Highlights
- x-ray radiation dose in pediatric patients is of specific concern as congenital heart disease is more often treated by interventional measures.
- the effect of advanced real time image noise reduction algorithms and optimized acquisition chain for fluoroscopy and exposure was studied.
- A state of the art image processing and reference acquisition chain was compared to the new imaging technology in 338 vs 329 consecutive patients.
- Patients were divided into three weight groups: A) below 10 kg, B) 10-40 kg, and C) over 40 kg according to clinical practice and procedure complexity.
- the novel X-ray imaging technology provided substantial radiation dose reduction of 56% or higher.
Abstract
Background
Pediatric catheterization exposes patients to varying radiation doses. Concerns over the effects of X-ray radiation dose on the patient population have increased in recent years. This study aims at quantifying the patient radiation dose reduction after the introduction of an X-ray imaging technology using advanced real time image noise reduction algorithms and optimized acquisition chain for fluoroscopy and exposure in a pediatric and adult population with congenital heart disease.
Methods
Patient and radiation dose data was retrospectively collected (July 2012–February 2013) for 338 consecutive patients treated with a system using state of the art image processing and reference acquisition chain (referred as “reference system”). The same data was collected (March–October 2013) for 329 consecutive patients treated with the new imaging technology (Philips AlluraClarity, referred as “new system”). Patients were divided into three weight groups: A) below 10 kg, B) 10–40 kg, and C) over 40 kg. Radiation dose was quantified using dose area product (DAP), while procedure complexity using fluoroscopy time, procedure duration and volume of contrast medium.
Results
The new system provides significant patient dose reduction compared to the reference system. Median DAP values were reduced in group A) from 140.6 cGy·cm2 to 60.7 cGy·cm2, in group B) from 700.0 cGy·cm2 to 202.2 cGy·cm2 and in group C) from 4490.4 cGy·cm2 to 1979.8 cGy·cm2 with reduction of 57%, 71% and 56% respectively (p < 0.0001 for all groups).
Conclusions
Despite no other changes in procedural approach, the novel X-ray imaging technology provided substantial radiation dose reduction of 56% or higher.
Keywords
X-ray imaging technology;Dose reduction;Congenital heart disease;DAP;Radiation exposure;Interventional therapy
1. Introduction
Patients with congenital heart defects frequently undergo numerous and repeated diagnostic and interventional catheterization procedures, in addition to other imaging studies such as chest-X-rays and CT studies. The growing number and complexity of interventional cardiology procedures have been significant in the past years as a result of advances made in transcatheter techniques and the armamentarium available (i.e. devices, stents, percutaneous valves, miniaturized balloons, coils, etc.) [1] ; [2]. While their benefits to the patients are undisputable, all these procedures contribute to high accumulated radiation doses to the patient population [3]; [4] ; [5]. This is particularly relevant for infants and children and even if the long term consequences of this exposure are not well understood and extremely difficult to estimate, there is now for many decades considerable concern about the possible stochastic effects, such as the incidence of solid tumors and leukemia [6]; [7]; [8]; [9]; [10] ; [11]. In fact, growing tissue in children is more radiosensitive than that in adults and, due to their small size, larger body parts are irradiated during cardiac catheterization including radiosensitive organs such as thyroid and eyes which are closer to the heart [12] ; [13]. Moreover, children with complex heart defects often need to undergo increasingly complex procedures many times during their lifetime, resulting in a high cumulative dose acquired [14]; [15] ; [16]. Minimizing radiation dose is therefore crucial for this vulnerable population, as children are likely to survive long enough through a possible latent period and develop or manifest late effects of early radiation exposure.
Successful patient radiation dose management can only be achieved by optimization of medical imaging technology together with best control of the equipment by the operator [17] ; [18]. In this respect, best practices are applied in our lab using “ALARA” radiation reduction principles in terms of patient radiation safety, such as short imaging time, avoidance of field overlap in repeated acquisitions, low SID, tight collimation, use of intra-procedural echocardiography, and a frame rate for fluoroscopy acquisitions of maximum 15 frames/s [19]; [20]; [21]; [22]; [23] ; [24]. Moreover, our cath lab has been recently upgraded to a novel X-ray imaging technology (AlluraClarity; Philips Healthcare, Best, Netherlands) developed for fluoroscopy and cine exposure for interventional cardiology for the entire patient size population, including pediatric. This technology enables significant patient entrance dose reduction achieved by a combination of advanced real-time image noise reduction algorithms with state-of-the-art hardware and an anatomy-specific optimized full acquisition chain (grid switch, beam filtering, pulse width, spot size, detector and image processing engine). Furthermore, image quality is further positively influenced by the use of smaller focal spot sizes and shorter pulses. Radiation dose reduction using this technology has already been investigated in other clinical and investigational domains outside congenital heart disease interventions by comparing state-of-the-art reference to new systems [51] ; [52]. In complex ablation procedures, patient dose and physician dose were reduced by 40% and 50%, respectively [25]. In interventional neuroradiology, 75% patient radiation dose reduction was demonstrated during digital subtraction angiography (DSA) without affecting image quality [26], and 60% procedural patient dose reduction was achieved [27]. However, results that quantify the reduction of patient radiation dose enabled by this technology for congenital heart disease are not available in literature. The study presented here was designed to quantify the procedural patient radiation dose reduction due to the novel X-ray imaging technology in a patient cohort with congenital heart diseases in comparison with our state-of-the-art angiography system.
2. Methods
2.1. Patients
All 667 consecutive patients with congenital heart defects referred between July 2012 and October 2013 were retrospectively included in the study. Patients who received catheter investigation with echocardiography only (i.e. Rashkind procedures) or under direct vision of the area to be treated (i.e. intraoperative hybrid procedures) were excluded from the analysis. In March 2013, the biplane flat panel angiography system was upgraded from Allura Xper FD20/10 (installed in 2011) equipped with state-of-the-art image processing (“reference system”) to the new AlluraClarity with Clarity IQ technology with advanced real time image noise reduction algorithms and optimized acquisition chain for all exposure techniques (Philips Healthcare, Best, The Netherlands). Patients were therefore assigned to the “reference” or “new” groups, and categorized according to clinical requirements and technical implementation to weight: A) below 10 kg, B) 10–40 kg, and C) over 40 kg.
The study was conducted in accordance with the provisions of the Declaration of Helsinki and was approved by the local ethical committee (approval Ref. No.: 21/2014). Procedure and dose data for all patients admitted with congenital heart disease (CHD) in the period July 2012–February 2013 were collected. As per institutional protocol and enforced by the European legislation, all relevant periprocedural data were continuously monitored and documented as part of the hospital quality assurance program. The data reported here include radiation dose, fluoroscopy time, procedure time, weight, height, BMI, and contrast medium use. After upgrading to the new system, procedure and dose data information for all consecutive patients were collected during March–October 2013. During the procedures the anti-scatter grid was not removed. The same cardiologists were employed and the same procedural techniques were used. All our catheter interventions were performed under deep conscious sedation and without general anesthesia as described in detail elsewhere [28].
2.2. Imaging technology
ClarityIQ is a novel X-ray imaging technology that combines advanced real-time image noise reduction algorithms, with state-of-the-art hardware to reduce patient entrance dose significantly. This is realized by anatomy-specific optimization of the full acquisition chain (grid switch, beam filtering, pulse width, spot size, detector and image processing engine) for every clinical task individually. The system has been optimized for all major disease areas using over a 1000 patients. In addition to patient entrance dose reduction, the image quality is improved through the use of the smaller focal spot sizes, and shorter pulses and the introduction of automatic real-time motion compensation in subtraction imaging.
