Highlights
- Novel data on the neurobiological effect of graphic cigarette warning labels (GWLs)
- GWLs produced activation indicating emotional and cognitive decision-making.
- Activation in response to GWLs also indicates memory formation.
- No differences in neural activity for GWLs on branded or plain cigarette packages
Abstract
Introduction
The study examined young adult smokers' neural response to graphic warning labels (GWLs) on cigarette packs using functional magnetic resonance imaging (fMRI).
Methods
Nineteen young adult smokers (M age 22.9, 52.6% male, 68.4% non-white, M 4.3 cigarettes/day) completed pre-scan, self-report measures of demographics, cigarette smoking behavior, and nicotine dependence, and an fMRI scanning session. During the scanning session participants viewed cigarette pack images (total 64 stimuli, viewed 4 s each) that varied based on the warning label (graphic or visually occluded control) and pack branding (branded or plain packaging) in an event-related experimental design. Participants reported motivation to quit (MTQ) in response to each image using a push-button control. Whole-brain blood oxygenation level-dependent (BOLD) functional images were acquired during the task.
Results
GWLs produced significantly greater self-reported MTQ than control warnings (p < .001). Imaging data indicate stronger neural activation in response to GWLs than the control warnings at a cluster-corrected threshold p < .001 in medial prefrontal cortex, amygdala, medial temporal lobe, and occipital cortex. There were no significant differences in response to warnings on branded versus plain cigarette packages.
Conclusions
In this sample of young adult smokers, GWLs promoted neural activation in brain regions involved in cognitive and affective decision-making and memory formation and the effects of GWLs did not differ on branded or plain cigarette packaging. These findings complement other recent neuroimaging GWL studies conducted with older adult smokers and with adolescents by demonstrating similar patterns of neural activation in response to GWLs among young adult smokers.
Keywords
Graphic warning label;Cigarettes;Neuroimaging;Young adults
1. Introduction
Graphic warning labels (GWLs) for cigarette packs have been implemented in more than 65 countries (Sanders-Jackson, Song, Hiilamo, & Glantz, 2013) based on evidence that they are more effective than text-only warnings for reducing smoking (Noar et al., 2015). Research can continue to inform GWL implementation in at least two important ways. Studies investigating optimal approaches to designing GWL messages can inform changes to GWLs to ensure sustained effectiveness. In contexts such as the U.S. where law requires GWLs (U.S. Congress, 2009) but lawsuits have delayed their implementation, research addressing concerns raised by the courts can support implementation (Kraemer & Baig, 2013).
Studies investigating GWLs have relied largely on self-report methods, demonstrating that GWLs generate stronger cognitive and emotional responses, are better recalled, and produce stronger motivation to quit smoking than text-only warnings (Azagba and Sharaf, 2013; Borland et al., 2009; Emery et al., 2014; Hammond et al., 2006; Nonnemaker et al., 2015 ; Peters et al., 2007). However, self-report measures of such constructs do not fully predict future behavior, and biobehavioral methods may help better understand GWL impact (Armitage et al., 2015; Falk et al., 2011 ; Webb and Sheeran, 2006).
Functional magnetic resonance imaging (fMRI) can ascertain information on smokers' responses to GWLs that is not readily captured by self-report (Falk, 2010). fMRI-measured neural activity in brain regions involved in emotional (i.e., amygdala) and cognitive (i.e., medial prefrontal cortex) processing of antismoking messages predicts cessation outcomes, explaining ≥ 20% additional variance in cessation behavior than self-report responses to messages (Chua et al., 2011; Falk et al., 2010; Falk et al., 2011; Jasinska et al., 2012 ; Wang et al., 2013). Two studies also showed that GWLs produce activation in brain regions involved with emotion, cognition, and memory formation among current smokers (Newman-Norlund et al., 2014 ; Wang et al., 2015). Other research links frontoinsular neural activity to craving reduction in response to GWLs (Do & Galvan, 2015) and demonstrates that neural responses in similar brain systems implicated in motivation, cognition, and memory are associated with population-level success of GWL-type messages for promoting cessation (Falk, O'Donnell, Tompson, et al., 2016).
