Abstract
German Shepherd Dogs are important in police and military work, and are a popular family pet. The debilitating joint disorders of hip dysplasia, cranial cruciate ligament tear (CCL) and elbow dysplasia can shorten a dogs useful working life and impact its role as a family member. For this study, veterinary hospital records were examined over a 14.5-year period on 1170 intact and neutered (including spaying) German Shepherd Dogs for joint disorders and cancers previously associated with neutering. The diseases were followed through 8 years of age, with the exception of mammary cancer (MC) in females that was followed through 11 years. The cancers followed, apart from mammary, were osteosarcoma, lymphoma, hemangiosarcoma and mast cell tumour. In intact males, 7% were diagnosed with one or more joint disorders, while in males neutered prior to a year of age, a significantly higher 21% were diagnosed with one or more joint disorders. In intact females, 5% were diagnosed with one or more joint disorders, while in females neutered prior to a year of age, this measure was significantly increased to 16%. The increased joint disorder incidence mostly associated with early neutering was CCL. MC was diagnosed in 4% of intact females compared with less than 1% in females neutered before 1 year. The occurrence of the other cancers followed through 8 years of age was not higher in the neutered than in the intact dogs. Urinary incontinence, not diagnosed in intact females, was diagnosed in 7% of females neutered before 1 year, a significant difference. These findings, profiling the increase in joint disorders associated with early neutering, should help guide the timing of neutering for this breed.
Introduction
The practices of spaying female and neutering male dogs, both referred to herein as neutering, have become routine in the U.S. in the last three decades (Trevejo et al. 2011). In Europe, the practice of neutering varies among countries. Increasingly, in the U.S., neutering is being performed prior to 6 months, as commonly advocated by both veterinarians and shelter personnel. The basis of this perspective is mostly in the interest of pet population control.
Neutering, however, especially in the first year, can come with its costs with regard to debilitating joint disorders, namely, hip dysplasia (HD), cranial cruciate ligament tear or rupture (CCL) and elbow dysplasia (ED). Across several breeds, a study of CCL found that neutered males and females were two to three times more likely than intact dogs to have this disorder (Witsberger et al. 2008). The same study also found a lesser, but significant, increase in HD in neutered compared with intact dogs. Neutering has also been shown to be associated with a threefold increase in excessive tibial plateau angle – a known risk factor for CCL (Duerr et al. 2007). Our recent studies on the effects of neutering in Golden and Labrador Retrievers (Torres de la Riva et al. 2013; Hart et al. 2014) showed an increase in the incidence of one or more of these joint disorders, in male and female Golden Retrievers, to 4–5 times the 5% occurrence in intact dogs, and in male and female Labrador Retrievers, a doubling of the 5% incidence of intact dogs.
An increase in some cancers sometimes is also of concern with neutering, particularly with osteosarcoma (OSA), hemangiosarcoma (HSA), lymphoma (LSA) and mast cell tumours (MCT). A study on OSA in several breeds found a twofold increase in neutered dogs relative to intact dogs (Ru et al. 1998). In spayed females, cardiac HSA was reported to be four times greater than that in intact females (Ware & Hopper 1999), and in spayed females splenic HSA was reported at two times greater than in intact females (Prymak et al. 1988). The occurrence of LSA was found to be higher in spayed than in intact females (Villamil et al. 2009). Cutaneous MCT, as studied in several dog breeds, was four times greater than that of intact females in neutered females (White et al. 2011).
In our studies involving the Golden Retriever (Torres de la Riva et al. 2013; Hart et al. 2014), we found that neutering at all neuter periods through 8 years of age increased the rate of at least one of the cancers by 3–4 times. Two other recent studies have also profiled the association of neutering with the occurrence of these cancers. A study utilising the U.S. nationwide Veterinary Medical Database found that neutered males and females were more likely to die of a cancer than intact dogs (Hoffman et al. 2013). A second study, utilising web-based, owner-reported disease occurrence in Vizslas, reported the incidence of cancers was higher in neutered dogs than in intact dogs (Zink et al. 2013). In the above papers, the occurrence of mammary cancer (MC) was low in females left intact, and not significantly reduced by neutering.
Drawing from the same database as used with the retrievers mentioned above (Torres de la Riva et al. 2013; Hart et al. 2014), the goal of this study was to statistically evaluate the relationships of neutering at different age periods with regard to the occurrence of major joint disorders, and the cancers mentioned above, in German Shepherd Dogs.