One of the features of ClarityIQ, temporal noise reduction, is implemented by averaging consecutive images. However, in interventional cardiology, cardiac and respiratory motion result in the appearance of “ghost images” of moving tissues or devices. This used to limit the number of images that could be averaged, hence requiring higher doses per frame. The implementation of a motion compensation algorithm in this technology enables alignment of moving objects before averaging, such that more consecutive images can be averaged to reduce noise more significantly.
In addition, a spatial noise reduction algorithm uses the random nature of noise to discern between noise and useful clinical information in a single image. When a pixel is identified as noise, its intensity is averaged with surrounding pixels in order to filter it out. Thanks to the powerful computational power, a larger neighborhood of pixels can be averaged, providing more noise reduction and increasing the confidence in the clinical information to maintain in the image [26] ; [27]. Finally, image enhancement processing reduces low frequency over- and under-exposed areas and enhances high frequency edges in order to sharpen contrast. The image enhancement enabled by these features made possible a further optimization of the X-ray acquisition chain; this was modified to achieve patient radiation dose reduction for both pediatric and adult population for fluoroscopy and cine exposure. The maximum patient entrance dose for fluoroscopy was reduced from 22 mGy/min, 44 mGy/min and 44 mGy/min in the reference system to 7 mGy/min, 12 mGy/min and 22 mGy/min in the new system for the fluoroscopy modes I, II and III respectively, with the same relative reduction over the entire patient thickness range. For the pediatric group, two cine applications are introduced based on patient weight: a) below 40 kg and b) above 40 kg. The below 40 kg group uses a small focal spot (0.4) to increase the sharpness of images. In addition, increase in copper filtration was implemented (0.4 mm Cu and 1.0 mm Al) compared to the reference pediatric cine acquisition chain (0.0 mm Cu and 0.0 mm Al). The practical aspects of adding copper filtration to reduce patient radiation dose have been already studied by other authors [13] ; [52]. Photons with lower energy do not contribute to the image quality, as they can't penetrate the patients body, but add needless radiation dose to the patient. Additional copper filtration enables the reduction of these photons, with consequent patient radiation dose reduction.
In conclusion, hardware imaging components such as copper filtration, power, pulse width and duration, and focal spot size enter a virtuous cycle with advanced and real-time image processing features in order to optimize the dose to image quality relationship for each clinical application.
2.3. Statistical analysis
Patient radiation dose was quantified as (cumulative) dose area product (DAP), measured by the internal transmission ionization chamber (KermaX plus; IBADosimetry, Schwarzenbruck, Germany) and displayed on the equipment as mGy·cm2; this was automatically converted into cGy·cm2 for documentation purposes. In addition, acquisition parameters, such as fluoroscopy time, procedure time, and volume of contrast medium were collected. The primary outcome of the study was radiation dose quantified as DAP. Secondary outcomes were the fluoroscopy time, procedure duration, and amount of contrast medium. These secondary outcomes were used as surrogates for case complexity, in order to exclude this as a potential incidental difference between the groups that could explain dose differences. The study hypotheses were that significant procedural dose reductions were achieved using the new technology in all three weight groups.
Descriptive statistics were used to describe patient and procedure characteristics, with differences between reference and new systems evaluated with a nonparametric t-test (assuming no Gaussian distribution) at a significance level of alpha = 0.05.
2.4. Subgroup analysis for ASD patients
Based on the absolute numbers, the timeframe of the analysis and the large variation in procedures performed, a comprehensive subgroup analysis per procedure type was not possible. However, as an illustrative case example, we performed a sub-analysis for patients with one of the most common procedures, interventional ASD closure. The protocol for ASD closure is very uniform and without relevant variations other than operator skills. This includes single plane fluoroscopy for the procedure and standard biplane cine angiography acquisitions for specific parts of the procedure such as documentation of the balloon sizing, the wiggle maneuver, the release process as well as a final documentation of the device position. All ASD closures are considered as technically simple interventions; due to the educational character of our institution, ASD closures are performed by registrars in training for pediatric cardiology or junior fellows as first operators with an experienced consultant supporting.
2.5. Standardizing dose reporting
Efforts were undertaken in this ASD analysis to make the large variability in procedures performed as well as in patients treated intuitively comparable. It is of no discussion that longer procedure times will expose patients to larger radiation doses; at the same time larger patients, i.e. those with a higher body weight and body mass index (BMI) will require larger radiation doses for the same procedure. Whereas a number of other important parameters also influence dose levels (such as projection angles), and whereas fluoroscopy time does not include cine exposure, these two factors were taken into account in a sub-analysis calculating dose exposure as radiation dose per weight, fluoroscopy time, and weight × fluoroscopy time [2]; [13] ; [20].
3. Results
3.1. Patients
In total, the complete data set of 667 consecutive patients with congenital heart disease were prospectively collected and retrospectively analyzed. There were 18 patients excluded from the dose reduction analysis because they were treated only with echocardiography or under direct vision as intraoperative hybrid procedure (13 patients from the < 10 kg group, 5 patients from the 10–40 kg group). Therefore, 649 patients (328 for the reference system and 321 for the new system) were included in the analysis.
Table 1 shows the distribution of patients among the three weight groups for both reference and new systems. The distribution between reference and new systems among the weight groups was similar, with a higher number of interventional than diagnostic procedures for all weight groups.
| ≤ 10 kg group | 10–40 kg group | > 40 kg group | ||||
|---|---|---|---|---|---|---|
| (n = 189) | (n = 234) | (n = 226) | ||||
| Reference system | New system | Reference system | New system | Reference system | New system | |
| Procedure type | ||||||
| Diagnostic + intervention | 100 | 89 | 119 | 115 | 109 | 117 |
| Diagnostic | 39 | 40 | 38 | 37 | 38 | 39 |
| Intervention | 61 | 49 | 81 | 78 | 71 | 78 |
The patient characteristics are listed in Table 2. The mean weight for all patients included in the analysis was 32.1 kg (SD: 27.6) and the BMI was 17.8 kg/m2 (SD: 5.0) for diagnostic and interventional procedures. In general there were no clinically important or statistically significant differences in the group size and distribution of weight or BMI between the Reference and New groups in any of the three weight subgroups or the subsequent variation regarding interventional procedures or diagnostic catheters.
| All patients | ≤ 10 kg group | 10–40 kg group | > 40 kg group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ref and New system | Ref system | New system | p value | Ref system | New system | p value | Ref system | New system | p value | |
| (n = 649) | (n = 100) | (n = 89) | (n = 119) | (n = 115) | (n = 109) | (n = 117) | ||||
| Weight [kg] | ||||||||||
| Mean (SD) | 32.1 (27.6) | 6.0 (1.9) | 6.3 (2.2) | 0.352 | 20.6 (8.5) | 20.6 (7.4) | 0.478 | 68.0 (19.5) | 63.6 (13.9) | 0.258 |
| Median | 19.0 | 6.0 | 6.1 | 17.0 | 18.0 | 62.0 | 64.0 | |||
| Min, Max | 2.2, 135.0 | 2.3, 10.0 | 2.2, 10.0 | 10.3, 39.0 | 11.0, 40.0 | 41.0, 135.0 | 42.0, 116.2 | |||
| Q1, Q3 | 8.70, 55.00 | 4.4, 7.5 | 4.7, 8.0 | 14.0, 27.2 | 15.0, 25.3 | 55.5, 78.0 | 53.0, 72.0 | |||
| BMI [kg/m2] | ||||||||||
| Mean (SD) | 17.8 (5.0) | 14.8 (2.6) | 14.3 (2.2) | 0.333 | 15.6 (2.3) | 15.4 (2.3) | 0.322 | 23.1 (5.2) | 22.4 (4.0) | 0.501 |
| Median | 16.4 | 14.8 | 14.2 | 15.6 | 15.4 | 22.0 | 21.9 | |||
| Min, Max | 4.8, 40.1 | 9.8, 30.0 | 5.2, 19.9 | 4.8, 21.9 | 7.1, 21.5 | 14.7, 40.1 | 15.2, 34.7 | |||
| Q1, Q3 | 14.5, 20.1 | 13.3, 16.1 | 13.3, 15.5 | 14.4,16.9 | 14.2, 16.4 | 19.7, 25.7 | 19.6, 24.6 | |||
- Ref system = Reference system, SD = Standard deviation, BMI = Body mass index.