This study extends this research by investigating young adult smokers' neural responses to GWLs and assessing whether effects differ by branded or plain cigarette packaging. Studies of neural responses to GWLs have been conducted with older adult smokers (Newman-Norlund et al., 2014 ; Wang et al., 2015) and adolescents (Do & Galvan, 2015). However, young adults are a priority for tobacco control due to high rates of smoking experimentation, frequent transitions to regular smoking, and the high prevalence of smoking in this group (Do and Galvan, 2015 ; Falk et al., 2016). Plain packaging is hypothesized to draw greater attention to and increase the effects of GWLs by eliminating tobacco industry branding, but this has not yet been tested using a neuroimaging paradigm. Examining young adult smokers' neural response to GWLs on branded and plain packaging can extend the evidence surrounding potential mechanisms of GWL action and inform future research and policy.
2. Methods
2.1. Participants and procedures
Participants were recruited through online and community-based advertisements and screened for eligibility. Eligible participants were ages 18 to 30 years, current smokers defined using validated epidemiologic measures and criteria as smoking ≥ 100 lifetime cigarettes and now smoking cigarettes all or some days (Agaku, King, Husten, et al., 2014). Participants also reported Camel, Marlboro, or Newport as their preferred cigarette brand. The latter criterion was imposed to tailor experimental stimuli to smokers' preferred brand, described below. All participants also met fMRI safety requirements (Kanal, Borgstede, Barkovich, et al., 2002). Eligible participants were scheduled for an in-person appointment to provide informed consent and complete a pre-scan, self-report assessment and fMRI scanning session. Prior to the appointment, participants were instructed to smoke as they normally would that day. All participants provided written informed consent, and all procedures were approved by an institutional review board.
2.2. Pre-scan measures
Pre-scan measures included demographics, cigarette smoking behaviors (Agaku et al., 2014), nicotine dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), and motivation to quit smoking (Mays et al., 2015a ; Mays et al., 2015b).
2.3. Experimental design
The study employed a two (graphic warning or control) by two (branded or plain cigarette pack) within-subjects design. Stimuli were adapted from a prior experiment (Mays et al., 2015a ; Mays et al., 2015b). GWLs tested were four of the warnings proposed for use in the U.S. by the Food and Drug Administration (FDA) communicating the smoking-associated risks of lung disease, cancer, stroke/heart attack, and mortality. These four warnings have been effective at eliciting cognitive and emotional responses in prior studies with young adults (Cameron et al., 2015 ; Hammond et al., 2013). Similar to another recent study (Wang et al., 2015), control warnings included the same warning text as GWLs but were composed of geometric shapes overlayed on the GWLs to produce a similar appearance while visually occluding graphic content.
GWLs and control warnings were displayed on cigarette pack images sized to the dimensions of a standard cigarette pack. The pack brand (Camel, Marlboro, or Newport) was tailored to smokers' preferred brand to account for brand preferences within the design (Bansal-Travers, Hammond, Smith, & Cummings, 2011). Branded packs were created using pack images available from an online database at the time of the study (Tobacco Labelling Resource Centre, n.d.). Plain packs displayed the brand name in standard font and were brown in color and stripped of all branding (Mays et al., 2015a ; Mays et al., 2015b). Stimuli were presented in randomized order such that the same warning did not appear consecutively and there were no more than two consecutive repeats from the same condition. Example GWL and control warnings are shown in Fig. 1; complete stimuli including pack images are available from the corresponding author.
|
|
|
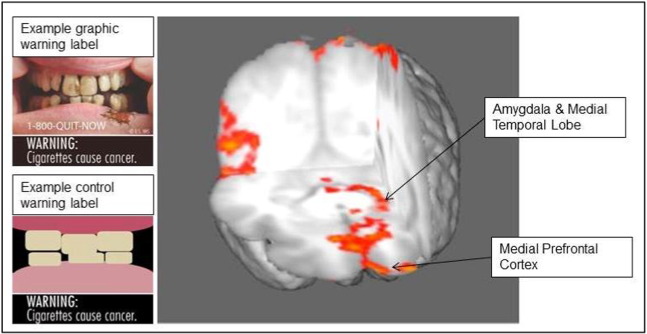
Fig. 1. Brain regions with graphic warning label activation > control at cluster corrected threshold of p < .001. Note: Brain regions based on contrast of graphic warning label > control at cluster corrected threshold of p < .001. Activity in medial temporal lobe, medial prefrontal cortex, amygdala, and occipital cortex was significantly greater in response to pack images displaying graphic warning labels than pack images displaying visually occluded control warnings. |
Participants viewed each pack image in the scanner for 4 s. During the scan participants used a push-button control to report how much each image motivated them to quit smoking, with response options from (1) Not At All to (4) A Lot (Mays et al., 2015a ; Mays et al., 2015b).
