Summary
Background/Objective
Recently, endoscopic and radiological procedures for various symptoms related to cirrhosis have improved. Thus, the role of Hassabs operation (gastroesophageal decongestion and splenectomy) has changed for cirrhotic patients.
Methods
Hassabs operation was performed on patients who had gastroesophageal varices that were difficult to control with balloon occluded retrograde transvenous obliteration or an endoscopic procedure, or had hypersplenism. Thirteen consecutive patients underwent this operation, and the outcomes of all patients were reviewed retrospectively.
Results
There was no operative morbidity or rebleeding varices. In the preoperative endoscopic injection sclerotherapy treated group (n = 6), only one patient (16.7%) developed recurrent varices. Mean platelet counts were significantly higher 6 months after surgery (201 ± 65 × 103/mm3) than preoperatively (64 ± 54 × 103/mm3). In patients with hepatocellular carcinoma, percutaneous therapies, such as radio frequency ablation, were safely performed with adequate therapeutic effect. Interferon therapy was given to patients with hepatitis C virus (HCV)-related cirrhosis without interruption.
Conclusion
Hassabs operation is a satisfactory approach to controlling varices, especially when combined with preoperative endoscopic treatment. Platelet counts were significantly higher after surgery. This therapy was important for cirrhotic patients contraindicated for liver transplantation in that they could continue their therapy for hepatocellular carcinoma (HCC) and HCV as needed.
Keywords
gastroesophageal varices;liver cirrhosis;splenectomy
1. Introduction
Bleeding of gastroesophageal varices is the most hazardous complication of portal hypertension, with a high mortality rate.1 Endoscopic procedures such as endoscopic injection sclerotherapy (EIS) and endoscopic variceal band ligation (EVL) are reasonably effective to control varices.2 Also, radiologic procedures such as transjugular intrahepatic portsystemic shunt (TIPS) and balloon occluded retrograde transvenous obliteration (B-RTO) have satisfactory results in controlling these varices.3 ; 4
Similarly, hypersplenism prevents safe application of the treatments for hepatitis C and hepatocellular carcinoma (HCC) because of thrombocytopenia. Recently, the usefulness of partial splenic embolization (PSE) for hypersplenism was reported.5 The usefulness of these procedures has changed the therapeutic modality for gastroesophageal varices and/or hypersplenism from surgical to endoscopic or radiological. Thus, Hassabs operation, which involves devascularization of perigastroesophageal vessels and splenectomy, has a new role as a procedure for cirrhotic patients. The surgical procedure is very important for cirrhotic patients as it treats several pathological processes caused by liver cirrhosis. In this study, the usefulness of Hassabs operation for cirrhotic patients was examined retrospectively.
2. Patients and methods
Our indications for Hassabs operation include gastroesophageal varices that are difficult to control with radiological and/or endoscopic procedures because of repeated recurrence, or thrombocytopenia (platelets count under 80 × 103/mm3) with gastroesophageal varices due to hypersplenism related to cirrhosis. Patients with uncontrollable bleeding from gastroesophageal varices were contraindicated. We used the surgical procedure of Hassabs operation that has been described previously without any modifications.6 Thirteen consecutive patients (male:female = 10:3) with a mean age of 52.7 (range, 38–66) years underwent Hassabs operation during the period from January 2004 to December 2008. The etiologies of liver cirrhosis were hepatitis C virus (HCV) in eight patients, hepatitis B virus (HBV) in two patients, idiopathic portal hypertension in two patients, and alcoholic cirrhosis in one patient. Preoperative therapies for gastroesophageal varices were EIS combined with EVL in three patients, EIS only in three patients, EVL only in two patients, and not treated by an endoscopic procedure preoperatively in five patients (Table 1). There were no perioperative hospital deaths during this period. Endoscopic findings were based on the general rules established by the Japanese Research Society for Portal Hypertension.7 According to the rules, the esophageal varices were classified into three grades: F1 (small, straight); F2 (moderate sized, tortuous); and F3 (large sized, nodular). The red color signs were defined into four grades: RC0, RC1 (limited), RC2, and RC3 (circular, many). Preoperative endoscopic examination and classification according to this rule was performed in all cases. Postoperative follow-up of gastroesophageal varices was performed by annual endoscopic examination. Our criteria for postoperative recurrent varices are greater than F2- or RC-positive cases. Written informed consent was obtained from all participating patients. In the statistical analysis, values are expressed as means ± standard deviation (SD). The significance of the differences obtained was tested using the Mann–Whitney U-test. Differences were considered statistically significant at a level of p < 0.05.
