Abstract
Aims
Subclinical diastolic dysfuntion in patients with preclinical heart failure with preserved ejection fraction (HFpEF) has been demonstrated in patients with Marfan syndrome (MFS). We investigated the relationship between diastolic dysfunction and NT-proBNP levels in patients with MFS.
Methods and results
NT-proBNP, C-reactive protein (CRP) and diastolic function were assessed in 217 patients with MFS (31 ± 16 y, 110 f. and in 339 patients referred for suspected MFS in whom the diagnosis was ruled out according to the Ghent nosology (30 ± 15 y, 154 f). Assessment of cardiovascular remodeling, diastolic function in echocardiography, and NT-proBNP was analyzed with univariate analysis and multi-parameter analysis of covariance (MANCOVA). NT-proBNP was 70.6 ± 74.8 pg/ml in patients with Marfan syndrome and 58.4 ± 100.3 pg/ml in controls (p = 0.002, Kolmogorov–Smirnov). There were significant intergroup differences regarding end-diastolic left ventricular volume (p < 0.001), and aortic diameter (p < 0.001). The ratio of early diastolic mitral flow velocity (E) to early relaxation velocity in tissue Doppler (e′), E/e′ (p < 0.001) was significantly higher in patients with Marfan syndrome than in controls, whereas e′ (p < 0.001) and the ratio of E to inflow velocity during atrial contraction (A), E/A (p = 0.012) was significantly lower. Besides age and gender, diagnosis of MFS, diastolic function (e′ and E/e′), Z-Score of aortic diameter, and left ventricular size were identified as significant independent parameters with impact on NT-proBNP levels.
Conclusions
MFS patients presenting with normal ejection fraction show disturbed diastolic function and higher NT-proBNP levels, which is partly explained by aortic Z-score. Assessment of diastolic function and NT-proBNP levels may therefore detect early abnormalities and guide surveillance and prevention management of patients with MFS.
Keywords
NT-proBNP ; Marfan syndrome ; Diastolic function ; Heart failure ; Cardiomyopathy
1. Introduction
Marfan syndrome is an autosomal, dominantly inherited disorder of connective tissue characterized by a high degree of clinical variability [1] . Although complications can involve the eye, the lung and the skeleton, the premature mortality of untreated patients results almost exclusively from cardiovascular complications, including aortic dissection and rupture [2] . Primary cardiomyopathy has been described in individuals with MFS [3] . Classical MFS is caused by heterozygous mutations in the gene coding for fibrillin-1 [4] . Fibrillins are major components of the microfibrils of the extracellular matrix. Microfibrils regulate the activity of transforming growth factor-β (TGF-β), which is associated with larger aortic diameters [5] , profibrotic processes in heart failure and are involved in myocardial remodeling processes [6] . Current treatment focusses on aortic dilation as a mortality-related end-point [7] and [8] .
B-type natriuretic peptide (BNP) is a cardiac neurohormone synthesized mainly by ventricular myocytes as a nonspecific response to wall stress [9] . Brain natriuretic peptide (BNP) opposes TGF-β-regulated gene expression related to fibrosis and myofibroblast conversion [10] . The N-terminal prohormone of BNP (NT-proBNP) is used as a diagnostic marker for cardiac insufficiency [11] , ventricular dysfunction [12] and [13] , and aortic dissection [14] . NT-proBNP levels correlate positively with age [15] , sex, and negatively with body mass index (BMI) and can be used to rule out heart failure with preserved or reduced ejection fraction (HFpEF, HFrEF) with similar accuracy [16] .
The aim of the present study was to evaluate left ventricular diastolic function, type of cardio-vascular hypertrophy and NT-proBNP levels in out-patients with MFS and a control group with similar clinical manifestations referred for evaluation of suspected MFS in whom the diagnosis was ruled out. We hypothesized that subclinical diastolic dysfunction in patients with preserved ejection fraction, left ventricular hypertrophy and elevated NT-proBNP levels are more frequent in patients with MFS and investigated the impacting factorson NT-proBNP levels in these patients.
2. Methods
2.1. Study design and patient population
The study is a monocentric consecutive cohort study of 863 patients seen in our Marfan clinic between 1/2010 and 7/2015. The diagnosis of Marfan syndrome was based on the revised Ghent nosology [17] including molecular genetic analysis. Clinical assessment according to the Ghent criteria was performed in all patients. All patients were routinely examined in our specialized multidisciplinary Marfan clinic. Patients that did not fulfill the Ghent criteria of MFS were used as controls.