2.4. Imaging data acquisition
Functional data were acquired in an event-related paradigm performed using a 3-T Allegra System (Siemens, Erlangen, Germany) to collect whole-brain T2*-weighted blood oxygenation level dependent (BOLD) functional images (asymmetric spin-echo echo-planar sequence; whole-brain repetition time, TR = 2000 ms; echo time = 25 ms; field of view = 256 mm; flip angle = 80°; matrix = 64 × 64; axial slices 4 mm thick). Sequential whole-brain volumes (32 contiguous slices) were collected during one event-related functional run. Sixty-four task trials were presented in total, lasting 4 s each with “jitter” interleaved between trials across a range from 250 to 4250 ms. The scanning run began with an unanalyzed fixation period equal to 3 TRs, which allowed the scanner to reach steady state.
2.5. Statistical analyses
fMRI data processing was carried out using FEAT (fMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIBs Software Library) (FSL, n.d.). General Linear Model-based analysis in FEAT uses FSL tools including Brain Extraction Tool (BET) (Smith, 2002), an affine registration tool, FMRIBs Linear Image Registration Tool (FLIRT) (Jenkinson et al., 2002 ; Jenkinson and Smith, 2001), and a motion-correction tool based on FLIRT (MCFLIRT) (Jenkinson et al., 2002). FEAT carries out standard-space registration after time-series statistics. FSL time-series statistics correct for temporal smoothness by applying pre-whitening (Woolrich, Ripley, Brady, & Smith, 2001). The following pre-statistics processing was applied: spatial smoothing using a Gaussian kernel of FWHM 5 mm; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). Registration to high resolution structural and, subsequently, standard space images was performed using FLIRT. At the individual subjects level, a design matrix was fitted to each subjects data as part of a general linear model with each condition modeled as events with a specified duration (i.e., the time from stimulus onset to onset of the response) convolved with a canonical hemodynamic response function. Higher-level analysis was performed using FMRIBs Local Analysis of Mixed Effects (Beckmann, Jenkinson, & Smith, 2003). Z (Gaussianized T/F) statistic images were thresholded using an exploratory cluster-corrected threshold of p < 0.001.
3. Results
Participants (n = 19) averaged 22.9 years of age (SD 3.3), smoked on average 4.3 (SD 2.5, range 0–10) cigarettes on a typical day, averaged 3.1 (SD 1.1, range 2–6) on the FTND, and 68.4% reported using at least one other tobacco product (e.g., electronic cigarettes, hookah) in the past 30 days (Supplementary Table 1). Overall, 52.6% of participants were male, and 68.4% were non-white race/ethnicity (Supplementary Table 1). Self-report motivation to quit smoking during the imaging task was significantly greater for GWLs (M 3.25, SD 0.65) than the control warnings (M 1.96, SD 0.79, t(18) = 8.15, p < .001).
Results of the whole-brain functional imaging analysis contrasting neural activity in response to GWLs versus control warnings are shown in Table 1. Compared to control warnings, there was significantly greater activation in response to GWLs in the left medial frontal gyrus, right middle occipital gyrus, right orbital gyrus, left parietal precuneus, areas of left medial temporal cortex, specifically, left parahippocampal gyrus (extending to hippocampus), and left amygdala (Table 1). Activation in two of these regions (medial prefrontal cortex, and amygdala; see Fig. 1) was of a priori interest based on their respective roles in emotional and cognitive decision-making (Falk et al., 2010; Falk et al., 2011; Jasinska et al., 2012; Newman-Norlund et al., 2014 ; Wang et al., 2015). Analyses were thus conducted for these regions using a priori-defined regions of interest (ROIs) using anatomical probabilistic atlas-derived masks and images from the Harvard-Oxford cortical atlas within the FSL suite. An additional post-hoc, exploratory analysis was conducted using the same ROI approach for medial temporal activity using an atlas-defined hippocampus + parahippocampus region. Activation averaged across voxels was extracted for each ROI for the Warning Labels > Control contrast. Prior research also suggests that activity in these brain regions is associated with tobacco-related decision-making, including cessation behavior (Falk et al., 2010; Falk et al., 2011; Jasinska et al., 2012; Newman-Norlund et al., 2014 ; Wang et al., 2015). To test this possibility, we conducted exploratory analyses examining whether activity in these three ROIs was correlated with in-scanner, self-reported motivation to quit in response to the pack images during the task. A multiple regression model using extracted activation for these three ROIs as regressors indicated that differences in amygdala activity in response to GWLs versus control warnings was significantly associated with in scanner-ratings of how much the warnings motivated them to quit smoking (β = .72, t(15) = 3.00, p = .009). The medial prefrontal and medial temporal ROI regressors did not reach significance in this model.