| Case | Sex | Age | Preoperative Tx. for varices | Preoperative | Postoperative | Additional treatment | Operative morbidity | ||
|---|---|---|---|---|---|---|---|---|---|
| EVa | GVa | EVa | GVa | ||||||
| 1 | M | 41 | EVL & EIS | F1 RC2 | (–) | F2 RC0 | (–) | (–) | PVT |
| 2 | F | 57 | None | F2 RC2 | F3 RC1 | n.a. | n.a. | (–) | (–) |
| 3 | M | 52 | None | F2 RC2 | F1 RC0 | F1 RC1 | F1 RC0 | (–) | PVT, Ileus |
| 4 | M | 57 | None | F2 RC1 | (–) | F2 RC1 | (–) | EVL | PVT |
| 5 | M | 49 | EVL | F3 RC2 | F3 RC0 | F2 RC1 | F1 RC0 | (–) | (–) |
| 6 | M | 66 | None | F3 RC2 | F2 RC1 | F2 RC0 | (–) | (–) | (–) |
| 7 | M | 38 | EIS | F2 RC0 | (–) | F1 RC0 | (–) | (–) | (–) |
| 8 | M | 45 | EVL | F2 RC2 | (–) | n.a. | n.a. | (–) | (–) |
| 9 | F | 62 | EIS | F3 RC3 | F3 RC0 | F1 RC0 | (–) | (–) | PVT |
| 10 | M | 60 | EIS | F1 RC0 | F4 RC0 | F1 RC0 | F1 RC0 | (–) | (–) |
| 11 | F | 46 | None | F2 RC2 | F2 RC0 | F1 RC1 | F1 RC0 | (–) | PVT |
| 12 | M | 62 | EVL & EIS | F1 RC1 | F1 RC0 | n.a. | n.a. | (–) | PVT |
| 13 | M | 50 | EVL & EIS | F1 RC2 | (–) | F1 RC1 | (–) | (–) | (–) |
EIS = endoscopic injection sclerotherapy; EV = esophageal varices; EVL = endoscopic variceal band ligation; GV = gastric varices; n.a. = not applicable; PVT = portal vein thrombosis; Tx = therapy.
a. Varices according to: The general rules for recording endoscopic findings of the Japanese Research Society for Portal Hypertension (2004).
3. Results
3.1. Change in hepatic reserve
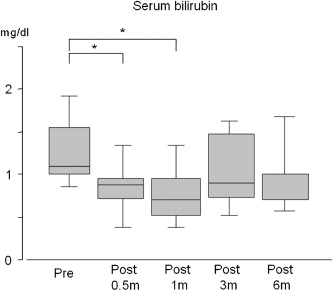
According to the Child–Pugh classification of functional status for liver disease, nine patients were class A and four patients were class B, preoperatively.8 Six months postoperatively, the patients' Child–Pugh classifications were unchanged. The total bilirubin level was 1.3 ± 0.4 mg/dL, and the serum albumin level was 3.5 ± 0.5 g/dL, preoperatively. Six months after the operation, the total bilirubin level was 1.0 ± 0.5 mg/dL, and the serum albumin level was 3.7 ± 0.5 g/dL (Figs. 1 and 2).
|
|
|
Figure 1. Changes in serum bilirubin after Hassabs operation. The serum bilirubin level was significantly lower than the preoperative serum bilirubin for 2 weeks to 1 month after surgery *(p < 0.05). |
|
|
|
Figure 2. Changes in serum albumin after Hassabs operation. The serum albumin level was significantly lower than the preoperative serum albumin for 2 weeks after surgery *(p < 0.05). |
3.2. Operative time and blood loss
The time required for operation was 361 ± 78 minutes. The blood loss was 985 ± 776 mL. The weight of the spleen was 665 ± 409 g.