3.2. Procedure characteristics
Table 3 shows the procedure characteristics for all patients in the three weight groups. Again, there were no statistically significant differences in fluoroscopy time between the reference and the new systems for the three weight groups, nor in procedure time and contrast medium for the < 10 kg and > 40 kg groups. However, for the 10–40 kg group, the procedure time was significantly lower (p = 0.028) for the new system while the parameters affecting X-ray radiation such as volume of contrast medium as well as fluoroscopy time were similar between the two systems.
| All patients | ≤ 10 kg group | 10–40 kg group | > 40 kg group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ref and New system | Ref system | New system | p value | Ref system | New system | p value | Ref system | New system | p value | |
| (n = 649) | (n = 100) | (n = 89) | (n = 119) | (n = 115) | (n = 109) | (n = 117) | ||||
| Procedure time [min] | ||||||||||
| Mean (SD) | 83.0 (50.8) | 78.0 (47.3) | 68.6 (34.7) | 0.227 | 92.3 (53.3) | 81.0 (50.7) | 0.029 | 89.3 (59.0) | 85.1 (51.0) | 0.787 |
| Median | 71.0 | 66.0 | 66.0 | 82.0 | 68.0 | 73.0 | 75.0 | |||
| Min, Max | 2.0, 392.0 | 22.0, 356.0 | 17.0, 177.0 | 2.0, 278.0 | 15.0, 270.0 | 4.0, 392.0 | 4.1, 255.0 | |||
| Q1, Q3 | 48.0, 103.0 | 48.0, 96.0 | 44.0, 86.0 | 53.5, 119.5 | 47.0, 101.5 | 53.0, 110.0 | 47.0, 110.0 | |||
| Fluoro time [min] | ||||||||||
| Mean (SD) | 13.9 (15.4) | 10.8 (8.1) | 13.2 (26.6) | 0.092 | 14.8 (13.1) | 12.4 (10.2) | 0.231 | 16.3 (17.4) | 15.2 (12.5) | 0.932 |
| Median | 9.8 | 9.4 | 7.1 | 10.3 | 9.4 | 10.7 | 11.7 | |||
| Min, Max | 0.1, 239.0 | 0.4, 56.0 | 0.1, 239.0 | 0.3, 76.5 | 1.2, 59.6 | 0.2, 119.2 | 0.1, 54.9 | |||
| Q1, Q3 | 5.7, 17.3 | 5.6, 13.7 | 3.8, 13.5 | 6.4, 19.8 | 5.8, 16.3 | 7.1, 18.1 | 6.2, 22.5 | |||
| CMV [ml] | ||||||||||
| n | 578 | 99 | 88 | 107 | 96 | 92 | 96 | |||
| Mean (SD) | 85.7 (66.5) | 39.1 (20.7) | 45.0 (46.6) | 0.717 | 94.5 (57.7) | 81.7 (42.5) | 0.145 | 124.2 (79.4) | 128.6 (75.3) | 0.652 |
| Median | 70.0 | 35.0 | 36.0 | 81.0 | 75.5 | 107.0 | 112.0 | |||
| Min, Max | 1.0, 495.0 | 6.0, 132.0 | 1.0, 416.0 | 10.0, 370.0 | 7.0, 210.0 | 5.0, 495.0 | 5.0, 357.0 | |||
| Q1, Q3 | 40.0, 110.0 | 24.0, 50.5 | 25.0, 51.8 | 60.0, 115.0 | 50.0, 109.3 | 70.8, 161.0 | 79.0, 170.5 | |||
- Ref = Reference system, SD = Standard deviation, CMV = contrast medium volume.
For diagnostic and intervention procedures separately, there were no differences between the new and reference systems for the below 10 kg and over 40 kg groups in terms of fluoroscopy time, procedure time and contrast. However for the 10–40 kg group, the contrast medium reaches significant difference with a higher volume of contrast used with the old system (median 80.5 ml) as compared to the new system (median 56.5 ml) in the diagnostic group (p = 0.018).
3.3. Radiation exposure
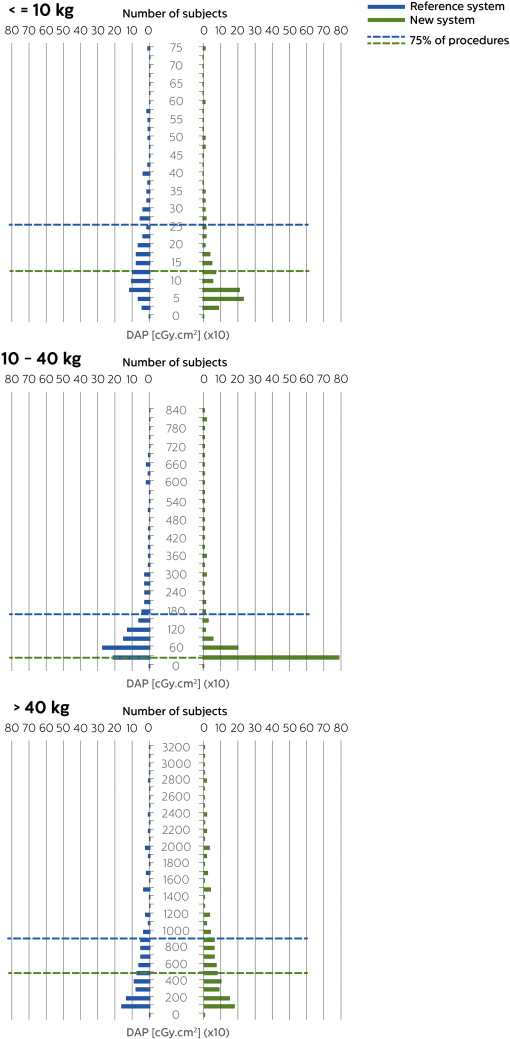
Median DAP values decreased with the new system from 140.6 cGy·cm2 to 60.7 cGy·cm2, from 700.0 cGy·cm2 to 202.2 cGy·cm2 and from 4490.4 cGy·cm2 to 1979.8 cGy·cm2 with reduction quantified at 57%, 71% and 56% for the below 10 kg, 10–40 kg and over 40 kg group respectively. Patient radiation dose was significantly reduced for the three groups (p < 0.0001) by using the new system. Fig. 1 shows the distribution of DAP values for the reference (left) and new system (right) for the below 10 kg, 10–40 kg and over 40 kg group respectively, for all procedures.
|
|
|
Fig. 1. Distribution of DAP values for all procedures for the < 10 kg (top), 10–40 kg (middle), > 40 kg (bottom) group for the reference system (left) and new system (right). Dashed lines represent the third quartile (Q3). For graph layout purposes, the x-axis might be limited with percent of displayed values greater than 97%. |
For diagnostic procedures the patient dose reduction was 46%, 79% and 46% while for the interventional procedures it was 61%, 64% and 61% for the below 10 kg, 10–40 kg and over 40 kg group respectively.