Methods
Study parameters
Using data from the computerised veterinary hospital records of the University of California-Davis, Veterinary Medical Teaching Hospital (VMTH), this study retrospectively examined the occurrence of joint disorders and cancers in male and female German Shepherd Dogs left intact and those neutered in the periods of <6 months, 6–11 months, 1 year (12–23 months) and 2–8 years. Joint disorders and cancers, except for MC, were tracked through the first 8 years of the dogs life. The reason that 8 years of age was chosen for the cut-off is that beyond 9 years of age, one could argue that the influence of neutering is fading and other, ageing-associated factors, such as inflammation, start to play a more influential role. With MC, most cases are diagnosed after 8 years of age, with the median age of diagnosis being 10.1 years in one study (Cohen et al. 1974), so we tracked this disease in females through 11 years. The occurrences of urinary incontinence (UI) and pyometra (PYO) in females were tracked through 8 years.
No animal care and use committee approval was required because, in conformity with the campus policy, faculty of the School of Veterinary Medicine are allowed use of the record system for research purposes. Strict confidentiality of the owners and their dogs was maintained.
Data collection and presentation
The VMTH is a primary care facility, as well as a secondary and tertiary care centre for referral cases. This hospital has a very large database, currently with approximately 50 000 cases per year. The subjects included were gonadally intact and neutered female and male German Shepherd Dogs admitted to the hospital between January 1, 2000 and June 30, 2014, representing 14.5 years of data. All cases of German Shepherd Dogs with information on date of birth, neuter status and age at neutering (if neutered) were included in the study. Extensive review of this database revealed that only 4% of 460 intact males and 14% of the 172 intact females were reported by the owner to be used for breeding.
As mentioned, age at neutering was categorised as <6 months, 6–11 months, 1 year (12 to <24 months) and 2–8 years (2 to <9 years). For some analyses, the cases for both <6 months and 6–11 months neuter periods were combined and referred to as early neutered (<12 months), because sometimes early neutering may refer to less than 1 year or less than 6 months. Only females were examined for MC, PYO and UI.
For all neutered dogs, records were reviewed to ensure that neutering occurred prior to the first clinical signs or diagnosis of any disease of interest. If a disease of interest occurred before neutering, the dog was considered as intact for that specific disease analysis, but was considered as neutered for a different disease that may have occurred after neutering. If a disease of interest occurred before January 1, 2000 or before 12 months of age, the case was deleted for that disease.
For cases where the hospital records on referral cases did not include age at neutering, telephone calls to the referring veterinarians were made to obtain the specific neutering dates. Because there were neutered dogs where age at neutering was not available from either the record or from the referring veterinarian and these cases could not be included, there were proportionately more intact cases in the final data set than would be expected in general. Nonetheless, the proportional differences on diseases between the intact and the various neutered dogs were not affected by the overrepresentation of intact dogs in the database.
The complete data set available totalled 1170 cases with 705 males, of which 245 were neutered and 460 were intact and 465 females, of which 293 were neutered and 172 intact. The number of cases reported for each disease varied somewhat because, as mentioned above, a case could be excluded for one disease analysis but included for another disease analysis.
In terms of inclusion criteria, a patient was considered as having a disease of interest if the diagnosis was made at the VMTH or by a referring veterinarian and later confirmed at the VMTH. Patients diagnosed with HD, ED and/or CCL presented with clinical signs such as difficulty moving, standing up, lameness and/or joint pain; diagnoses were confirmed based on radiographic evidence, orthopaedic physical examination and/or surgical confirmation. Diagnoses of the various cancers (LSA, HSA, OSA, MCT, MC) were accompanied by clinical signs such as enlarged lymph nodes, lumps on the skin or presence of masses, and confirmed based on imaging, appropriate blood cell analyses, chemical panels, histopathology and/or cytology. PYO was confirmed by ultrasonic evidence and/or post-surgically after removal of the uterus. UI was confirmed by clinical signs, urinalyses and exclusion of other diseases, such as urinary tract infection. When a diagnosis was listed in the record as ‘suspected’ based on clinical signs, but the diagnostic tests were inconclusive, the case was excluded from the analysis for that specific disease.