Exclusion criteria were history of cardiac surgery or aortic dissection at any time, mitral or aortic regurgitation, Loeys-Dietz-Syndrome, no definite diagnosis, and incomplete dataset (Fig. 1 ). No patient in this cohort was diagnosed with atrial fibrillation.
|
|
|
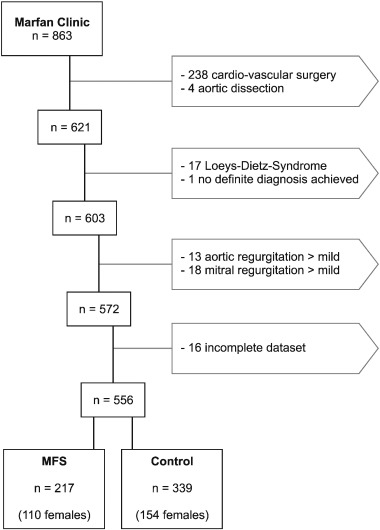
Fig. 1. Patient exclusion flow-chart. We analyzed 863 individual patients from 1/2010 through 7/2015. We excluded 238 patients because they had had cardiac surgery at some time in the past, and four patients that had experienced a type-B aortic dissection which was treated conservatively. Any patient with more than mild aortic or mitral regurgitation was excluded as well, as were patients who were diagnosed with Loeys-Dietz-Syndrome. Seventeen patients had incomplete datasets. The remaining 556 patients consisted of 217 with MFS, and 339 controls.
|
2.2. Clinical examination
History, physical examination, genetic testing, laboratory tests, orthopedic and ophthalmologic counseling and cardiovascular imaging were performed according to current recommendations. Blood creatinine, C-reactive protein and NT-proBNP levels were collected as a part of routine laboratory examination. Children were defined as patients with age < 18 years.
Two-dimensional, pulsed, and color-Doppler and color tissue-Doppler echocardiograms were acquired using phased array probes on a Vivid 7 Vingmed General Electric Ultrasound scanner (GE Vingmed Ultrasound Horton Norway) following a standardized protocol. M-mode and two dimensional recordings just beneath the mitral leaflet tips in the long axis for at least 3 beats were used for linear measurements. Left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), thickness of the posterior wall (PWD) and the septum (IVD) were measured and fractional shortening (FS), left ventricular end-diastolic volume (LVEDV), and ejection fraction (EF) according to the Teichholz' equation [18] , relative wall thickness (RWT) and LV mass (cube formula) were calculated according to recent recommendations on chamber quantification [19] . LV-geometry was defined according to the above recommendations. Left atrial diameter, aortic root diameters, early diastolic mitral flow velocity (E) and the inflow velocity during atrial contraction (A), as well as early relaxation velocity in tissue Doppler (e′, as mean of septal and lateral e′) were measured, and the E/A and E/e′ ratios were calculated. Z-scores were calculated for aortic diameter [20] . Aortic or mitral regurgitation and mitral valve prolapse were graded according to recommendations for assessment of valvular regurgitation [21] .
2.3. NT-proBNP measurement
Venous blood was collected by peripheral venous puncture as part of the routine clinical evaluation in an ammonium-heparin monovette (Sarstedt, Nuembrecht, Germany). Plasma was separated by centrifugation at 4000 rpm (2700 × g ) at 4 °C. Aliquots were stored at − 80 °C and thawed immediately prior to NT-proBNP determination. Measurements were performed with a non-competitive sandwich electrochemiluminescent immunoassay (ECLIA) on an Elecsys Modular E 170 platform (Roche Diagnostics, Mannheim, Germany).
2.4. Statistical analysis
SPSS® Statistics version 23, (IBM® corporation) was used. Continuous data are given as mean ± standard deviation. Categorical data are presented as percentages and analyzed by cross-table analysis. Since the NT-proBNP data were not normally distributed and cover a large range, a non-parametric test (Kolmogogorov–Smirnov) and logarithmic transformation were used as appropriate. Significance level was 0.05. Binary cluster analysis was used to identify morphological and functional cardiac features that are characteristic for group differences. Univariate linear regression with single covariates was used to pre-select cofactors and covariates for linear modeling by multiple covariate analysis of variance (MANCOVA) using stepwise forward and backward selection. Medical treatment was excluded from the multivariate analysis in order to avoid bias, as any association could be caused by diagnosis-associated treatment strategy.
2.5. Ethical standards
This study complies with the requirements of our institutional Ethics Committee Review Board and is in compliance with the Helsinki standards of human medical studies. All patients gave informed consent.
3. Results
For this study we recruited 863 patients seen in our Marfan outpatient clinic. Because NT-proBNP levels are known to be raised after surgery or aortic dissection [14] and [22] , 238 patients were excluded because they had had cardio-vascular surgery at any time before. Four additional who had experienced an aortic dissection treated conservatively, 17 patients with diagnosis of Loeys-Dietz-Syndrome, one patient in whom definite diagnosis could not be established, 13 with more than mild aortic regurgitation, 18 who had a more than mild degree of mitral regurgitation, and 16 patients in whom datasets were incomplete, were excluded as well. We investigated NT-proBNP levels and echocardiographic diastolic function parameters in 217 (110 females) patients with MFS. As a control group we investigated 339 (154 females) patients seen for suspected MFS in whom the diagnosis was subsequently ruled out (Table 1 ).