3.4. Subgroup analysis for ASD patients
A total number of 19 patients were referred to our cath lab for interventional occluder closure of a secundum atrial septal defect (ASD) while we were using the reference system and 31 patients while we were using the new system. Based on the relatively small absolute patient numbers, the whole cohort was analyzed without subdivision into the different weight groups. Again there were no clinically important or statistically significant differences between the patients or procedure characteristics (see Table 5). DAP decreased from 678.0 cGy·cm2 to 112.3 cGy·cm2 for the new system (reduction of 83%; p = 0.0002).
| DAP [cGy·cm2] | ||||
|---|---|---|---|---|
| Mean (SD) | Median | Min, Max | Q1, Q3 | |
| All patients | ||||
| Reference and new system (n = 649) | 2343.5 (5848.9) | 396.3 | 0.1, 71271.2 | 126.2, 1998.1 |
| ≤ 10 kg group | ||||
| Reference system (n = 100) | 189.0 (156.0) | 140.6 | 10.8, 891.7 | 82.2, 261.9 |
| New system (n = 89) | 109.1 (125.3) | 60.7 | 0.2, 739.0 | 41.0, 129.0 |
| p value | < 0.0001 | |||
| 10–40 kg group | ||||
| Reference system (n = 119) | 1649.3 (3522.7) | 700.0 | 33.9, 35042.3 | 380.8, 1688.2 |
| New system (n = 115) | 420.9 (880.9) | 202.2 | 17.4, 8011.0 | 110.4, 350.4 |
| p value | < 0.0001 | |||
| > 40 kg group | ||||
| Reference system (n = 109) | 7303.4 (10.381.6) | 4490.4 | 45.9, 71271.2 | 1680.1, 8617.6 |
| New system (n = 117) | 3840.1 (6302.6) | 1979.8 | 0.1, 45393.2 | 658.1, 4977.3 |
| p value | < 0.0001 | |||
| All patients | All weight groups | |||
|---|---|---|---|---|
| Ref and new system | Ref system | New system | p value | |
| (n = 50) | (n = 19) | (n = 31) | ||
| Weight [kg] | ||||
| Mean (SD) | 35.7 (21.7) | 35.7 (22.7) | 35.7 (21.4) | 0.604 |
| Median | 30.0 | 32.0 | 26.0 | |
| Min, Max | 10.7, 80.0 | 10.7, 80.0 | 13.0, 80.0 | |
| Q1, Q3 | 17.3, 49.8 | 14.5, 52.8 | 19.0, 48.0 | |
| BMI [kg/m2] | ||||
| Mean (SD) | 17.8 (3.7) | 17.9 (3.7) | 17.8 (3.7) | 0.849 |
| Median | 16.4 | 16.3 | 16.4 | |
| Min, Max | 12.9, 27.0 | 13.3, 24.7 | 12.9, 27.0 | |
| Q1, Q3 | 15.0, 20.6 | 15.0, 21.4 | 15.0, 20.0 | |
| Procedure time [min] | ||||
| Mean (SD) | 49.7 (22.9) | 56.6 (27.3) | 45.5 (18.9) | 0.141 |
| Median | 43.0 | 47.0 | 42.0 | |
| Min, Max | 15.0, 120.0 | 19.0, 104.0 | 15.0, 120.0 | |
| Q1, Q3 | 35.0, 56.8 | 36.5, 75.5 | 35.0, 53.5 | |
| Fluoro time [min] | ||||
| Mean (SD) | 7.3 (5.6) | 8.4 (7.0) | 6.6 (4.6) | 0.263 |
| Median | 5.7 | 6.9 | 5.5 | |
| Min, Max | 1.2, 31.8 | 1.2, 31.8 | 1.2, 24.2 | |
| Q1, Q3 | 3.9, 9.3 | 4.6, 9.0 | 3.8, 9.3 | |
| CMV [ml] | ||||
| Mean (SD) | 33.6 (26.0) | 11.5 (9.2) | 42.4 (25.5) | 0.781 |
| Median | 22.0 | 11.5 | 50.0 | |
| Min, Max | 5.0, 70.0 | 5.0, 18.0 | 10.0, 70.0 | |
| Q1, Q3 | 14.0, 55.0 | 8.3, 14.8 | 22.0, 60.0 | |
| DAP [cGy·cm2] | ||||
| Mean (SD) | 574.0 (991.6) | 1048.1 (1445.2) | 283.5 (350.9) | 0.0002 |
| Median | 273.7 | 678.0 | 112.3 | |
| Min, Max | 30.2, 6368.0 | 33.9, 6368.0 | 30.2, 1475.0 | |
| Q1, Q3 | 83.4, 694.5 | 352.0, 1014.6 | 68.3, 367.1 | |
| DAP/fluoro time [cGy·cm2/min] | ||||
| Mean (SD) | 76.9 (83.2) | 132.1 (103.0) | 43.0 (42.8) | p < 0.0001 |
| Median | 42.3 | 118.6 | 20.4 | |
| Min, Max | 9.9, 413.8 | 10.3, 413.8 | 9.9, 186.8 | |
| Q1, Q3 | 17.2, 113.6 | 55.3, 176.9 | 14.9, 59.5 | |
| DAP/weight [cGy·cm2/kg] | ||||
| Mean (SD) | 24.1 (71.9) | 49.6 (112.3) | 8.5 (14.4) | p < 0.0001 |
| Median | 7.9 | 15.9 | 4.4 | |
| Min, Max | 1.3, 505.4 | 2.3, 505.4 | 1.3, 81.9 | |
| Q1, Q3 | 3.4, 15.9 | 10.7, 38.5 | 2.9, 7.4 | |
| DAP/weight/fluoro time [cGy·cm2/kg/min] | ||||
| Mean (SD) | 2.6 (3.7) | 4.8 (5.2) | 1.2 (1.1) | p < 0.0001 |
| Median | 1.5 | 3.2 | 0.9 | |
| Min, Max | 0.2, 19.8 | 0.7, 19.8 | 0.2, 6.6 | |
| Q1, Q3 | 0.8, 2.3 | 1.7, 5.5 | 0.7, 1.5 | |
- Ref system = Reference system, SD = Standard deviation, BMI = Body mass index, CMV = Contrast medium volume, DAP = Dose area product.
As the patient group with congenital heart defects may vary substantially regarding body composition (i.e. weight, length, BMI) as well as with regard to the procedure performed (interventional vs. diagnostic, complex vs. “simple”) and finally by the time needed to perform the procedure, a more detailed analysis is needed for comparison and to estimate and judge the influence of possible radiation reduction efforts. For this group extra efforts were undertaken to standardize the radiation dose according to patient (i.e. weight) and procedure characteristics (i.e. fluoroscopy time). There was a significant reduction of radiation dose (DAP) applied as calculated by DAP/weight (reduction 72%), DAP/fluoroscopy time (reduction 83%) and DAP/weight/fluoroscopy time (reduction 72%).
4. Discussion
The aim of the study was to quantify the patient dose reduction achieved in a patient cohort with congenital heart disease enabled by an X-ray imaging technology combining advanced real time image noise reduction with an optimized acquisition chain for fluoroscopy and exposure in interventional cardiology for the entire population size. The results confirmed a patient dose reduction of 57%, 71% and 56% for the below 10 kg, 10–40 kg and over 40 kg group.