| Anatomical region | Brodmann area | z | Peak Talairach coordinates | Voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left medial frontal gyrus | 10 | 3.96 | − 4 | 54 | 14 | 43 |
| Right middle occipital gyrus | 18 | 3.80 | 30 | − 93 | 6 | 29 |
| Right orbital gyrus | 11 | 3.49 | 5 | 40 | − 22 | 52 |
| Left parietal precuneus | 31 | 3.43 | − 10 | − 54 | 30 | 16 |
| Left parahippocampal gyrus | 34 | 3.36 | − 26 | 5 | − 18 | 19 |
| Left amygdala | NA | 3.29 | − 24 | − 4 | − 22 | 11 |
There were no significant differences in neural activation between branded or plain packs, and the interaction between warning label (graphic or control) and pack branding (plain or branded) was not significant. In sensitivity analyses using imaging data from branded packs only (data not shown), findings reported for GWLs versus control warnings were consistent.
4. Discussion
Among young adult smokers GWLs produced greater activation in brain regions involved in cognitive and emotional decision-making and memory formation compared with visually occluded control warnings. These findings are consistent with other investigations, converging on potential neurobiological mechanisms that may underlie behavioral response to graphic content in GWLs.
That GWLs produced greater amygdala activation is consistent with research with adult smokers indicating highly emotionally salient GWLs, similar to those tested, elicit the strongest amygdala response (Newman-Norlund et al., 2014 ; Wang et al., 2015). Similar responses to GWLs have been associated with craving reduction post-exposure (Do & Galvan, 2015), and greater amygdala activation to anti-smoking messages has predicted cessation (Jasinska et al., 2012). Our correlational analysis of ROI activity with in-scanner, self-reported motivation to quit in response to GWLs provides additional support for this idea, indicating that greater amygdala activity in response to GWLs is correlated with stronger self-reported motivation to quit smoking. The consistency of these observations with general studies of emotional response to high-arousal images (Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012) also suggests that emotional content of GWLs is not being eliminated by young smokers, who may be motivated to disregard anti-smoking messages through counter-arguing or other defensive mechanisms.
That GWLs activated medial temporal regions including the hippocampus is congruent with evidence indicating that emotionally salient GWLs generate the strongest hippocampal activation and are better recalled by adult smokers (Newman-Norlund et al., 2014 ; Wang et al., 2015). The hippocampus plays a critical role in memory formation, and the amygdala is within a network of brain regions thought to mediate encoding of emotional stimuli (Wang et al., 2015). This suggests a cognitive-affective neurobiological pathway through which GWL messages are encoded, extending self-report evidence on GWL effects (Noar et al., 2015).
We also found greater activation in response to GWLs in areas of the medial prefrontal cortex that are involved in self-related processing and positive valuation of external stimuli (Bartra et al., 2013 ; Denny et al., 2012). In prior studies, medial prefrontal activation in response to smoking cessation messages is a stronger predictor of future cessation behavior than self-report responses to such messages (Chua et al., 2011 ; Falk et al., 2011). Similarly, research also indicates that medial prefrontal activation in response to GWL-type images on public health messages is a stronger predictor of population-level effects of these messages for promoting cessation (Falk et al., 2016) than self-report responses. Although activity in the medial prefrontal and medial temporal ROIs was not significantly correlated with in-scanner, self-reported motivation to quit, this may not necessarily be a strong indication that activity in these regions in response to GWLs is not associated with future behavior change. In previous studies neural activity in response to similar cessation messages, particularly in the medial prefrontal region, is a stronger predictor of future quitting behavior than self-report measures (Falk et al., 2010 ; Falk et al., 2011). Overall, our findings are consistent with the idea that GWLs may motivate cessation among young smokers by integrating affective salience and self-related processing.