3.3. Therapeutic effect for varices
The mean duration of follow-up was 37.9 months. Of the 13 patients, three patients rejected postoperative endoscopic follow-up. The variceal rebleeding rate was 0%. Only one patient received additional endoscopic treatment (EVL) for varices during the postoperative period. Six patients developed recurrent varices after surgery. Of the six patients who were treated with EIS preoperatively, only one developed recurrent varices.
3.4. Change in platelet counts
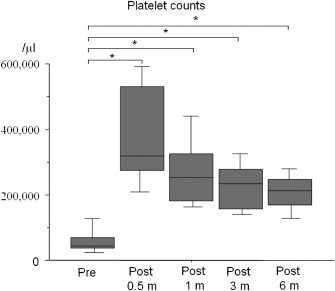
The postoperative platelet counts were significantly higher than the preoperative platelets counts for 2 weeks to 6 months after surgery. The preoperative platelet count was 64 ± 54 × 103/mm3. Six months after surgery, the platelet count was 201 ± 65 × 103/mm3 (Fig. 3).
|
|
|
Figure 3. Changes in platelets counts after Hassabs operation. The platelet counts were significantly higher than the preoperative platelets counts for 2 weeks to 6 months after surgery *(p < 0.05). |
3.5. Operative morbidity and postoperative hospital stay
There were no episodes of decompensated liver dysfunction, such as increased ascites or encephalopathy. Asymptomatic portal vein thrombosis arising from the stump of the splenic vein, which was graded as Grade 1 according to the classification of Yerdel et al, occurred in six patients, and ileus occurred in one patient.9 These complications were all successfully treated with medication. Postoperative hospital stay was 30.2 ± 8.3 days.
3.6. Postoperative therapy for HCC
In three patients, HCC was found at operation. All lesions were successfully treated with radiofrequency ablation (RFA) or percutaneous ethanol injection therapy without any complications.
3.7. Postoperative therapy for HCV
In four of eight HCV-positive patients, antiviral therapy was performed using peginterferon plus ribavirin. No patients were withdrawn from therapy due to thrombocytopenia and leukocytopenia.
4. Discussion
The development of endoscopic therapy affects the management of gastroesophageal varices, and many cases have been treated with EIS and/or EVL.2 Interventional radiology (IVR) for these patients has also improved. Thus, symptoms such as gastric varices were treated by IVR, for example, B-RTO and TIPS.3; 4; 5 ; 10 Therefore, the role of Hassabs operation has recently changed from devascularization itself to controlling various clinical settings due to liver cirrhosis. In fact, some cases are resistant to or recurrent after endoscopic or interventional therapy.4 ; 11 Furthermore, it is reasonable to control gastroesophageal varices using a combination of endoscopic, interventional, and surgical procedures. Thus, the indications for Hassabs surgery at our institute have changed to include patients who were contraindicated for liver transplantation due to social and/or medical reasons with gastroesophageal varices that are difficult to control with IVR or an endoscopic procedure, and those who have thrombocytopenia owing to hypersplenism complicated by gastroesophageal varices.
In the present series, no patient had postoperative variceal bleeding, but the variceal recurrence rate was high (46.2%). However, in the subgroup of patients treated preoperatively with EIS, only one patient (16.7%) developed recurrent varices. These data support the use of combination therapy involving Hassabs operation and EIS.12 Unlike the Sugiura–Futagawa procedure, Hassabs operation devascularizes only the extramural vessels; intramural vessels are not treated.13 Thus, EIS is needed for embolization of the intramural vessels and venous network. This combination therapy was reasonable and effective. The concept of the simultaneous combination of Hassabs and EIS therapy has been reported previously, but there was operative mortality.14 The reason for the operative mortality was thought to have been the compromised nature of cirrhotic patients. Thus, two-stage therapy involving the combination of preoperative EIS and Hassabs operation is better because of its safety and effectiveness.
