 4 D. Haridass, N. Narain, M. Ott; Hepatocyte transplantation: waiting for stem cells; Curr Opin Organ Transplant, 13 (2008), pp. 627–632
- 5 G.R. Cherrick, S.W. Stein, C.M. Leevy, C.S. Davidson; Indocyanine green: observations of its physical properties, plasma decay, and hepatic extraction; J Clin Invest, 39 (1960), pp. 592–600
- 6 T. Yamada, M. Yoshikawa, S. Kanda, et al.; In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green; Stem Cells, 20 (2002), pp. 146–154
- 7 S. Agarwal, K.L. Holton, R. Lanza; Efficient differentiation of functional hepatocytes from human embryonic stem cells; Stem Cells, 26 (2008), pp. 1117–1127
- 8 K. Ito, H. Suzuki, T. Horie, Y. Sugiyama; Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport; Pharm Res, 22 (2005), pp. 1559–1577
- 9 S.D. Campbell, S.M. de Morais, J.J. Xu; Inhibition of human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia; Chem Biol Interact, 150 (2004), pp. 179–187
- 10 J. König, A. Seithel, U. Gradhand, M.F. Fromm; Pharmacogenomics of human OATP transporters; Naunyn-Schmiedebergs Arch Pharmacol, 372 (2006), pp. 432–443
- 11 B.F. Scharschmidt, J.G. Waggoner, P.D. Berk; Hepatic organic anion uptake in the rat; J Clin Invest, 56 (1978), pp. 1280–1292
- 12 L. Huang, M. Vore; Multidrug resistance P-glycoprotein 2 is essential for the biliary excretion of indocyanine green; Drug Metab Dispos, 29 (2001), pp. 634–637
- 13 F.R. Simon, M. Iwahashi, L.J. Hu, et al.; Hormonal regulation of hepatic multidrug resistance-associated protein 2 (Abcc2) primarily involves the pattern of growth hormone secretion; Am J Physiol Gastrointest Liver Physiol, 290 (2006), pp. G595–G608
- 14 K. Takahashi, K. Hakamada, E. Totsuka, Y. Umehara, M. Sasaki; Warm ischemia and reperfusion injury in diet-induced canine fatty livers; Transplantation, 69 (2000), pp. 2028–2034
- 15 R.R. Mitry, R.D. Hughes, M.M. Aw, et al.; Human hepatocyte isolation and relationship of cell viability to early graft function; Cell Transplant, 12 (2003), pp. 69–74
- 16 R.R. Mitry, A. Dhawan, R.D. Hughes, et al.; One liver, three recipients: segment IV from split-liver procedures as a source of hepatocytes for cell transplantation; Transplantation, 77 (2004), pp. 1614–1616
- 17 R.R. Mitry, C.E. Sarraf, R. Havlik, N.A. Habib; Detection of adenovirus and initiation of apoptosis in hepatocellular carcinoma cells after Ad-p53 treatment; Hepatology, 31 (2000), pp. 885–889
- 18 R.R. Mitry, R.D. Hughes, S. Bansal, S.C. Lehec, J.A. Wendon, A. Dhawan; Effects of serum from patients with acute liver failure due to paracetamol overdose on human hepatocytes in vitro; Transplant Proc, 37 (2005), pp. 2391–2394
- 19 P. Green, R. Dawson Jr., D.R. Wallace, J. Owens; Treatment of rat brain membranes with taurine increases radioligand binding; Adv Exp Med Biol, 442 (1998), pp. 377–383
- 20 S. Soric, M.P. Belanger, N. Askin, C. Wittnich; Impact of female sex hormones on liver tissue lactic acidosis during ischemia; Transplantation, 84 (2007), pp. 763–770
- 21 S. Wilkening, F. Stahl, A. Bader; Comparison of primary human hepatocytes and hepatoma cell line HepG2 with regard to their biotransformation properties; Drug Metab Dispos, 31 (2003), pp. 1035–1042
- 22 S. Kawahara, Y. Hata, M. Miura, et al.; Intracellular events in retinal glial cells exposed to ICG and BBG; Invest Ophthalmol Vis Sci, 48 (2007), pp. 4426–4432
- 23 A.I. Musat, C.A. Sattler, G.L. Sattler, H.C. Pitot; Reestablishment of cell polarity of rat hepatocytes in primary culture; Hepatology, 18 (1993), pp. 198–205
- 24 J. Das, J. Ghosh, P. Manna, P.C. Sil; Taurine provides antioxidant defense against NaF-induced cytotoxicity in murine hepatocytes; Pathophysiology, 15 (2008), pp. 181–190
- 25 M.T. Donato, A. Lahoz, S. Montero, et al.; Functional assessment of the quality of human hepatocyte preparations for cell transplantation; Cell Transplant, 17 (2008), pp. 1211–1219
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?