We did not observe differences in neural activation to GWLs based on branded versus plain cigarette packaging. One hypothesis proposed previously is that removing branding may reduce the degree to which packs stimulate anticipated reward among smokers, decreasing neural activation in brain regions involved in reward anticipation (e.g., ventral striatum) (Martin, 2014). The lack of differences could be due to the modest sample size and insufficient statistical power to detect subtle effects, or the low levels of nicotine dependence in the sample (i.e., less craving in response to pack images to produce a clear contrast) (Munafo, Roberts, Bauld, & Leonards, 2011). These issues should be examined in future studies.
This study has important limitations. The sample included young adults who smoked on average about 5 cigarettes per day and with relatively low levels of nicotine dependence. The findings observed within this relatively heterogeneous sample of young smokers may not generalize to older, heavier smoking populations. However, as noted the results are generally consistent with recent neuroimaging GWL studies conducted with older, heavier smoking adults and adolescents, suggesting that these studies may converge on similar findings. We did not gather additional information on the sample that may have affected our findings, such as psychiatric comorbidities and detailed information on use of tobacco products other than cigarettes. The contrast of primary interest in the present study was between GWLs and visually occluded control warnings. Given our results and the related literature described herein, it appears likely that the intended featural differences between GWLs and control warnings (e.g., emotional salience, self-relevance) rather than incidental features (e.g., color or luminance) are primarily responsible for the observed regional effects. However, the possibility that non-identical incidental visual features in control vs. GWL images had some effect on our data cannot be ruled out. Finally, we did not investigate other questions germane to policy that will be important to examine in the future, such as whether varying GWL message design (e.g., level of graphicness, message content) or size (e.g., 30% of pack surface versus 50%) impacts observed neural activation. Future research that directly links such neural activation to downstream, smoking-relevant cognitions and behaviors is also important.
Despite these limitations, our findings contribute to an apparent convergence of evidence on neurobiological mechanisms involving emotional cognitive decision-making and memory formation in response to GWLs among smokers. Our study uniquely adds to this evidence by demonstrating that even in a sample of young adult smokers that may be motivated to disregard GWL messages, their effects are consistent with broader research on emotionally salient, aversive images and are evident with plain and branded packaging. These data begin to delineate a neural underlay for recent self-report investigations indicating that GWLs are an important intervention for motivating young adult smokers to quit (Cameron et al., 2015; Magnan and Cameron, 2015; Mays et al., 2015b ; Villanti et al., 2015). Future studies can advance this area of research by prospectively examining whether neural activation in response to GWLs predicts future quitting behavior and examining differences based on aspects of warning message content relevant to policy decision-making (Newman-Norlund et al., 2014 ; Wang et al., 2015).
The following are the supplementary data related to this article.
Supplementary Table 1.
Sample characteristics (n = 19).
Role of funding sources
Data collection for this study was supported in part through a contract from the Truth Initiative (formerly Legacy). Manuscript preparation was supported in part by the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) Center for Tobacco Products (CTP) under NIH grant number CA172217 (D. Mays). This work was also supported in part by the Georgetown Lombardi Comprehensive Cancer Center Support Grant under NIH grant number P30CA051008. The study sponsors had no role in the study design; in the collection, analysis and interpretation data; in the writing of the report; and in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Contributors
Authors Adam E. Green and Darren Mays designed the study, led data collection and analysis, and led writing of the manuscript. Authors Emily Falk, Natalie Gallagher, Amanda Richardson, and Raymond Niaura contributed to the study design, interpretation of the findings, and manuscript preparation. Authors Donna Vallone, Kenneth P. Tercyak, and David B. Abrams contributed to the study design and manuscript preparation. All authors have approved the final manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Acknowledgments
Findings of this research were presented in part at the 2014 Annual Meeting of the Society for Research on Nicotine and Tobacco, Philadelphia, PA. The authors thank Andrea Johnson and Sarah Murphy for their assistance with data collection.