The increase in the platelet count postsplenectomy has practical benefit for patients undergoing interferon therapy for hepatitis C virus and percutaneous radio frequency ablation (RFA) for HCC. The usefulness of PSE in such situations has been reported, but the quantitative evaluation of embolized volume during the procedure is still difficult.5 ; 15 An important limitation of excessive embolization is a high incidence of splenic abscess.16 ; 17 Furthermore, the risk of insufficient embolization as a result of attempting to avoid excessive embolization exists. In our experience with patients after PSE, splenectomy is difficult, because of strong adhesions between the spleen and peritoneum caused by inflammatory changes owing to PSE itself. Thus, PSE is not performed in our institute. Our main therapy for thrombocytopenia is splenectomy, including Hassabs operation. However, splenectomy is more invasive than PSE, thus, the introduction of a less invasive surgical approach, such as a laparoscopic procedure, is preferable for cirrhotic patients.18
The occurrence of portal vein thrombosis was much higher in the present study than in previous series.19 ; 20 This may be caused by our postoperative protocol to take contrast enhanced CT to evaluate postoperative changes of the surgical site and detect asymptomatic portal vein thrombosis about 7–10 days after surgery. Thus, we had no cases with symptomatic portal vein thrombosis, such as massive ascites or gastrointestinal bleeding. Our protocols for anticoagulation therapy immediately after operation are systemic heparinization and replacement antithrombin three to keep its activity above 80% starting from about the 3rd postoperative day. If portal vein thrombosis was seen on contrast-enhanced CT, the heparin dosage was increased to attain an activated partial thromboplastin time (APTT) about two times longer than the normal range. After confirming the contraction of the thrombus, warfarin was started to maintain the prothrombin time–international normalized ratio (PT–INR) at about two. This anticoagulation therapy is performed under hospitalization, and affects the length of the hospital stay. Thus, our result suggests that more safe and effective anticoagulation is needed to manage these patients.
Postoperative changes in hepatic reserve after Hassabs operation were recently reported to include changes in hepatic hemodynamic status that had a beneficial effect on hepatic functional reserve.21 The details of the mechanisms of liver regeneration after splenectomy have been reported in a cirrhotic rat model.22 ; 23 However, in the present series, immediately after operation, hepatic functional reserve deteriorated according to the Child–Pugh classification, and no definite change compared with the preoperative status was observed 6 months postoperatively. These exacerbations of hepatic reserve are suspected to be caused by surgical stress and the time lag of the recovering cirrhotic liver itself. The cause of the deterioration of albumin is also suspected to be surgical stress. However, the reasons for the postoperative change of bilirubin were unexplained. Furthermore, the detailed mechanisms of the beneficial effect of this procedure in humans are still unclear. Thus, further investigations are needed.
In conclusion, Hassabs operation is still meaningful, not only for controlling gastroesophageal varices, but also to treat some conditions related to cirrhosis for the patients contraindicated for liver transplantation. In our institute, asymptomatic portal vein thrombosis occurred at a high rate, but combination therapy with EIS was effective to control varices. Studies involving more patients and more detailed research, especially by hepatic reserve, are needed for definite conclusions.