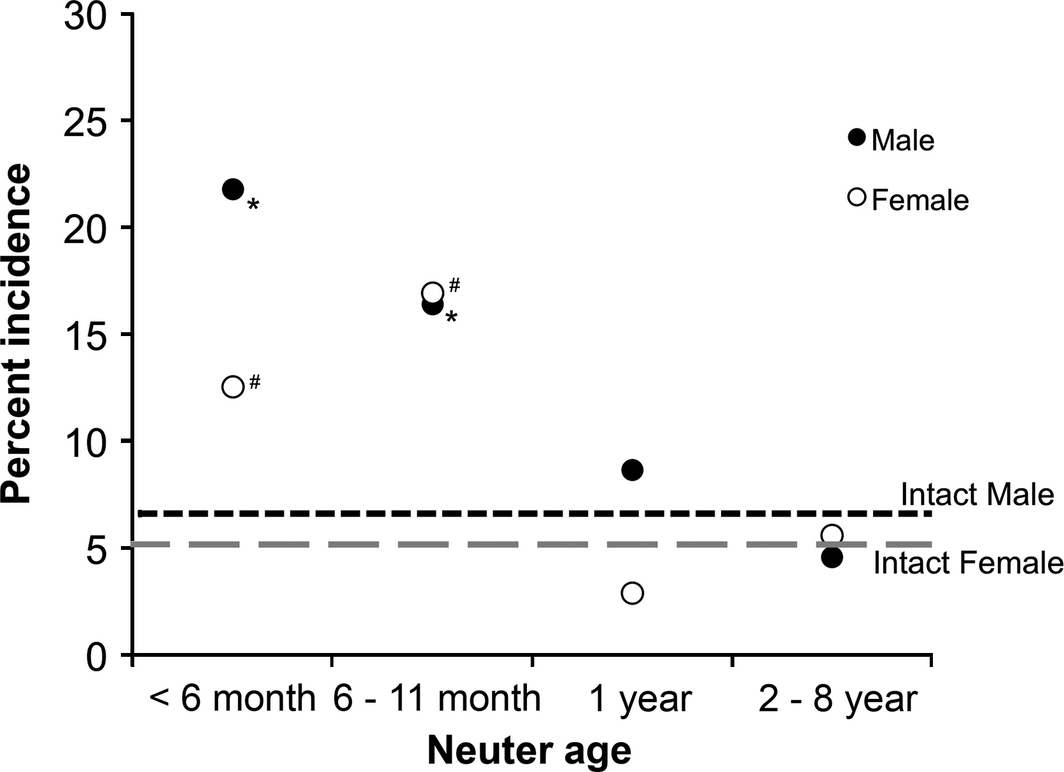
Figure 1 portrays single data points, corresponding to the various neuter ages, representing the incidence of dogs diagnosed with at least one joint disorder (after controlling for multiple diagnoses). The data for the incidence of individual joint disorders, cancers, PYO and UI at different neuter ages, and in intact dogs, are presented in Tables 1-3.
|
|
|
Figure 1. Incidences of the occurrences of at least one joint disorder in male and female German Shepherd Dogs, as a function of age at neutering. The horizontal lines show the occurrences in intact males and females for the same measures. Asterisks adjacent to a point indicate significant differences from the intact level. The pound sign next to two adjacent points indicates that, while neutering at the <6 months and 6–11 months periods alone did not reach significance, when cases for the two early neuter periods were combined, the result was significantly higher than that of intact females. The main joint disorder associated with neutering was cranial cruciate ligament tear or rupture (Table 1). |
| Neuter age | HD | CCL | ED | At least one |
|---|---|---|---|---|
| Male <6 months | 2/23 (8.7) | 3/24 (12.5) | 1/23 (4.35) | 5/24 (20.83) |
| Male 6–11 months | 3/55 (5.45) | 5/60 (8.33) | 3/57 (5.26) | 9/55 (16.36) |
| (Male <12 months) | 5/78 (6.41) | 8/84 (9.52) | 4/80 (5) | 14/79 (17.72) |
| Male 1 year | 2/58 (3.45) | 1/61 (1.64) | 2/60 (3.33) | 5/58 (8.62) |
| Male 2–8 years | 2/90 (2.22) | 1/97 (1.03) | 1/99 (1.01) | 4/89 (4.49) |
| Male intact | 21/445 (4.72) | 3/458 (0.66) | 8/456 (1.75) | 29/440 (6.59) |
| Female <6 months | 3/41 (7.32) | 2/44 (4.55) | 0/43 (0) | 5/40 (12.5) |
| Female 6–11 months | 6/80 (7.5) | 7/84 (8.33) | 0/80 (0) | 13/77 (16.88) |
| (Female <12 months) | 9/121 (7.44) | 9/128 (7.03) | 0/123 (0) | 18/110 (16.36) |
| Female 1 year | 1/35 (2.86) | 0/36 (0) | 0/36 (0) | 1/35 (2.86) |
| Female 2–8 years | 4/91 (4.4) | 1/95 (1.05) | 0/96 (0) | 5/90 (5.56) |
| Female intact | 6/157 (3.82) | 1/159 (0.63) | 1/158 (0.63) | 8/156 (5.13) |
| The occurrences of joint disorders are portrayed for the different neuter periods; the periods <6 months and 6–11 months are combined in the <12 months period, representing early neutering. The columns represent hip dysplasia (HD), cranial cruciate ligament tear or rupture (CCL), and elbow dysplasia (ED). Shown are number of cases over number in the pool, with percentages given in parentheses. When bolded the incidence is significantly above that of intact dogs (see text). | ||||
| Neuter age | LSA | MCT | HSA | OSA | At least one |
|---|---|---|---|---|---|
| Male <6 months | 1/24 (4.17) | 0/24 (0) | 0/24 (0) | 0/24 (0) | 1/24 (4.17) |