References
- Agaku et al., 2014 I.T. Agaku, B.A. King, C.G. Husten, et al.; Tobacco product use among adults—United States, 2012–2013; MMWR. Morbidity and Mortality Weekly Report, 63 (2014), pp. 542–547 (pii: mm6325a3)
- Armitage et al., 2015 C.J. Armitage, P. Norman, S. Alganem, M. Conner; Expectations are more predictive of behavior than behavioral intentions: Evidence from two prospective studies; Annals of Behavioral Medicine, 49 (2015), pp. 239–246 http://doi.org/10.1007/s12160-014-9653-4
- Azagba and Sharaf, 2013 S. Azagba, M.F. Sharaf; The effect of graphic cigarette warning labels on smoking behavior: Evidence from the Canadian experience; Nicotine & Tobacco Research, 15 (2013), pp. 708–717 http://doi.org/10.1093/ntr/nts194
- Bansal-Travers et al., 2011 M. Bansal-Travers, D. Hammond, P. Smith, K.M. Cummings; The impact of cigarette pack design, descriptors, and warning labels on risk perception in the U.S.; American Journal of Preventive Medicine, 40 (2011), pp. 674–682 http://doi.org/10.1016/j.amepre.2011.01.021
- Bartra et al., 2013 O. Bartra, J.T. McGuire, J.W. Kable; The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value; NeuroImage, 76 (2013), pp. 412–427 http://doi.org/10.1016/j.neuroimage.2013.02.063
- Beckmann et al., 2003 C.F. Beckmann, M. Jenkinson, S.M. Smith; General multilevel linear modeling for group analysis in FMRI; NeuroImage, 20 (2003), pp. 1052–1063 http://doi.org/10.1016/S1053-8119(03)00435-X
- Borland et al., 2009 R. Borland, N. Wilson, G.T. Fong, et al.; Impact of graphic and text warnings on cigarette packs: Findings from four countries over five years; Tobacco Control, 18 (2009), pp. 358–364 http://doi.org/10.1136/tc.2008.028043
- Cameron et al., 2015 L.D. Cameron, J.K. Pepper, N.T. Brewer; Responses of young adults to graphic warning labels for cigarette packages; Tobacco Control, 24 (2015), pp. e14–e22 http://doi.org/10.1136/tobaccocontrol-2012-050645
- Chua et al., 2011 H.F. Chua, S.S. Ho, A.J. Jasinska, et al.; Self-related neural response to tailored smoking-cessation messages predicts quitting; Nature Neuroscience, 14 (2011), pp. 426–427 http://doi.org/10.1038/nn.2761
- Denny et al., 2012 B.T. Denny, H. Kober, T.D. Wager, K.N. Ochsner; A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex; Journal of Cognitive Neuroscience, 24 (2012), pp. 1742–1752 http://doi.org/10.1162/jocn_a_00233
- Do and Galvan, 2015 K.T. Do, A. Galvan; FDA cigarette warning labels lower craving and elicit frontoinsular activation in adolescent smokers; Social Cognitive and Affective Neuroscience, 10 (2015), pp. 1484–1496 http://doi.org/10.1093/scan/nsv038
- Emery et al., 2014 L.F. Emery, D. Romer, K.M. Sheerin, K.H. Jamieson, E. Peters; Affective and cognitive mediators of the impact of cigarette warning labels; Nicotine & Tobacco Research, 16 (2014), pp. 263–269 http://doi.org/10.1093/ntr/ntt124
- Falk, 2010 E.B. Falk; Communication neuroscience as a tool for health psychologists; Health Psychology, 29 (2010), pp. 355–357 http://doi.org/10.1037/a0020427
- Falk et al., 2010 E.B. Falk, E.T. Berkman, T. Mann, B. Harrison, M.D. Lieberman; Predicting persuasion-induced behavior change from the brain; The Journal of Neuroscience, 30 (2010), pp. 8421–8424 http://doi.org/10.1523/JNEUROSCI.0063-10.2010
- Falk et al., 2011 E.B. Falk, E.T. Berkman, D. Whalen, M.D. Lieberman; Neural activity during health messaging predicts reductions in smoking above and beyond self-report; Health Psychology, 30 (2011), pp. 177–185 http://doi.org/10.1037/a0022259
- Falk et al., 2016 E.B. Falk, M.B. O'Donnell, S. Tompson, et al.; Functional brain imaging predicts public health campaign success; Social Cognitive and Affective Neuroscience, 11 (2016), pp. 204–214
- FSL, n.d FSL. FMRIB Software Library v5.0. Created by the Analysis Group, FMRIB, Oxford, UK. http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/. (Accessed September 2, 2015).