References
- 1 L.R. Roberts, P.S. Kamath; Pathophysiology and treatment of variceal hemorrhage; Mayo Clin Proc, 71 (1996), pp. 973–983
- 2 G.V. Stiegmann, M. Yamamoto; Approach to the endoscopic treatment of esophageal varices; World J Surg, 16 (1992), pp. 1034–1041
- 3 J.C. García-Pagán, K. Caca, C. Bureau, et al.; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding; N Engl J Med, 362 (2010), pp. 2370–2379
- 4 T. Akahoshi, M. Hashizume, M. Tomikawa, et al.; Long-term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: A 10-year experience; J Gastroenterol Hepatol, 23 (2008), pp. 1702–1709
- 5 H. Yoshida, Y. Mamada, N. Taniai, T. Tajiri; Partial splenic embolization; Hep Res, 38 (2008), pp. 225–233
- 6 M.A. Hassab; Gastroesophageal decongestion and splenectomy in the treatment of esophageal varices in bilharzial cirrhosis: further studies with a report on 355 operations; Surgery, 61 (1967), pp. 169–176
- 7 The Japan Society for Portal Hypertension; The General Rules for Study of Portal Hypertension; Kanehara Syuppan, Tokyo (2004) 37–41
- 8 Z.J. Haskal, C.R. Rees, E.J. Ring, R. Saxon, D. Sacks; Reporting standards for transjugular intrahepatic portosystemic shunts; J Vasc Interv Radiol, 14 (2003), pp. S419–S426
- 9 M.A. Yerdel, B. Gunson, D. Mirza, et al.; Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome; Transplantation, 69 (2000), pp. 1873–1881
- 10 N. Hiraga, H. Aikata, S. Takaki, et al.; The long-term outcome of patients with bleeding gastric varices after balloon-occluded retrograde transvenous obliteration; J Gastroenterol, 42 (2007), pp. 663–672
- 11 M. Yuki, H. Kazumori, S. Yamamoto, T. Shizuku, Y. Kinoshita; Prognosis following endoscopic injection sclerotherapy for esophageal varices in adults: 20-year follow-up study; Scand J Gastroenterol, 43 (2008), pp. 1269–1274
- 12 M. Tomikawa, M. Hashizume, K. Okita, et al.; Endoscopic injection sclerotherapy in the management of 2105 patients with esophageal varices; Surgery, 131 (2002), pp. S171–S175
- 13 M. Sugiura, S. Futagawa; Results of six hundred thirty-six esophageal transections with paraesophagogastric devascularization in the treatment of esophageal varices; J Vasc Surg, 1 (1984), pp. 254–260
- 14 H. Nakamura, N. Goseki, Y. Dobashi, T. Igari, M. Endo; Hassab operation with intraoperative endoscopic injection sclerotherapy (“Hassab-EIS”) for esophagogastric varices: with an autopsied case after excessive gastric vascular damage; Hepatogastroenterology, 43 (1996), pp. 980–986
- 15 J.I. Bibao, B. Sangro, J.M. Longo, et al.; Splenic embolization prior to myelosuppressive treatment in hepatocarcinoma and active chronic hepatitis; Eur J Radiol, 15 (1992), pp. 211–214
- 16 T. Sakai, K. Shiraki, H. Inoue, et al.; Complications of partial splenic embolization in cirrhotic patients; Dig Dis Sci, 47 (2002), pp. 388–391
- 17 K.B. Jones, P.T. de Koos; Postembolization splenic abscess in a patient with pancreatitis and splenic vein thrombosis; South Med J, 77 (1984), pp. 390–393
- 18 M. Hashizume, K. Tanoue, M. Morita, M. Ohta, M. Tomikawa, K. Sugimachi; Laparoscopic gastric devascularization and splenectomy for sclerotherapy-resistant esophagogastric varices with hypersplenism; J Am Coll Surg, 187 (1998), pp. 263–270
- 19 M. Yoshida, Y. Watanabe, A. Horiuchi, Y. Yamamoto, H. Sugishita, K. Kawachi; Portal and splenic venous thrombosis after splenectomy in patients with hypersplenism; Hepatogastroenterology, 56 (2009), pp. 538–541
- 20 M.A. Amin, M.M. el-Gendy, I.E. Dawoud, A. Shoma, A.M. Negm, T.A. Amer; Partial splenic embolization versus splenectomy for the management of hypersplenism in cirrhotic patients; World J Surg, 33 (2009), pp. 1702–1710
- 21 Y. Zhan, T. Wen, L. Yan, et al.; The changes of hepatic hemodynamics and functional hepatic reserve after splenectomy with periesophagogastric devascularization; Hepatogastroenterology, 56 (2009), pp. 835–839
- 22 K. Murata, K. Shiraki, K. Sugimoto, et al.; Splenectomy enhances liver regeneration through tumor necrosis factor (TNF)-α following dimethylnitrosamine-induced cirrhotic rat model; Hepatogastroenterology, 48 (2001), pp. 1022–1027
- 23 S. Ueda, A. Yamanoi, Y. Hirakawa, D.K. Dhar, M. Tachibana, N. Nagasue; Transforming growth factor-beta1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats; Lab Invest, 83 (2003), pp. 1593–1603
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?