These results are in agreement with prior literature regarding this technology in different patient populations and clinical domains. Procedural patient and staff occupational dose reduction, enabled by the technology investigated, has already been proven outside congenital heart disease interventions by comparing state-of-the-art reference to new systems. In complex ablation procedures, patient dose and physician dose were reduced by 40% and 50%, respectively [25]. In interventional neuroradiology, 75% patient radiation dose reduction was demonstrated during digital subtraction angiography (DSA) without affecting image quality [26], and 60% procedural patient dose reduction was achieved [27]. A preclinical study on a piglet model proved that DSA radiation dose for pediatrics can be reduced by a factor four without deterioration of image quality [51].
Patient radiation dose reduction is of extreme importance in patients with congenital heart disease as they are subjected to repeated procedures over the lifetime. As interventional procedures become more and more complex and procedural complexity is correlated with increasing fluoroscopy exposure time, adequate radiation reduction is of specific importance [29] ; [30].
Baysson et al. reported on an epidemiological study launched in France to provide further knowledge about the potential cancer risks with pediatric catheterization procedures in 8000 patients [6]. Even though the long-term risks of fatal malignancy following a single pediatric catheter investigation seems very low, the effect of repetitive exposure is unclear [7] ; [31]. In general, DAP values measured during pediatric catheterizations show an excellent correlation with the entrance radiation dose and skin doses measured with thermoluminescent dosemeters (TLDs) or can be transferred by calculation models to effective doses and are therefore valid to obtain a real-time measurement of the total amount of radiation used [4]; [13]; [32]; [33]; [34] ; [35]. Similar results are obtained when calculating effective doses from DAP as published by the International Commission on Radiology Protection and measuring the effects on DNA damage [8]. Indirect cancer risk estimations and direct DNA-damage data emphasize the strict radiation dose optimization in children [36]. Using Monte Carlo simulations effective and equivalent organ doses can be derived by the use of DAP in children undergoing cardiac catheterization and estimated risk of exposure-induced death values can be calculated [9]. Therefore the substantial reduction of DAP values as presented here will be helpful to reduce the cumulative radiation risk for the pediatric patients with congenital heart defects undergoing diagnostic and interventional catheter investigations as well as for the operators performing the procedures [37].
Table 6 provides a comparison in terms of patient radiation dose with literature. Unfortunately, variability in patient size and procedure types, together with the different ways of reporting results in other studies makes comparison with literature very cumbersome. For example some authors have made an attempt of providing dose reference by classifying the population in standardized age groups [16]; [34] ; [38], while others in weight groups [15]; [39]; [40] ; [41] or fluoroscopy time [42] ; [43]. Moreover, when making comparisons in terms of patient dose, procedure complexity should always be assessed. Procedure complexity should be classified based on a combination of parameters such as fluoroscopy time, procedure time and number of exposure images or amount of contrast medium used during angiography acquisitions, beside information on frame rates used for both fluoroscopy and cine acquisitions.
| Weight [kg] | Nr of patients | Fluoro time [min] | DAP [cGy·cm2] | Reference | ||
|---|---|---|---|---|---|---|
| Median | [Q1, Q3] | Median | [Q1, Q3] | |||
| Diagnostic | ||||||
| ≤ 10 kg group | ||||||
| 6.1 | 39 | 9.5 | 4.8, 12.6 | 103.9 | 82.2, 261.9 | This Study — Reference system |
| 6.0 | 40 | 6.6 | 3.5, 13.6 | 56.3 | 41.0, 129.4 | This study — New system |
| 4.5 | 242 | 26 | 743 | 433, 1433 | [16] | |
| < 5 | 15 | 9, 23 | 224 | 116, 349 | [15] | |
| 5–12.5 | 16 | 11, 26 | 418 | 272, 663 | [15] | |
| 3.6 | 510 | 14 | 8, 22 | 228 | 122, 420 | [2] |
| 9.5 | 1429 | 14 | 9, 20 | 540 | 286, 957 | [2] |
| 10–40 kg group | ||||||
| 17.0 | 38 | 8.8 | 5.2, 14.5 | 706.85 | 385.7, 1130.2 | This Study — Reference system |
| 16.4 | 37 | 7.4 | 4.6, 14.9 | 151.60 | 97.7, 237.3 | This study — New system |
| 11.9 | 134 | 26 | 1399 | 852, 2222 | [16] | |
| 23.2 | 85 | 20 | 1647 | 904, 2494 | [16] | |
| 12.5–25 | 13 | 9, 20 | 945 | 575, 1.552 | [15] | |
| 25–45 | 14 | 8, 22 | 2722 | 1.563, 4.290 | [15] | |
| 27.4 | 498 | 12 | 7, 17 | 1449 | 674, 2674 | [2] |
| > 40 kg group | ||||||
| 61.0 | 38 | 9.6 | 4.0, 14.00 | 3144.15 | 1123.4, 5766.0 | This Study — Reference system |
| 63.2 | 39 | 8.8 | 3.6, 13.6 | 1697.20 | 225.25, 3094.00 | This study — New system |
| 50.5 | 130 | 19 | 3415 | 1584, 6029 | [16] | |
| 68.7 | 212 | 25 | 8284 | 3431, 18402 | [16] | |
| 45–65 | 14 | 10, 25 | 5595 | 3432, 9071 | [15] | |
| > 65 | 16 | 10, 21 | 8959 | 4919, 14784 | [15] | |
| 58 | 314 | 11 | 7, 18 | 4006 | 1569, 8168 | [2] |
| 92 | 76 | 12 | 7, 19 | 10347 | 2811, 15874 | [2] |
| Intervention | ||||||
| ≤ 10 kg group | ||||||
| 5.8 | 61 | 9.3 | 5.9, 14.1 | 167.70 | 86.0, 276.5 | This Study — Reference system |
| 6.9 | 49 | 6.9 | 4.1, 13.5 | 65.20 | 47.0, 136.7 | This study — New system |
| < 5 | 19 | 12, 32 | 258 | 171, 367 | [15] | |
| 5–12.5 | 26 | 14.5, 41 | 656 | 346, 1380 | [15] | |
| 3.4 | 680 | 18 | 11, 31 | 276 | 118, 610 | [2] |
| 10–40 kg group | ||||||
| 17.9 | 81 | 10.5 | 6.8, 21.2 | 700 | 384.5, 1750.8 | This Study — Reference system |
| 19.0 | 78 | 10.8 | 6.3, 17.1 | 251.60 | 121.3, 397.4 | This study — New system |
| 12.5–25 | 24 | 13, 44 | 1296 | 634, 3128 | [15] | |
| 25–45 | 38 | 24, 59 | 6586 | 243, 9693 | [15] | |
| 11.2 | 2231 | 19 | 11, 32 | 737 | 336, 1541 | [2] |
| 28 | 767 | 19 | 11, 31 | 1922 | 837, 3780 | [2] |
| > 40 kg group | ||||||
| 63.0 | 71 | 12.6 | 8.3, 22.9 | 5681.40 | 1889.6, 9738.7 | This Study — Reference system |
| 64.0 | 78 | 12.80 | 7.1, 24.5 | 2223.95 | 791.6, 5826.2 | This study — New system |
| 45–65 | 24 | 16, 45 | 8514 | 3520, 14404 | [15] | |
| > 65 | 36 | 19, 51 | 15841 | 8758, 38969 | [15] | |
| 57 | 500 | 19 | 12, 31 | 5462 | 2370, 10418 | [2] |
| 88.4 | 90 | 20 | 12, 34 | 11600 | 6509, 20225 | [2] |
- DAP = Dose area product.
In accordance to other published studies reporting patient radiation doses and procedure complexity [15]; [16] ; [30], this study reports consistent fluoroscopy times used for diagnostic and interventional procedures with the two systems across the weight groups, regardless of procedure complexity.