| Male 6–11 months | 0/60 (0) | 0/60 (0) | 0/60 (0) | 0/60 (0) | 0/60 (0) |
| Male 1 year | 1/61 (1.64) | 0/61 (0) | 0/61 (0) | 0/61 (0) | 1/61 (1.64) |
| Male 2–8 years | 1/100 (1) | 0/99 (0) | 0/100 (0) | 1/100 (1) | 2/99 (2.02) |
| Male intact | 7/452 (1.55) | 2/458 (0.44) | 3/457 (0.66) | 1/458 (0.22) | 13/450 (2.89) |
| Female <6 months | 0/43 (0) | 0/43 (0) | 0/44 (0) | 1/44 (2.27) | 1/42 (2.38) |
| Female 6–11 months | 0/86 (0) | 0/85 (0) | 0/86 (0) | 0/86 (0) | 0/85 (0) |
| Female 1 year | 0/35 (0) | 0/35 (0) | 0/36 (0) | 0/36 (0) | 0/34 (0) |
| Female 2–8 years | 1/95 (1.05) | 1/96 (1.04) | 0/94 (0) | 0/95 (0) | 2/93 (2.15) |
| Female intact | 1/158 (0.63) | 0/159 (0) | 0/159 (0) | 0/159 (0) | 1/158 (0.63) |
| The occurrences of cancers are portrayed for the different neuter periods; the periods <6 months and 6–11 months are combined in the <12 months period, representing early neutering. The columns represent the occurrences of lymphosarcoma (LSA), mast cell tumour (MCT), hemangiosarcoma (HSA), osteosarcoma (OSA) and the occurrence of at least 1 of the cancers. Shown are number of cases over number in the pool, with percentages given in parentheses. | |||||
| Neuter age | MC | PYO | UI |
|---|---|---|---|
| Female <6 months | 0/46 (0) | 0/44 (0) | 2/43 (4.65) |
| Female 6–11 months | 1/91 (1.11) | 0/86 (0) | 6/83 (7.22) |
| Female 1 year | 1/37 (2.7) | 0/33 (0) | 1/36 (2.78) |
| Female 2–8 years | 5/102 (4.9) | 0/91 (0) | 1/95 (1.05) |
| Female intact | 7/173 (4.1) | 4/163 (2.45) | 0/156 (0) |
| The numbers of cases are shown, over the numbers in the pool, with percentages given in parentheses. For MC, the occurrence was tracked through 11 years of age (see text), whereas with PYO and UI, the occurrence was tracked through 8 years of age. When bolded the incidence is significantly above that of intact dogs (see text). | |||
Body weights and body condition scores (BCS) are reported to be a factor in the occurrence of joint disorders (Duval et al. 1999; Comhaire & Snaps 2008). Body weights are difficult to compare among dogs because of the confounding factor of variations in body height, so BCSs were used in comparing neutered dogs with and without a joint disorder for those conditions where analyses showed a significant association between neutering and the joint disorder. The BCS used by the VMTH is the standard 1–9 range where a score of 5 is optimal and a score of 9 represents the most overweight (Baldwin et al. 2010). Typically, the BCS is assigned at the time of a patients visit to the hospital. The BCSs at the time of diagnosis of neutered dogs with a joint disorder were compared with BCSs of neutered dogs without the disorder at an age that fell within the range representing 80% of the ages of dogs with the disorder at the time of diagnosis.