| MFS | Control | Significance | |
|---|---|---|---|
| Mean, ± SD | Mean, ± SD | p | |
| Age (yrs) | 31.05±16.16 | 30.27±14.61 | ns |
| HR (bpm) | 71.40±11.85 | 72.86±13.46 | ns |
| Syst BP (mmHg) | 125.08±22.50 | 122.96±17.98 | ns |
| Diastol BP (mmHg) | 75.21±12.70 | 74.76±12.06 | ns |
| MAD (mmHg) | 91.90±14.21 | 90.33±12.72 | ns |
| Height (cm) | 179.95±19.37 | 177.60±15.40 | ns |
| Weight (cm) | 71.32±22.46 | 67.23±17.33 | 0.023 |
| BSA | 1.89±0.38 | 1.83±0.28 | 0.036 |
| BMI | 21.46±4.8 | 21.17±4.54 | ns |
| LVEDD (mm) | 50.52±7.06 | 48.18±5.83 | <0.001 |
| LVESD (mm) | 30.93±5.91 | 29.38±4.79 | 0.001 |
| IVSd (mm) | 10.45±2.67 | 10.00±2.35 | 0.015 |
| PWd (mm) | 9.12±2.23 | 8.92±2.01 | ns |
| RWT | 0.39±2.09 | 0.39±0.08 | ns |
| FS (%) | 38.44±7.16 | 38.83±6.79 | ns |
| EF (%) | 67.84±8.90 | 68.69±8.31 | ns |
| EDV (ml) | 124.70±40.94 | 111.28±30.38 | <0.001 |
| iEDV | 66.10±16.94 | 60.49±12.72 | <0.001 |
| Aortic diameter (mm) | 38.10±6.71 | 32.41±6.46 | <0.001 |
| Z-score aorta | 1.99±2.14 | 1.1±1.51 | <0.001 |
| Left atrium (mm) | 32.35±7.15 | 31.83±6.18 | ns |
| E | 76.51±19.66 | 83.80±19.23 | <0.001 |
| A | 57.13±16.42 | 55.94±15.26 | ns |
| E/A ratio | 1.43±0.48 | 1.59±0.52 | <0.001 |
| e′ Sept | 10.71±2.95 | 13.16±3.19 | <0.001 |
| e′ Lat | 12.37±4.1 | 15.99±4.21 | <0.001 |
| e′ Mean | 11.54±3.3 | 14.58±3.38 | <0.001 |
| E/e′ ratio | 6.84±1.95 | 5.88±1.45 | <0.001 |
| NT-proBNP | 70.55±74.76 | 58.35±101.09 | <0.002* |
| ln NT-proBNP | 3.78±1.02 | 3.5±1.05 | 0.001 |
| Creatinine | 0.79±0.14 | 0.77±0.17 | ns |
| CRP | 0.22±0.33 | 0.2±0.31 | ns |
| IVRTm | 81.95±18.40 | 71.56±14.74 | 0.009 |
Legend: * Kolmogorov–Smirnov test. Results are represented as mean ± standard deviation(SD). BMI, body mass index; BSA, body surface area; syst BP and dias BP, systolic and diastolic blood pressure, MAD, mean arterial blood pressure; LVEDD, LVESD, left ventricular end-diastolic and end-systolic diameter, FS, fractional shortening; EF, ejection fraction; EDV, end-diastolic volume, EDVI, indexed end-diastolic volume; E, early diastolic mitral flow velocity; A, inflow velocity during atrial contraction; E/A ratio of E over A, e′ sept, e′ lat, septal and lateral early relaxation velocity in tissue Doppler; E/e′, ratio of E over e′; NT-proBNP, N-terminal pro hormone of brain natriuretic peptide; Ln NT-proBNP, natural logarithm of NT-proBNP; CRP c-reactive protein; IVRTm, mean isovolumetric relaxation time. (Table 2 ) Univariate linear analysis, LnBNP.
There were no significant group differences regarding age, height, BMI, blood pressure and heart rate. Weight was slightly but significantly higher (p = 0.023) in patients with MFS.
There were about 20% children with MFS and 17% among controls (n.s). There was a trend to more males presenting with MFS (50% vs. 45% males). Both trends were not significant in cross-table analysis. More patients were medically treated in the MFS group (50% vs. 26%, p < 0.001). Especially treatment with angiotensin II receptor blockers (ARBs) (25% vs. 3%, p < 0.001) or angiotensin converting enzyme (ACE) inhibitors (6% vs. 2%, p < 0.001) and beta-blockers (16% vs. 5%, p < 0.001) were more frequent in patients with MFS. Treatment with beta-blockers was associated with significantly increased NT-proBNP levels in both groups (p < 0.001). Mitral valve prolapse (80% vs. 44%, p < 0.001), mild mitral regurgitation (11% vs. 4%, p < 0.001) and mild aortic regurgitation (20% vs. 7%, p < 0.001) were found more often in patients with MFS.