Moreover, the application of best practices in our lab to minimize fluoroscopy times and cine images to clinically acceptable levels, according guidelines [44], provide fluoroscopy times which are lower than the one reported by others [15] ; [16], see Table 6. In light of these best practice behaviors, it is not possible to conclusively determine whether the lower radiation dose observed in our study on either system compared with literature reflects system or operator effects. Additional standardization of the radiation dose applied to patient and procedural factors may be helpful to differentiate these effects. We may suggest that previous studies used state-of-the-art systems, and could therefore be compared with our reference group, highlighting behavioral influence on dose. As the same conditions (i.e. operators, complexity, etc.) were present throughout our study, we infer that the dose reduction shown here between the reference and new systems in turn reflects only system capabilities.
4.1. ASD patients sub-analysis
We have tried to accomplish the task of standardization by analyzing the results of dose reduction and combining them with the confounding factors exposure time, weight and BMI and the combination thereof. Similar efforts were undertaken by Walters et al. who compared the DAP in patients during interventional electrophysiology and included the time factor to analyze the effects of collimation on radiation exposure, thus generating DAP per minute of fluoroscopy in adult patients [20]. As body size usually does not vary that substantially in adults, this additional calculation seemed adequate. Kobayashi recently published the results of a multicenter study by the Congenital Cardiovascular Interventional Study Consortium (CCISC) where the authors tried to standardize radiation dose reporting in the pediatric cardiac cath lab [2]. They analyzed the influence of weight as well as radiation exposure time and recommended that Kerma area product/body weight should be used as the standard in documenting radiation usage in pediatric laboratories and as a baseline to compare and evaluate strategies to lower radiation dosage in pediatric patients undergoing cardiac catheterizations. Chida and coworkes could demonstrate a good correlation between DAP and the weight of the patient as well as the weight-fluoroscopic time product indicating that body weight is important for determining radiation dose to children undergoing cardiac catheterization [10]. Similar assumptions were reported by Verghese et al. and Glatz and coworkers who could show an influence of age, weight and the type of intervention on the radiation dose measured by air Kerma or DAP [15] ; [16]. As age reflects body weight and the fluoroscopy time is influenced by the procedure type these influences must be taken into account when comparing different radiation doses of two systems.
Despite the fact that most of the procedures were performed by registrars in training, the overall procedure time (mean 49.7 min, SD 22.9 min, median 43 min) and fluoroscopy time (mean 7.3 min, SD 5.6 min, median 5.7 min) were considerably shorter than previously reported. Fischer et al. reported a single center series using the Amplatzer Septal Occluder (ASO) in 200 patients with a median fluoroscopy time of 12 min [45]. The MAGIC atrial septal defect study presented the results of an unrestricted multi-institution routine community use of the ASO for ASD closure in 458 patients; here the mean overall fluoroscopy time was 18.46 min (SD 12.10), for simple ASDs it was 17.66 (SD 11.07) and for complex cases 20.85 (SD 14.55) [46]. Similar fluoroscopy times exceeding a mean of 10 min are reported by many authors [47]; [48]; [49] ; [50].
Papadopoulou et al. assessed patient radiation doses in 16 patients during transcatheter ASD closure. The mean body weight was 28.7 kg (median 21,0) and the mean fluoro-time 22.3 min (median 16.5 min) [4]. They reported mean DAP doses of 1.071 cGy·cm2 (median 873 cGy·cm2). This is a considerably longer fluoroscopy time and higher DAP values as compared to our cohort with the new system (weight: mean 35.7 kg, median 26 kg, fluoro time: mean 6.6 min, median 5.5 min, DAP mean 283.5, median 112.3 cGy·cm2). Standardization of radiation doses allows us to reveal a dose reduction of the new system as compared to the data published by Papadopoulou of 74% [4]. When taking the different fluoroscopy times as well as the different body weight of the patients into account the dose reduction is equally evident; median dose/time: 20.4 vs 118.6 cGy·cm2/min = 83%; median dose/weight: 4.4 vs 15.9 cGy·cm2/kg = 72%; median dose/weight/fluoroscopy time 0.9 vs 3.2 cGy·cm2/kg/min = 72%.
4.2. Limitations of this study
The first potential limitation was that this study was not a prospective randomized controlled trial, but a retrospective all-comer design, whereby the two groups' data were acquired sequentially. This theoretically could introduce learning curve and bias effects. However, all data was collected in a prospective manner according to the hospitals policy of data management and based on our quality assurance program, making inadequate data acquisition extremely unlikely. In addition, all cases were performed by experienced operators, making learning curve effects also unlikely. Therefore the possibility of inadequate data acquisition seems extremely low.
Second this study does not show the possibility to adequately match patients. Nevertheless, the data presented and the number of patients treated in a consecutive way adequately represents the usual patient and case load of a state of the art cath lab with a large percentage of interventions. Therefore the mix of patients, diagnoses, treatment modalities and other possible confounding factors is unlikely to have influenced the results. In order to learn about differences in radiation across types of procedures, further investigation is warranted using a paired study design to ensure equivalent patient and intervention characteristics.
Third, the clinical image quality was not taken into account. Based on the personal experience of all operators involved, there was no clinically important difference in image quality. Based on the amount of contrast medium applied, inadequate image quality should have led to a more extensive use of contrast medium or additional fluoroscopy time to obtain additional information not available by initial standard visualization; this was not the case. In addition patient outcomes did not change therefore proving that image quality was sufficient to perform the clinical task as well as before.
Finally, the mode of analyzing the radiation dose in combination with body composition (weight, BMI) as well as radiation time for standardization may be criticized. Based on the usual and large variation of patients in the field of congenital heart defects, it seems a very logical approach to analyze the influence of possible radiation reduction efforts. Only the combination of absolute radiation dose with the confounding factors provides the capability of comparing different systems without the need of extremely large patient numbers and clearly matched pairs. In addition and in congruence with other authors, this approach may contribute as a clinically relevant surrogate of radiation exposure in this very variable patient population.
Although we showed reduced exposure in our patient population, no data is available on the potential long term consequences with respect to cancer risk. Further investigation using a longitudinal study design over the decades may be warranted. However, causal relationships between exposure at a young age and later occurrence of adverse effects would be difficult to interpret. Perhaps specific blood markers measured a few months after treatment may be an acceptable surrogate indicator of future malignancy.
5. Conclusion
In general, an increasing number of interventional catheter procedures are performed in children and patients with congenital heart defects; the justification for these interventions are evident because they are able to avoid, postpone, replace or facilitate complex cardiac surgery. The complexity of these procedures may however often result in higher radiation doses. With the introduction of the novel X-ray imaging technology to our cath lab, we could demonstrate a significant and substantial reduction of the overall radiation dose applied to the patients, quantified at 57%, 71% and 56% for the below 10 kg, 10–40 kg and over 40 kg group respectively despite no other relevant changes in the procedural approach, the complexity of the patients or procedures involved. When analyzing specific subgroups of patients or procedures (i.e. ASD closure) this effect may become even more evident. We therefore believe that these results give confidence that this technology will add substantially to the ongoing initiatives to further limit radiation exposure and thereby reduce the risk to this potentially vulnerable patient population.
Funding sources
By hospital funding only.
Conflict of interest
Maria Mauti and Cherif Sahyoun are employees of Philips Healthcare.
All other authors declare no conflict of interest.
Acknowledgments
None.