Statistical analyses
A survival analysis was used to compare the incidence rates of each disease between groups of animals defined in terms of their age at neutering. Patients were diagnosed at different ages and with varying years at risk from the effects of gonadal hormone removal. Animals that were intact at the time that a disease was diagnosed were treated as intact for the purposes of the analysis of that disease, even though they may subsequently have been neutered. Animals that were neutered prior to diagnosis were considered at risk for the period between neutering and diagnosis.
For the survival analyses, Cox proportional hazard models (Cox 1972; Rothman & Greenland 1998) were used to test for differences with respect to the hazard of a disease among the neutered and intact groups while adjusting for the differences in time at risk between animals. In instances where the Cox models had computational issues when there were zero cases within one or more neutering groups, those groups were compared using a Kaplan–Meier life table analysis (Kalbfleisch & Prentice 1980). Analyses were run using the SAS software package, version 9.3. Post hoc comparisons among the subgroups were based on least squares means of the hazard within each subgroup.
In the Results section, when significant differences were found between a neutered and an intact group, the P-values, based on the proportional hazard models, and the hazard ratio with 95% confidence intervals (CI) are reported. For all statistical tests, the two-tailed statistical level of significance was set at P < 0.05.
Results
Joint disorders, males
Figure 1 and Table 1 present the incidence of dogs having at least one of the joint disorders. The occurrence of at least one joint disorder in intact males was 6.6%. At neuter age <6 months, at least one of the joint disorders occurred in 20.8% of the males, three times that of intact males (Cox: P = 0.0025, hazard ratio = 4.47 [95% CI = 1.70, 11.80]). At neuter age 6–11 months, this incidence was 16.4%, more than double that of intact males (P = 0.0015, hazard ratio = 3.50 [1.62, 7.57]). Not surprisingly, combining cases in both of these early neuter periods also revealed a highly significant increase above that of intact males (P < 0.0001, hazard ratio = 3.79 [1.95, 7.37]).
As shown in Table 1, the main joint disorder in intact males was HD, occurring in about 5%. However, the main joint disorder increased by neutering in males was CCL. In intact males, CCL occurred in less than 1%, but for the <6 months and 6–11 months neuter periods, this joint disorder occurred in 12.5% and 8.3% of dogs, respectively, significantly higher than that of intact males (P < 0.001, hazard ratio = 26.16 [5.55, 123.29]) for both periods combined. At neuter period <6 months, HD was higher than intact males, but not significantly so. The median age of diagnosis of CCL in neutered males was 5 years, and for HD was 4.5 years. The occurrence of ED in intact males was about 2%, which was increased (non-significantly) to 4.4% and 5.3%, respectively, with the <6 months and 6–11 months periods. The median age of diagnosis of ED in neutered males was 1.5 years. The median BCSs of neutered males with CCL, HD and ED were all 5.0, which were the same as the median BCSs of neutered males without these joint disorders.
Joint disorders, females
Figure 1 and Table 1 portray the incidence of females having at least one of the joint disorders at different neuter periods as well as in intact females. The occurrence of at least one joint disorder in intact females was 5.1%. At neuter age <6 months, at least one of the joint disorders occurred in 12.5% of dogs, more than double that of the intact females, and at the 6–11 months neuter age, this incidence increased to almost 17%, three times that of intact females. Combining cases in both of these early neuter periods revealed a significant increase above that of intact females (P = 0.0063, hazard ratio = 3.97 [1.48, 10.71]).