3.1. Echocardiography and laboratory values
Chamber dimensions, wall thickness and parameters of systolic and diastolic function were within normal range in both groups. There were no significant group differences for, left atrial size, fractional shortening (FS: 39 ± 7%), ejection fraction (EF: 68 ± 9%), and mitral inflow velocities(see Table 1 ). Aortic diameter, end-diastolic left ventricular (LV) (indexed) volumes and masses, end-systolic and end-diastolic diameter, septal thickness, and the E/e′ ratio (p < 0.001) were significantly higher in patients with MFS than in controls, while e′ (p < 0.001) and the E/A ratio (p = 0.001) were significantly reduced. Aortic diameters and their z-scores (p < 0.001) were – as expected – larger in MFS patients. NT-proBNP was 71 ± 75 pg/ml in patients with MFS and 58 ± 100 pg/ml in controls (p < 0.002; Kolmogorov–Smirnov test) (Table 1 ). CRP and creatinine levels did not differ significantly.
3.2. Type of cardiovascular hypertrophy in MFS
There were more dilated (24% vs. 14%, p < 0.001) and hypertrophied (35% vs. 18%, p < 0.001) ventricles in patients with MFS, however. The groups did not differ with respect to type of hypertrophic remodeling comprising about 30% excentric cases in both groups. Impaired ejection fraction was found in 21 patients with MFS and 19 controls.
In binary two-step cluster analysis larger aortic diameter (38 ± 6 mm vs. 32 ± 6 mm, p < 0.001), and reduced early relaxation in tissue Doppler (e′) (11.5 ± 3.3 cm/s vs. 14.6 ± 3.4 cm/s, p < 0.001) turned out to be the best classifying variables.
3.3. Analysis of cofactors and covariates predicting NT-proBNP by linear regression modeling
For linear modeling a logarithmic transformation of NT-proBNP was used.
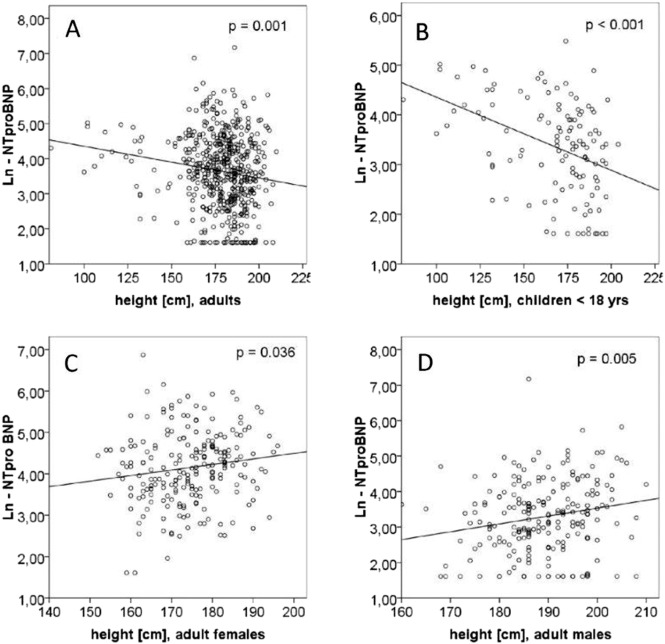
Age (p < 0.001), gender (p < 0.001), height (p < 0.001), BSA (p < 0.001), CRP (p = 0.001), diagnosis of Marfan syndrome (p = 0.001), A-wave (p < 0.001), E/A ratio (p = 0.017), E/e′ ratio (p < 0.001), tissue Doppler measurements of septal e′ (p < 0.001) and lateral e′ (p = 0.014) and its calculated average e′ mean (p = 0.001), aortic regurgitation (p = 0.003), mitral regurgitation (p < 0.001), mitral valve prolapse (p < 0.028), aortic z-score (p = 0.002), indexed end-diastolic volume (iEDV, p = 0.032), LV hypertrophy (p = 0.003) and concentric type of hypertrophy (p = 0.039) but not LV linear dimensions or ejection fraction or creatinine were significant covariates or cofactors in predicting NT-proBNP by univariate linear analysis (see Table 2 ). The paradoxically negative correlation of height with NT-proBNP in the total sample is explained by hidden effects of gender and inclusion of children as demonstrated in Fig. 2 . The interaction of gender and age on e' is illustrated in Fig. 3 .