References
- [1] T.F. Feltes, E. Bacha, R.H. Beekman 3rd, J.P. Cheatham, J.A. Feinstein, A.S. Gomes, et al.; Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association; Circulation, 123 (22) (2011 Jun 7), pp. 2607–2652 http://dx.doi.org/10.1161/CIR.0b013e31821b1f10
- [2] D. Kobayashi, J. Meadows, T.J. Forbes, P. Moore, A.J. Javois, C.A. Pedra, et al.; Standardizing radiation dose reporting in the pediatric cardiac catheterization laboratory — a multicenter study by the CCISC (Congenital Cardiovascular Interventional Study Consortium); Catheter Cardiovasc Interv (2014 Feb 28) http://dx.doi.org/10.1002/ccd.25467
- [3] J.L. Georges, L. Belle, C. Ricard, S. Cattan, F. Albert, J.L. Hirsch, et al.; RAY'ACT investigators. Patient exposure to X-rays during coronary angiography and percutaneous transluminal coronary intervention: results of a multicenter national survey; Catheter Cardiovasc Interv, 83 (5) (2014 Apr 1), pp. 729–738 http://dx.doi.org/10.1002/ccd.25327
- [4] D.I. Papadopoulou, E.N. Yakoumakis, T.K. Makri, P.H. Sandilos, B.D. Thanopoulos, E.K. Georgiou; Assessment of patient radiation doses during transcatheter closure of ventricular and atrial septal defects with Amplatzer devices; Catheter Cardiovasc Interv, 65 (3) (2005 Jul), pp. 434–441
- [5] A.C. Glatz, K.S. Purrington, A. Klinger, A.R. King, J. Hellinger, X. Zhu, et al.; Cumulative exposure to medical radiation for children requiring surgery for congenital heart disease; J Pediatr, 164 (4) (2014 Apr) http://dx.doi.org/10.1016/j.jpeds.2013.10.074 [789-794.e10]
- [6] H. Baysson, J.L. Réhel, Y. Boudjemline, J. Petit, B. Girodon, B. Aubert, et al.; Risk of cancer associated with cardiac catheterization procedures during childhood: a cohort study in France; BMC Public Health, 13 (2013 Mar 22), p. 266 http://dx.doi.org/10.1186/1471-2458-13-266
- [7] B. Modan, L. Keinan, T. Blumstein, S. Sadetzki; Cancer following cardiac catheterization in childhood; Int J Epidemiol, 29 (3) (2000 Jun), pp. 424–428
- [8] L. Beels, K. Bacher, D. De Wolf, J. Werbrouck, H. Thierens; gamma-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks?; Circulation, 120 (19) (2009 Nov 10), pp. 1903–1909 http://dx.doi.org/10.1161/CIRCULATIONAHA.109.880385
- [9] E1. Yakoumakis, H. Kostopoulou, T. Makri, A. Dimitriadis, E. Georgiou, I. Tsalafoutas; Estimation of radiation dose and risk to children undergoing cardiac catheterization for the treatment of a congenital heart disease using Monte Carlo simulations; Pediatr Radiol, 43 (3) (2013 Mar), pp. 339–346 http://dx.doi.org/10.1007/s00247-012-2510-3
- [10] K. Chida, T. Ohno, S. Kakizaki, M. Takegawa, H. Yuuki, M. Nakada, et al.; Radiation dose to the pediatric cardiac catheterization and intervention patient; AJR Am J Roentgenol, 195 (5) (2010 Nov), pp. 1175–1179
- [11] D.J. Vince; Medical radiation to children with congenital heart disease; Can Med Assoc J, 91 (1964 Dec 26), pp. 1345–1349
- [12] K.J. Strauss; Pediatric interventional radiography equipment: safety considerations; Pediatr Radiol, 36 (Suppl. 2) (2006), pp. 126–135
- [13] K. Bacher, E. Bogaert, R. Lapere, D. De Wolf, H. Thierends; Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization; Circulation, 111 (2005), pp. 83–89
- [14] R.H. Beekman, B.W. Duncan, D.J. Hagler, T.K. Jones, J.D. Kugler, J.W. Moore, et al.; Pathways to approval of pediatric cardiac devices in the United States: challenges and solutions; Pediatrics, 124 (2009), pp. e155–e162
- [15] A.C. Glatz, A. Patel, X. Zhu, Y. Dori, B.D. Hanna, M.J. Gillespie, et al.; Patient radiation exposure in a modern, large-volume, pediatric cardiac catheterization laboratory; Pediatr Cardiol, 35 (5) (2014 Jun), pp. 870–878
- [16] G.R. Verghese, D.B. McElhinney, K.J. Strauss, L. Bergersen; Characterization of radiation exposure and effect of a radiation monitoring policy in a large volume pediatric cardiac catheterization lab; Catheter Cardiovasc Interv, 79 (2012), pp. 294–301
- [17] K.J. Strauss, S.C. Kaste; The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients — a white paper executive summary; Pediatr Radiol, 36 (Suppl. 2) (2006), pp. 110–112
- [18] B.G. Smith, S.M. Tibby, S.A. Qureshi, E. Rosenthal, T. Krasemann; Quantification of temporal, procedural, and hardware-related factors influencing radiation exposure during pediatric cardiac catheterization; Catheter Cardiovasc Interv, 80 (6) (2012 Nov 15), pp. 931–936
- [19] N.J. Sutton, J. Lamour, L.A. Gellis, R.H. Pass; Pediatric patient radiation dosage during endomyocardial biopsies and right heart catheterization using a standard “ALARA” radiation reduction protocol in the modern fluoroscopic era; Catheter Cardiovasc Interv, 83 (1) (2014 Jan 1), pp. 80–83 http://dx.doi.org/10.1002/ccd.25058
- [20] T.E. Walters, P.M. Kistler, J.B. Morton, P.B. Sparks, K. Halloran, J.M. Kalman; Impact of collimation on radiation exposure during interventional electrophysiology; Europace, 14 (11) (2012 Nov), pp. 1670–1673 http://dx.doi.org/10.1093/europace/eus095
- [21] P. Arasaratnam, H.H. Ho, W. Low, N. Wilkinson, D. Foo, P.J. Ong; Radiation dose surveillance using a novel automated, remote-site dose monitoring tool in cardiac catheterization laboratory: a feasibility study; Int J Cardiol, 167 (5) (2013 Sep 1), pp. 2338–2339 http://dx.doi.org/10.1016/j.ijcard.2012.11.007
- [22] C.E. Chambers, K.A. Fetterly, R. Holzer, P.J. Lin, J.C. Blankenship, S. Balter, et al.; Radiation safety program for the cardiac catheterization laboratory; Catheter Cardiovasc Interv, 77 (4) (2011 Mar 1), pp. 546–556
- [23] L.A. Gellis, S.R. Ceresnak, G.J. Gates, L. Nappo, R.H. Pass; Reducing patient radiation dosage during pediatric SVT ablations using an “ALARA” radiation reduction protocol in the modern fluoroscopic era; Pacing Clin Electrophysiol, 36 (6) (2013 Jun), pp. 688–694
- [24] J.M. Sawdy, T.M. Kempton, V. Olshove, M. Gocha, J.L. Chisolm, S.L. Hill, et al.; Use of a dose-dependent follow-up protocol and mechanisms to reduce patients and staff radiation exposure in congenital and structural interventions. Catheter Cardiovasc Interv. 2011 Jul 1;78(1):136-42; (Erratum in) Catheter Cardiovasc Interv, 78 (3) (2011 Sep 1), p. 491