As with intact males, and shown in Table 1, the main joint disorder of intact females was HD, occurring in about 4%. Also, like males, the main joint disorder associated with neutering was CCL. The occurrence of CCL, which was diagnosed in less than 1% of intact females, occurred in 4.6% of females neutered at <6 months and in 8.3% in those neutered at 6–11 months. Combining cases in both of these early neuter periods for CCL reveals a significant increase above intact females (P = 0.0328, hazard ratio = 9.49 [1.20, 74.90]). As with males, the incidence of HD associated with the early neuter periods increased, but did not reach significance compared with intact females. The median age of diagnosis of CCL in neutered females was 6 years, and 3 years for HD. There was no occurrence of ED in neutered females, and in the intact females, the occurrence was less than 1%. The median BCS of neutered females with HD was 4.5 and for neutered females without HD was 5. The median BCS of neutered females with CCL was 5.75 and for neutered females without CCL was 5.
Cancers, males
The underlying rate of intact males having at least one of the cancers was about 3%. As revealed in Table 2, neutering at any age period was not associated with any evident increase in cancer occurrence above the level of intact males. With LSA, occurring in 1.5% of intact males, there was a non-significant increase in incidence at the <6 months neuter period to 4.2%.
Cancers, females
The occurrence of at least one of the cancers in intact females was less than 1%. As in males, neutering at any age period was not associated with any evident increase in cancer occurrence above the level of intact females (Table 2). The occurrence of MC in intact females, and those neutered at various periods, is shown in Table 3. MC was diagnosed in 4.1% of intact females and in none of those neutered at <6 months, not a significant difference. For neutering beyond 6 months, MC occurred in a modestly increasing percentage of females, ranging up to 5% for females neutered at 2–8 years.
Urinary incontinence and pyometra
There was no occurrence of UI in intact females. In females neutered at <6 months, the incidence of UI was 4.7%. For those neutered at 6–11 months the incidence was 7.3%, which was a significant increase compared with intact females (Kaplan–Meier: P < 0.05). The mean age of onset of UI in early neutered females was 5.2 years. The occurrence of PYO in intact females was 2.5%.
Discussion
The findings reported here on the popular German Shepherd Dog with regard to joint disorders are particularly important because joint disorders, such as CCL and HD, are painful for the dog, create a burden for those caring for the dog, and can disqualify the dog as a working partner in military and police work.
This study reveals that in males, neutering within the first year of life is associated with a highly significant, threefold risk of acquiring at least one joint disorder: up to 21% compared with 7% in males left intact or neutered beyond the first year. In females, neutering within the first year is also associated with a highly significant threefold risk of acquiring at least one joint disorder: up to 17% compared with 5% in females left intact or neutered beyond 1 year.
Agencies acquiring a German Shepherd as a police or military working companion, or a family adopting a German Shepherd puppy companion pet, are wise to take into account the genetic variables in joint disorders. This breed has been the focus of several studies on the occurrence of HD, referred to as the most common hereditary skeletal disorder (Smith et al. 2001). In fact, genetic studies on HD relate the occurrence to single-nucleotide polymorphisms in several quantitative trait loci (Fels & Distl 2014). Interestingly, our study reveals that while CCL is rather infrequent in intact males and females, it is CCL rather than HD that is especially associated with early neutering. As shown in this study, delaying neutering until the dog is at least a year of age appears to avoid the increase in risks of joint disorders is associated with neutering. This is a consideration for joint disease control that is immediately available.
The results of this study on CCL are consistent with those on the Golden Retriever and Labrador Retriever from this centre (Torres de la Riva et al. 2013; Hart et al. 2014) and other studies mentioned in the Introduction. The limitations of this study are that the reported results regarding joint disorders and cancers in the intact dogs will not necessarily represent an accurate occurrence of the disease syndromes among the various strains of German Shepherd Dogs. One might also wonder about differences in dogs presented to the hospital and used for the database compared with those seen in primary practices. From our investigations, we have not detected any differences.
With regard to a mechanism by which early gonadectomy may be related to an increased occurrence in joint disorders, we suggest a hypothesis stemming from studies on the closure of long-bone growth plates by gonadal hormone secretion as the animal approaches maturity (Salmeri et al. 1991; Perry et al. 2008). The hypothesis is that neutering much before bone growth plate closure is complete allows the bones to grow a little longer than normal, and that this disturbs the joint alignment in some dogs sufficiently to lead to a clinically apparent joint disorder that would not have been evident had the dog been neutered beyond puberty.