| R2 | F | p | |
|---|---|---|---|
| Diagnosis MFS | 0.025 | 13.83 | <0.001 |
| Age | 0.15 | 96.80 | <0.001 |
| Gender | 0.180 | 119.75 | <0.001 |
| MVP | 0.021 | 11.62 | 0.001 |
| HTN | 0.016 | 8.47 | 0.004 |
| Z-score aorta | 0.018 | 10.11 | 0.002 |
| LA | 0.000 | 0.02 | ns |
| iEDV | 0.007 | 3.58 | ns |
| E | 0.000 | 0.14 | ns |
| A | 0.035 | 19.36 | <0.001 |
| E/A-ratio | 0.015 | 7.80 | 0.005 |
| e′ Sept | 0.042 | 20.18 | <0.001 |
| e′ Lat | 0.029 | 13.84 | <0.001 |
| e′ Mean | 0.040 | 19.24 | <0.001 |
| E/e′-ratio | 0.058 | 28.15 | <0.001 |
| Mild AR | 0.029 | 16.10 | <0.001 |
| Mild MR | 0.028 | 15.84 | <0.001 |
| CRP | 0.023 | 9.61 | 0.002 |
| BSA all | 0.009 | 4.95 | 0.027 |
| BSA adult females | 0.005 | 1.14 | ns |
| BSA adult males | 0.025 | 5.37 | 0.021 |
| BSA children <18yrs | 0.160 | 22.47 | <0.001 |
| BMI | 0.000 | 0.02 | ns |
| BMI children <18yrs | 0.067 | 8.51 | 0.004 |
| Height | 0.022 | 12.22 | 0.001 |
| Height all | 0.019 | 8.27 | 0.004 |
| Height adult females | 0.021 | 4.47 | 0.036 |
| Height adult males | 0.038 | 8.11 | 0.005 |
| Height children <18yrs | 0.16 | 22.34 | <0.001 |
| Weight all adults | 0.004 | 1.90 | ns |
| Weight children <18yrs | 0.15 | 20.25 | <0.001 |
| Beta blockers | 0.059 | 29.20 | <0.001 |
| Beta blockers, <18yrs | 0.027 | 2.733 | ns |
| Losartan | 0.026 | 12.24 | 0.01 |
| Losartan, children | 0.009 | 0.09 | ns |
Legend: MVP, mitral valve prolapse; HTN, Hypertension; Z-score aorta, normalized diameters of aortic diameter; LA, left atrium; iEDV, indexed end-diastolic volume; BSA, body surface area; E, early diastolic mitral flow velocity; A, inflow velocity during atrial contraction; e′ sept, e′ lat, e′ mean, septal and lateral early relaxation velocity in tissue Doppler, and their mean; E/e′, ratio of E, early diastolic mitral flow velocity, over e′; MR, mitral regurgitation; AR, aortic regurgitation; CRP, C-reactive protein; BSA, body surface area; < 18, under 18 years of age; ns, not significant.
|
|
|
Fig. 2. Regression analysis of height. A negative regression in all patients (not shown) and adults (A) is explained by the higher NTproBNP levels younger and therefore smaller children (B) and the higher NT-proBNP levels in females (C, D), who are smaller than males. This outweighs the effect of greater height of patients with MFS who do have higher NT-proBNP levels.
|
|
|
|
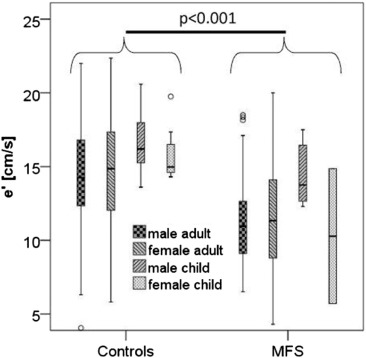
Fig. 3. Box plot of early relaxation velocity (e′) in tissue Doppler (average of e′ septal and e′ lateral) on top and ratio of aortic diameter to e′ at bottom for controls and patients with MFS stratified with respect to gender and age below 16 years. Lines: median, boxes: 75% confidence interval (CI), whiskers: 95% CI.
|
In a first step of multiple parameter univariate ANCOVA (MANCOVA) linear analysis, gender (p < 0.001), age (p < .0.001), height (p = 0.001), and diagnosis of MFS (p = 0.001) were found to be significant covariates and cofactors of NT-proBNP (F-value of model 52, R2 = 0.27). The diagnosis of MFS was a significant cofactor for NT-proBNP levels, as was e′, which can be regarded as a good indicator of diastolic function. MFS patients had significantly lower e′ values as compared to controls. Aortic z-score was significanty larger in the MFS group. Therefore, e′ or aortic diameter z-score were potential confounders. However, the diagnosis of MFS remained a significant cofactor even after the aortic z-score was introduced into the model, suggesting that aortic z-score has an influence on NT-proBNP levels indepently of the diagnosis of MFS. Finally in MANCOVA model adjusted for demographic parameters we investigated which parameters explain the effects of diagnosis MFS (F = 10.05) on NT-proBNP. We found that parameters of diastolic cardiac function as E/e′ (F = 10.92) and e′ mean (F = 11.72), iEDV (F = 7.70), and aortic diameter (F = 10.04) were significant and replaced diagnosis of MFS best. This analysis suggests that the impact of MFS on NT-proBNP is largely mediated by impaired diastolic relaxation as well as aortic dilatation, and to a smaller amount by other factors such as concentric hypertrophy and mitral regurgitation.