- Hammond et al., 2006 D. Hammond, G.T. Fong, A. McNeill, R. Borland, K.M. Cummings; Effectiveness of cigarette warning labels in informing smokers about the risks of smoking: findings from the International Tobacco Control (ITC) Four Country Survey; Tobacco Control, 15 (Suppl. 3) (2006), pp. iii19–iii25 http://doi.org/10.1136/tc.2005.012294
- Hammond et al., 2013 D. Hammond, J.L. Reid, P. Driezen, C. Boudreau; Pictorial health warnings on cigarette packs in the United States: An experimental evaluation of the proposed FDA warnings; Nicotine & Tobacco Research, 15 (2013), pp. 93–102 http://doi.org/10.1093/ntr/nts094
- Heatherton et al., 1991 T.F. Heatherton, L.T. Kozlowski, R.C. Frecker, K.O. Fagerstrom; The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire; British Journal of Addiction, 86 (1991), pp. 1119–1127
- Jasinska et al., 2012 A.J. Jasinska, H.F. Chua, S.S. Ho, T.A. Polk, L.S. Rozek, V.J. Strecher; Amygdala response to smoking-cessation messages mediates the effects of serotonin transporter gene variation on quitting; NeuroImage, 60 (2012), pp. 766–773 http://doi.org/10.1016/j.neuroimage.2011.12.064
- Jenkinson and Smith, 2001 M. Jenkinson, S. Smith; A global optimisation method for robust affine registration of brain images; Medical Image Analysis, 5 (2001), pp. 143–156 (pii: S1361841501000366)
- Jenkinson et al., 2002 M. Jenkinson, P. Bannister, M. Brady, S. Smith; Improved optimization for the robust and accurate linear registration and motion correction of brain images; NeuroImage, 17 (2002), pp. 825–841 (pii: S1053811902911328 [pii])
- Kanal et al., 2002 E. Kanal, J.P. Borgstede, A.J. Barkovich, et al.; American College of Radiology white paper on MR safety; AJR. American Journal of Roentgenology, 178 (2002), pp. 1335–1347 http://doi.org/10.2214/ajr.178.6.1781335
- Kraemer and Baig, 2013 J.D. Kraemer, S.A. Baig; Analysis of legal and scientific issues in court challenges to graphic tobacco warnings; American Journal of Preventive Medicine, 45 (2013), pp. 334–342 http://doi.org/10.1016/j.amepre.2013.05.004
- Lindquist et al., 2012 K.A. Lindquist, T.D. Wager, H. Kober, E. Bliss-Moreau, L.F. Barrett; The brain basis of emotion: A meta-analytic review; The Behavioral and Brain Sciences, 35 (2012), pp. 121–143 http://doi.org/10.1017/S0140525X11000446
- Magnan and Cameron, 2015 R.E. Magnan, L.D. Cameron; Do young adults perceive that cigarette graphic warnings provide new knowledge about the harms of smoking?; Annals of Behavioral Medicine, 49 (2015), pp. 594–604 http://doi.org/10.1007/s12160-015-9691-6
- Martin, 2014 L.E. Martin; Effects of plain packaging on decision-making and reward for nicotine cigarettes; Neuroscience and Neuroeconomics, 3 (2014), pp. 63–73 http://doi.org/10.2147/NAN.S35911
- Mays et al., 2015a D. Mays, R.S. Niaura, W.D. Evans, D. Hammond, G. Luta, K.P. Tercyak; Cigarette packaging and health warnings: The impact of plain packaging and message framing on young smokers; Tobacco Control, 24 (2015), pp. e87–e92 http://doi.org/10.1136/tobaccocontrol-2013-051234
- Mays et al., 2015b D. Mays, M.M. Turner, X. Zhao, W.D. Evans, G. Luta, K.P. Tercyak; Framing pictorial cigarette warning labels to motivate young smokers to quit; Nicotine & Tobacco Research, 17 (2015), pp. 769–775 http://doi.org/10.1093/ntr/ntu164
- Munafo et al., 2011 M.R. Munafo, N. Roberts, L. Bauld, U. Leonards; Plain packaging increases visual attention to health warnings on cigarette packs in non-smokers and weekly smokers but not daily smokers; Addiction, 106 (2011), pp. 1505–1510 http://doi.org/10.1111/j.1360-0443.2011.03430.x