Body weight, when elevated much above normal for the breed, can also be related to the occurrence of joint disorders (Duval et al. 1999; Comhaire & Snaps 2008). In this study, BCSs of neutered dogs with the joint disorders related to neutering were compared with BCSs of neutered dogs without the joint disorders. The median BCSs for both male and female neutered dogs with a joint disorder were the same as, or close to, the ideal score of 5, as were BCSs of neutered dogs without the disorder. There were similar findings in the Golden and Labrador Retrievers (Hart et al. 2014). Thus, while body weight probably plays a role in the development of a joint disorder in overweight dogs, the findings presented here are consistent with the perspective that the increase in joint disorders in neutered dogs is at least partially due to the effect of gonadal hormonal removal in delaying long-bone growth plate closure.
With regard to cancers, in contrast to the Golden Retriever where the female is particularly vulnerable to the effects of neutering on the occurrence of cancers (Torres de la Riva et al. 2013; Hart et al. 2014), this study on the German Shepherd Dog reveals that whether the dog was intact or neutered, there was a low level of occurrence of the particular cancers that were followed. The caveat is the cancers were only tracked through 8 years for the reasons explained in the methods.
One of the frequently mentioned advantages of early neutering of female dogs is protection against MC (Root Kustritz 2007). While none of the females neutered at <6 months were diagnosed with MC, we found that only 4% of intact females followed through 11 years were diagnosed with MC. Neutering at 1 year and beyond resulted in an incidence level about the same as intact females. There may be important genetic breed-line differences in the occurrence of MC that are not portrayed in the database. But, the relatively low level of MC occurrence in the intact females suggests that MC is not a major disease for this breed, at least through 11 years of age. Relevant to the discussion of MC is the recent meta-analysis of published studies on neutering females and MC, finding that the evidence linking neutering to a reduced risk of MC is weak (Beauvais et al. 2012).
UI in neutered females is a concern of many dog caregivers as the dog ages. For the dogs tracked through 8 years of age in this study, UI reached a level of 7% in females neutered before 1 year. In females neutered at 1 year and beyond, the incidence of this problem dropped, and was not diagnosed at all in intact females.
In conclusion, with the current focus on avoiding joint disorders in German Shepherd Dogs, the findings provided here offer some evidence-based guidelines in deciding upon the age to neuter a puppy to reduce the risk of one or more joint disorders.
Acknowledgements
Special thanks are extended to Marty Bryant, Valerie Caceres, Sara Sewell, Emily Romanko and Aaron Frankel. We appreciate the epidemiological consultation provided by Hsin-Yi Weng.
Source of Funding
Supported by the Canine Health Foundation (#01488-A) and the Center for Companion Animal Health, University of California, Davis (# 2009-54-F/M).
Conflicts of interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributions
Conceived and designed study: BH, LH, AT; collected and complied and analysed data: AT, BH, LH; statistical analyses: NW; drafted and edited manuscript: BH, LH, AT, NW.
References
- Baldwin K., Bartges J., Buffington T., Freeman L.M., Grabow M., Legred J. & Ostwald D. (2010) AAHA nutritional assessment guidelines for dogs and cats. Journal of the American Animal Hospital Association46, 285–296.
- Beauvais W., Cardwell J.M. & Brodbelt D.C. (2012) The effect of neutering on the risk of mammary tumours in dogs – a systematic review. Journal of Small Animal Practice53, 314–322.
- Cohen D., Reif J.S., Brodey R.S. & Keiser H. (1974) Epidemiological analysis of the most prevalent sites and types of canine neoplasia observed in a veterinary hospital. Cancer Research34, 2859–2868.
- Comhaire F.H. & Snaps F. (2008) Comparison of two canine registry databases on the prevalence hip dysplasia by breed and body weight and height. American Journal of Veterinary Research232, 330–333.
- Cox D.R. (1972) Regression models and life tables (with discussion). Journal of the Royal Statistical Society34, 187–220.
- Duerr F.M., Duncan C.G., Savicky R.S., Park R.D., Egger E.L. & Palmer R.H. (2007) Risk factors for excessive tibial plateau angle in large-breed dogs with cranial cruciate disease. Journal of the American Veterinary Medical Association231, 1688–1691.