- [25] L.R.C. Dekker, P.H. van der Voort, T.A. Simmers, X.A.A.M. Verbeek, R.W.M. Bullens, M. van't Veer, et al.; New image processing and noise reduction technology allows reduction of radiation exposure in complex electrophysiologic interventions while maintaining optimal image quality: a randomized clinical trial; Heart Rhythm, 10 (11) (2013 Nov), pp. 1678–1682
- [26] M. Söderman, S. Holmin, T. Andersson, C. Palmgren, D. Babić, B. Hoornaert; Clinical results with an image noise reduction algorithm for digital subtraction angiography; Radiology, 269 (2) (2013 Nov), pp. 553–560
- [27] M. Soderman, M. Mauti, S. Boon, A. Omar, M. Marteinsdóttir, T. Andersson, et al.; Radiation dose in neuroangiography using image noise reduction technology: a population study based on 614 patients; Neuroradiology, 55 (2013), pp. 1365–1372
- [28] A. Hanslik, A. Moysich, K.T. Laser, E. Mlczoch, D. Kececioglu, N.A. Haas; Percutaneous closure of atrial septal defects in spontaneously breathing children under deep sedation: a feasible and safe concept; Pediatr Cardiol, 35 (2) (2014 Feb), pp. 215–222
- [29] M.H. El Sayed, A.M. Roushdy, H. El Farghaly, A. El Sherbini; Radiation exposure in children during the current era of pediatric cardiac intervention; Pediatr Cardiol, 33 (1) (2012 Jan), pp. 27–35
- [30] L. Bergersen, K. Gauvreau, D. McElhinney, S. Fenwick, D. Kirshner, J. Harding, et al.; Capture of complexity of specialty care in pediatric cardiology by work RVU measures; Pediatrics, 131 (2) (2013 Feb), pp. 258–267
- [31] M.A. Clay, R.M. Campbell, M. Strieper, P.A. Frias, M. Stevens, W.T. Mahle; Long-term risk of fatal malignancy following pediatric radiofrequency ablation; Am J Cardiol, 102 (7) (2008 Oct 1), pp. 913–915
- [32] D. Papadopoulou, E. Yakoumakis, P. Sandilos, V. Thanopoulos, T. Makri, G. Gialousis, et al.; Entrance radiation doses during paediatric cardiac catheterisations performed for diagnosis or the treatment of congenital heart disease; Radiat Prot Dosimetry, 117 (1–3) (2005), pp. 236–240
- [33] E. Bogaert, K. Bacher, K. Lemmens, M. Carlier, W. Desmet, X. De Wagter, et al.; A large-scale multicentre study of patient skin doses in interventional cardiology: dose-area product action levels and dose reference levels; Br J Radiol, 82 (976) (2009 Apr), pp. 303–312
- [34] O. Dragusin, M. Gewillig, W. Desmet, K. Smans, L. Struelens, H. Bosmans; Radiat Prot Dosimetry, 129 (1–3) (2008), pp. 91–95
- [35] B. Herron, J. Strain, T. Fagan, L. Wright, H. Shockley; X-ray dose from pediatric cardiac catheterization: a comparison of materials and methods for measurement or calculation; Pediatr Cardiol, 31 (8) (2010 Nov), pp. 1157–1161
- [36] L. Ait-Ali, M.G. Andreassi, I. Foffa, I. Spadoni, E. Vano, E. Picano; Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease; Heart, 96 (4) (2010 Feb), pp. 269–274
- [37] K.P. Kim, D.L. Miller, S. Balter, R.A. Kleinerman, M.S. Linet, D. Kwon, et al.; Occupational radiation doses to operators performing cardiac catheterization procedures; Health Phys, 94 (3) (2008 Mar), pp. 211–227
- [38] A.N. Al-Haj, A.M. Lobriguito, W. Rafeh; Variation in radiation doses in paediatric cardiac catheterisation procedures; Radiat Prot Dosimetry, 129 (1–3) (2008), pp. 173–178
- [39] A. Boothroyd, E. McDonald, B.M. Moores, V. Sluming, H. Carthy; Radiation exposure to children during cardiac catheterization; Brit J Radiol, 70 (1997), pp. 180–185
- [40] X. Jiang, L.R.C. Dekker; Observations and considerations on patient X-ray exposure in the electrophysiology lab; Arrhythm Electrophysiol Rev, 2 (2) (2013), pp. 141–144
- [41] D.G. Onnasch, F.K. Schröder, G. Fischer, H.H. Kramer; Diagnostic reference levels and effective dose in paediatric cardiac catheterization; Br J Radiol, 80 (951) (2007 Mar), pp. 177–185
- [42] H. Iida, J. Horii, M. Chabatake, E. Taka, M. Shimizu, T. Mizushima; Evaluation and estimation of entrance skin dose in patients during diagnostic and interventional radiology procedures; Nihon Hoshasen Gijutsu Gakkai Zasshi, 60 (1) (2004 Jan), pp. 126–135
- [43] J.D. Moore, D. Shim, J. Sweet, K.L. Arheart, R.H. Beekman 3rd.; Radiation exposure to children during coil occlusion of the patent ductus arteriosus; Catheter Cardiovasc Interv, 47 (4) (1999 Aug), pp. 449–454
- [44] H. Justino; The ALARA concept in pediatric cardiac catheterization: techniques and tactics for managing radiation dose; Pediatr Radiol, 36 (Suppl. 2) (2006 Sep), pp. 146–153
- [45] G. Fischer, J. Stieh, A. Uebing, U. Hoffmann, G. Morf, H.H. Kramer; Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single centre study in 236 consecutive patients; Heart, 89 (2) (2003 Feb), pp. 199–204
- [46] A.D. Everett, J. Jennings, E. Sibinga, C. Owada, D.S. Lim, J. Cheatham, et al.; Community use of the amplatzer atrial septal defect occluder: results of the multicenter MAGIC atrial septal defect study; Pediatr Cardiol, 30 (3) (2009 Apr), pp. 240–247 http://dx.doi.org/10.1007/s00246-008-9325-x
- [47] M. Chessa, M. Carminati, G. Butera, R.M. Bini, M. Drago, L. Rosti, et al.; Early and late complications associated with transcatheter occlusion of secundum atrial septal defect; J Am Coll Cardiol, 39 (6) (2002 Mar 20), pp. 1061–1065
- [48] K. Lopez, B.V. Dalvi, D. Balzer, J.L. Bass, T. Momenah, Q.L. Cao, et al.; Transcatheter closure of large secundum atrial septal defects using the 40 mm Amplatzer septal occluder: results of an international registry; Catheter Cardiovasc Interv, 66 (4) (2005 Dec), pp. 580–584
- [49] D. Hohmann, S. Schoof, A. Wessel, H. Bertram; Mechanical support of the left atrial disc during transcatheter closure of large atrial septal defects in children; Pediatr Cardiol, 30 (4) (2009 May), pp. 513–515 http://dx.doi.org/10.1007/s00246-008-9340-y
- [50] B.R. Kannan, E. Francis, K. Sivakumar, S.R. Anil, R.K. Kumar; Transcatheter closure of very large (> or = 25 mm) atrial septal defects using the Amplatzer septal occluder; Catheter Cardiovasc Interv, 59 (4) (2003 Aug), pp. 522–527
- [51] J. Racadio, K. Strauss, T. Abruzzo, et al.; Significant dose reduction for pediatric digital subtraction angiography without impairing image quality: preclinical study in a piglet model; Am J Roentgenol, 203 (2014 Oct), pp. 904–908
- [52] K.A. Fetterly; Investigation of the practical aspects of an additional 0.1 mm copper x-ray spectral filter for cine acquisition mode imaging in a clinical care setting; Health Phys, 99 (5) (2010 Nov), pp. 624–630
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?