4. Discussion
This is the largest clinical study on NT-proBNP levels and diastolic function parameters expressing a subclinical intrinsic cardiomyopathy in patients with MFS. Subclinical cardiomyopathy in patients with MFS has been found in several clinical echocardiographic [23] , [24] and [25] and magnetic resonance and combined imaging studies [3] , [6] and [26] . Diastolic dysfunction has been described to be impaired in MFS [27] and [28] but NT-proBNP measurements in this context have not been published before. In agreement with these studies we found a relatively impaired diastolic left ventricular relaxation as compared to controls. Left ventricular dilatation and hypertrophy without differences in type of hypertrophic remodeling and as expectedly aortic dilatation were more often seen in ths MFS group.
Moreover, in this study we demonstrate significantly higher NT-proBNP levels in patients with MFS as compared to control patients with similar phenotype in whom MFS had been ruled out. This has not been described before. The relative elevation of NT-proBNP in MFS as opposed to controls was not explained by known demographic cofactors and covariates. The strongest predictor of NT-proBNP elevation was diastolic function follwed by Z-score of aortic diameter suggesting that primary disease related cardiomyopathy in MFS manifests predominantly as diastolic relaxation impairment. Patients with valvular dysfunction with left ventricular loading were excluded from our study. Therefore, our results might be attributed to a MFS-related myocardial impairment as would be the case in primary cardiomyopathy, which in turn leads to diastolic dysfunction and dilatation of cardiac cavities and large vessels with resultant NT-proBNP elevation.
According to the Laplace law aortic wall stress is proportional to the aortic diameter. Our finding of a significant and independent correlation of z-scores of aortic diameters and NT-proBNP therefore may be in part caused by ventricular afterload mismatch resulting from increased aortic stiffness. Thus some modulating role of the aortic pathology in MFS in the development of cardiomyopathy in patients with MFS is likely. Afterload impedance mismatch due to higher aortic stiffness may be related to decreased aortic elastic properties in young patients with MFS as compared to a control group [29] . Fibrillin-1 deficiency activates TGF-ß signaling pathways, leading to elevated collagen synthesis and matrix metalloproteinase-mediated disruption of the elastic fibers in the vessel wall, thereby increasing aortic stiffness and decreasing vasoreactivity [30] . Increased levels of TGF-ß have been detected in aneurysmatic aortic wall of patients with MFS [31] and circulating TGF-ß has been proposed as prognostic biomarker in MFS [5] and [32] .
Treatment with beta-blockers was associated with significantly increased NT-proBNP levels in both groups (p < 0.001) as has been descibed before [33] and [34] . Impairment of renal function has been shown to increase NT-proBNP levels [35] . No patient in our study had severe renal dysfunction. Blood creatinine levels were investigated and did not differ between groups and were not significant in linear modeling.
5. Limitations and conclusions
This is a non-randomized retrospective consecutive cohort study with several limitations. Data on potential clinical correlates of diastolic dysfunction, such as exercise intolerance, were not collected, and echocardiographic evaluation was performed using a minimal data set without sophisticated echocardiographic data on diastolic and systolic biventricular function as well as left and right atrial volumes, aortic compliance. Circulating TGF-ß and matrix metalloproteinases were not measured. Nonetheless, our study has shown for the first time that circulating NT-proBNP levels are relatively increased in patients with MFS. Presumably this observation is related to the presence of subclinical diastolic cardiomyopathy in MFS, which conceivably could be related to the underlying genetic defect in MFS or could be a secondary effect of the increased afterload associated with increased aortic stiffness or both. Further research will be needed to characterize a potential clinical role of NT-proBNP measurements in the management of persons with MFS, and to better characterize the prevalence and clinical relevance of diastolic dysfunction in this patient group.
Funding
None.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgements
We thank Ms. Astrid Benhennour for bibliographical assistance.