- Newman-Norlund et al., 2014 R.D. Newman-Norlund, J.F. Thrasher, J. Fridriksson, et al.; Neural biomarkers for assessing different types of imagery in pictorial health warning labels for cigarette packaging: A cross-sectional study; BMJ Open, 4 (2014), Article e006411 http://doi.org/10.1136/bmjopen-2014-006411
- Noar et al., 2015 S.M. Noar, M.G. Hall, D.B. Francis, K.M. Ribisl, J.K. Pepper, N.T. Brewer; Pictorial cigarette pack warnings: A meta-analysis of experimental studies [published online ahead of print May 6, 2015]; Tobacco Control (2015) http://doi.org/10.1136/tobaccocontrol-2014-051978
- Nonnemaker et al., 2015 J.M. Nonnemaker, C.J. Choiniere, M.C. Farrelly, K. Kamyab, K.C. Davis; Reactions to graphic health warnings in the United States; Health Education Research, 30 (2015), pp. 46–56 http://doi.org/10.1093/her/cyu036
- Peters et al., 2007 E. Peters, D. Romer, P. Slovic, et al.; The impact and acceptability of Canadian-style cigarette warning labels among U.S. smokers and nonsmokers; Nicotine & Tobacco Research, 9 (2007), pp. 473–481 http://doi.org/10.1080/14622200701239639
- Sanders-Jackson et al., 2013 A.N. Sanders-Jackson, A.V. Song, H. Hiilamo, S.A. Glantz; Effect of the Framework Convention on Tobacco Control and voluntary industry health warning labels on passage of mandated cigarette warning labels from 1965 to 2012: Transition probability and event history analyses; American Journal of Public Health, 103 (2013), pp. 2041–2047 http://doi.org/10.2105/AJPH.2013.301324
- Smith, 2002 S.M. Smith; Fast robust automated brain extraction; Human Brain Mapping, 17 (2002), pp. 143–155 http://doi.org/10.1002/hbm.10062
- Tobacco Labelling Resource Centre, n.d Tobacco Labelling Resource Centre. http://www.tobaccolabels.ca/. (Accessed July 27, 2015).
- U.S.Congress, 2009 U.S. Congress; Public Law 111-31. H.R. 1256. Family Smoking Prevention and Tobacco Control Act; https://www.govtrack.us/congress/bills/111/hr1256 (Accessed July 27, 2015) (2009)
- Villanti et al., 2015 A.C. Villanti, J.L. Pearson, J. Cantrell, D.M. Vallone, J.M. Rath; Patterns of combustible tobacco use in U.S. young adults and potential response to graphic cigarette health warning labels; Addictive Behaviors, 42 (2015), pp. 119–125 http://doi.org/10.1016/j.addbeh.2014.11.011
- Wang et al., 2015 A.L. Wang, S.B. Lowen, D. Romer, M. Giorno, D.D. Langleben; Emotional reaction facilitates the brain and behavioural impact of graphic cigarette warning labels in smokers; Tobacco Control, 24 (2015), pp. 225–232 http://doi.org/10.1136/tobaccocontrol-2014-051993
- Wang et al., 2013 A.L. Wang, K. Ruparel, J.W. Loughead, et al.; Content matters: Neuroimaging investigation of brain and behavioral impact of televised anti-tobacco public service announcements; The Journal of Neuroscience, 33 (2013), pp. 7420–7427 http://doi.org/10.1523/JNEUROSCI.3840-12.2013
- Webb and Sheeran, 2006 T.L. Webb, P. Sheeran; Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence; Psychological Bulletin, 132 (2006), pp. 249–268 http://doi.org/10.1037/0033-2909.132.2.249
- Woolrich et al., 2001 M.W. Woolrich, B.D. Ripley, M. Brady, S.M. Smith; Temporal autocorrelation in univariate linear modeling of FMRI data; NeuroImage, 14 (2001), pp. 1370–1386 http://doi.org/10.1006/nimg.2001.0931
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?