- Duval J.M., Budsberg S.C., Flo G.L. & Sammarco J.L. (1999) Breed, sex, and body weight as risk factors for rupture of the cranial cruciate ligament in young dogs. Journal of the American Veterinary Medical Association215, 811–814.
- Fels L. & Distl O. (2014) Identification and validation of quantitative trait loci (QTL) in German Shepherd Dogs. PLoS One9, 5. doi:10.1371/journal.pone.0096618.
- Hart B.L., Hart L.A., Thigpen A.P. & Willits N.H. (2014) Long-term health effects of neutering dogs: comparison of Labrador Retrievers with Golden Retrievers. PLoS One9, 7. doi:10.1371/journal.pone.0102241.
- Hoffman J.M., Creevy K.E. & Promislow D.E.L. (2013) Reproductive capability is associated with lifespan and cause of death in companion dogs. PLoS One8, 4. doi:10.1371.
- Kalbfleisch J.D. & Prentice R.L. (1980) The Statistical Analysis of Failure Time Data. Wiley & Sons: New York.
- Perry R.J., Farquharson C. & Ahmed S.F. (2008) The role of sex steroids in controlling pubertal growth. Clinical Endocrinology68, 4–15.
- Prymak C., McKee L.J., Goldschmidt M.H. & Glickman L.T. (1988) Epidemiologic, clinical, pathologic, and prognostic characteristics of splenic hemangiosarcoma and splenic hematoma in dogs: 217 cases (1985). Journal of the American Veterinary Medical Association193, 706–712.
- Root Kustritz M.V. (2007) Determining the optimal age for gonadectomy of dogs and cats. Journal of the American Veterinary Medical Association231, 1665–1675.
- Rothman K.J. & Greenland S. (1998) Modern Epidemiology. Lippincott Williams & Wilkins: Philadelphia.
- Ru G., Terracini B. & Glickman L.T. (1998) Host related risk factors for canine osteosarcoma. The Veterinary Journal156, 31–39.
- Salmeri K.R., Bloomberg M.S., Scruggs S.L. & Shille V. (1991) Gonadectomy in immature dogs: effects on skeletal, physical, and behavioral development. Journal of the American Veterinary Medical Association1991, 1193–1203.
- Smith G.K., Mayhew P.D., Kapatkin A.S., McKelvie P.J. & Shofer F.S. (2001) Evaluation of risk factors for degenerative joint disorders associated with hip dysplasia in German Shepherd dogs, golden retrievers, labrador retrievers, and rottweilers. Journal of the American Veterinary Medical Association219, 1719–1724.
- Torres de la Riva G., Hart B.L., Farver T.B., Oberbauer A.M., McV Messam L.L., Willits N. & Hart L.A. (2013) Neutering dogs: effects on joint disorders and cancers in golden retrievers. PLoS One8, 2. doi:10.1371/journal.pone.0055937.
- Trevejo R., Yang M. & Lund E.M. (2011) Epidemiology of surgical castration of dogs and cats in the United States. Journal of the American Veterinary Medical Association238, 898–904.
- Villamil J.A., Henry C.J., Hahn A.W., Bryan J.N., Tyler J.W. & Caldwell C.W. (2009) Hormonal and sex impact on the epidemiology of canine lymphoma. Journal of Cancer Epidemiology2009, 1–7. doi:10.1155/2009/591753.
- Ware W.A. & Hopper D.L. (1999) Cardiac tumors in dogs: 1982–1995. Journal of Veterinary Internal Medicine13, 95–103.
- White C.R., Hohenhaus A.E., Kelsey J. & Procter-Grey E. (2011) Cutaneous MCTs: associations with spay/neuter status, breed, body size, and phylogenetic cluster. Journal of the American Animal Hospital Association47, 210–216.
- Witsberger T.H., Villamil J.A., Schultz L.G., Hahn A.W. & Cook J.L. (2008) Prevalence of, and risk factors for, hip dysplasia and cranial cruciate ligament deficiency in dogs. Journal of the American Veterinary Medical Association232, 1818–1824.
- Zink M.C., Farhoody P., Elser S.E., Ruffini L.D., Gibbons T.A. & Rieger R.H. (2013) Evaluation of the risk and age of onset of cancer and behavioral disorders in gonadectomized Vizslas. Journal of the American Veterinary Medical Association244, 309–319.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?