References
- [1] P.N. Robinson, M. Godfrey; The molecular genetics of Marfan syndrome and related microfibrillopathies; J. Med. Genet., 37 (1) (Jan 2000), pp. 9–25
- [2] Y. von Kodolitsch, M. Rybczynski, C. Detter, P.N. Robinson; Diagnosis and management of Marfan syndrome; Futur. Cardiol., 4 (1) (Jan 2008), pp. 85–96
- [3] F. Alpendurada, J. Wong, A. Kiotsekoglou, W. Banya, A. Child, S.K. Prasad, D.J. Pennell, R.H. Mohiaddin; Evidence for Marfan cardiomyopathy; Eur. J. Heart Fail., 12 (10) (Oct 2010), pp. 1085–1091
- [4] H.C. Dietz, R.E. Pyeritz, B.D. Hall, R.G. Cadle, A. Hamosh, J. Schwartz, D.A. Meyers, C.A. Francomano; The Marfan syndrome locus: confirmation of assignment to chromosome 15 and identification of tightly linked markers at 15q15–q21.3.; Genomics, 9 (2) (Feb 1991), pp. 355–361
- [5] R. Franken, A.W. den Hartog, V. de Waard, L. Engele, T. Radonic, R. Lutter, J. Timmermans, A.J. Scholte, M.P. van den Berg, A.H. Zwinderman, M. Groenink, B.J. Mulder; Circulating transforming growth factor-beta as a prognostic biomarker in Marfan syndrome; Int. J. Cardiol., 168 (3) (Oct 3, 2013), pp. 2441–2446
- [6] J.F. De Backer, D. Devos, P. Segers, D. Matthys, K. Francois, T.C. Gillebert, A.M. De Paepe, J. De Sutter; Primary impairment of left ventricular function in Marfan syndrome; Int. J. Cardiol., 112 (3) (Oct 10, 2006), pp. 353–358
- [7] R.V. Lacro, H.C. Dietz, L.A. Sleeper, A.T. Yetman, T.J. Bradley, S.D. Colan, G.D. Pearson, E.S. Selamet Tierney, J.C. Levine, A.M. Atz, D.W. Benson, A.C. Braverman, S. Chen, J. De Backer, B.D. Gelb, P.D. Grossfeld, G.L. Klein, W.W. Lai, A. Liou, B.L. Loeys, L.W. Markham, A.K. Olson, S.M. Paridon, V.L. Pemberton, M.E. Pierpont, R.E. Pyeritz, E. Radojewski, M.J. Roman, A.M. Sharkey, M.P. Stylianou, S.B. Wechsler, L.T. Young, L. Mahony; Atenolol versus losartan in children and young adults with Marfans syndrome; N. Engl. J. Med., 371 (22) (Nov 27, 2014), pp. 2061–2071
- [8] O. Milleron, F. Arnoult, J. Ropers, P. Aegerter, D. Detaint, G. Delorme, D. Attias, F. Tubach, S. Dupuis-Girod, H. Plauchu, M. Barthelet, F. Sassolas, N. Pangaud, S. Naudion, J. Thomas-Chabaneix, Y. Dulac, T. Edouard, J.E. Wolf, L. Faivre, S. Odent, A. Basquin, G. Habib, P. Collignon, C. Boileau, G. Jondeau; Marfan Sartan: a randomized, double-blind, placebo-controlled trial; Eur. Heart J., 36 (32) (Aug 21, 2015), pp. 2160–2166
- [9] E.R. Levin, D.G. Gardner, W.K. Samson; Natriuretic peptides; N. Engl. J. Med., 339 (5) (Jul 30, 1998), pp. 321–328
- [10] A.M. Kapoun, F. Liang, G. O'Young, D.L. Damm, D. Quon, R.T. White, K. Munson, A. Lam, G.F. Schreiner, A.A. Protter; B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation; Circ. Res., 94 (4) (Mar 5, 2004), pp. 453–461
- [11] C.J. Coats, V. Parisi, M. Ramos, K. Janagarajan, C. O'Mahony, A. Dawnay, R.H. Lachmann, E. Murphy, A. Mehta, D. Hughes, P.M. Elliott; Role of serum N-terminal pro-brain natriuretic peptide measurement in diagnosis of cardiac involvement in patients with Anderson–Fabry disease; Am. J. Cardiol., 111 (1) (Oct 4, 2013), pp. 111–117
- [12] E. Lubien, A. DeMaria, P. Krishnaswamy, P. Clopton, J. Koon, R. Kazanegra, N. Gardetto, E. Wanner, A.S. Maisel; Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings; Circulation, 105 (5) (Feb 5, 2002), pp. 595–601
- [13] W.J. Paulus, C. Tschope, J.E. Sanderson, C. Rusconi, F.A. Flachskampf, F.E. Rademakers, P. Marino, O.A. Smiseth, G. De Keulenaer, A.F. Leite-Moreira, A. Borbely, I. Edes, M.L. Handoko, S. Heymans, N. Pezzali, B. Pieske, K. Dickstein, A.G. Fraser, D.L. Brutsaert; How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the European Society of Cardiology; Eur. Heart J., 28 (20) (Oct 2007), pp. 2539–2550
- [14] E. Sbarouni, P. Georgiadou, A. Marathias, S. Geroulanos, D.T. Kremastinos; D-dimer and BNP levels in acute aortic dissection; Int. J. Cardiol., 122 (2) (Nov 15, 2007), pp. 170–172
- [15] P. Hildebrandt, P.O. Collinson, R.N. Doughty, A. Fuat, D.C. Gaze, F. Gustafsson, J. Januzzi, J. Rosenberg, R. Senior, M. Richards; Age-dependent values of N-terminal pro-B-type natriuretic peptide are superior to a single cut-point for ruling out suspected systolic dysfunction in primary care; Eur. Heart J., 31 (15) (Aug 2010), pp. 1881–1889
- [16] E. Litton, K.M. Ho; The use of pre-operative brain natriuretic peptides as a predictor of adverse outcomes after cardiac surgery: a systematic review and meta-analysis; Eur. J. Cardiothorac. Surg., 41 (3) (Mar 2012), pp. 525–534
- [17] B.L. Loeys, H.C. Dietz, A.C. Braverman, B.L. Callewaert, J. De Backer, R.B. Devereux, Y. Hilhorst-Hofstee, G. Jondeau, L. Faivre, D.M. Milewicz, R.E. Pyeritz, P.D. Sponseller, P. Wordsworth, A.M. De Paepe; The revised Ghent nosology for the Marfan syndrome; J. Med. Genet., 47 (7) (Jul 2010), pp. 476–485
- [18] L.E. Teichholz, T. Kreulen, M.V. Herman, R. Gorlin; Problems in echocardiographic volume determinations: echocardiographic–angiographic correlations in the presence of absence of asynergy; Am. J. Cardiol., 37 (1) (Jan 1976), pp. 7–11
- [19] N. Abrahamsson, B.E. Engstrom, M. Sundbom, F.A. Karlsson; Gastric bypass surgery elevates NT-ProBNP levels; Obes. Surg., 23 (9) (Sep 2013), pp. 1421–1426
- [20] M.D. Pettersen, W. Du, M.E. Skeens, R.A. Humes; Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study; J. Am. Soc. Echocardiogr., 21 (8) (Aug 2008), pp. 922–934
- [21] P. Lancellotti, C. Tribouilloy, A. Hagendorff, B.A. Popescu, T. Edvardsen, L.A. Pierard, L. Badano, J.L. Zamorano; Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging; Eur. Heart J. Cardiovasc. Imaging., 14 (7) (Jul 2013), pp. 611–644
- [22] L. Vetrugno, M.G. Costa, L. Pompei, P. Chiarandini, D. Drigo, F. Bassi, N. Gonano, R. Muzzi, R.G. Della; Prognostic power of pre- and postoperative B-type natriuretic peptide levels in patients undergoing abdominal aortic surgery; J. Cardiothorac. Vasc. Anesth., 26 (4) (Aug 2012), pp. 637–642
- [23] M.J. Angtuaco, H.V. Vyas, S. Malik, B.N. Holleman, J.M. Gossett, R. Sachdeva; Early detection of cardiac dysfunction by strain and strain rate imaging in children and young adults with Marfan syndrome; J. Ultrasound Med., 31 (10) (Oct 2012), pp. 1609–1616
- [24] A. Kiotsekoglou, S. Saha, J.C. Moggridge, V. Kapetanakis, M. Govindan, F. Alpendurada, M.J. Mullen, D.K. Nassiri, J. Camm, G.R. Sutherland, B.H. Bijnens, A. Child; Impaired biventricular deformation in Marfan syndrome: a strain and strain rate study in adult unoperated patients; Echocardiography., 28 (4) (Apr 2011), pp. 416–430
- [25] R.W. Scherptong, H.W. Vliegen, E.E. van der Wall, Y. Hilhorst-Hofstee, J.J. Bax, A.J. Scholte, V. Delgado; Biventricular performance in patients with Marfan syndrome without significant valvular disease: comparison to normal subjects and longitudinal follow-up; J. Am. Soc. Echocardiogr., 24 (12) (Dec 2011), pp. 1392–1399 (e1)
- [26] P. de Witte, J.J. Aalberts, T. Radonic, J. Timmermans, A.J. Scholte, A.H. Zwinderman, B.J. Mulder, M. Groenink, M.P. van den Berg; Intrinsic biventricular dysfunction in Marfan syndrome; Heart, 97 (24) (Dec 2011), pp. 2063–2068
- [27] A. Kiotsekoglou, J.C. Moggridge, B.H. Bijnens, V. Kapetanakis, F. Alpendurada, M.J. Mullen, S. Saha, D.K. Nassiri, J. Camm, G.R. Sutherland, A.H. Child; Biventricular and atrial diastolic function assessment using conventional echocardiography and tissue-Doppler imaging in adults with Marfan syndrome; Eur. J. Echocardiogr., 10 (8) (Dec 2009), pp. 947–955
- [28] M. Rybczynski, D.H. Koschyk, M.A. Aydin, P.N. Robinson, T. Brinken, O. Franzen, J. Berger, T. Hofmann, T. Meinertz, Y. von Kodolitsch; Tissue Doppler imaging identifies myocardial dysfunction in adults with Marfan syndrome; Clin. Cardiol., 30 (1) (Jan 2007), pp. 19–24
- [29] D. Baumgartner, C. Baumgartner, G. Matyas, B. Steinmann, J. Loffler-Ragg, E. Schermer, U. Schweigmann, I. Baldissera, B. Frischhut, J. Hess, I. Hammerer; Diagnostic power of aortic elastic properties in young patients with Marfan syndrome; J. Thorac. Cardiovasc. Surg., 129 (4) (Apr 2005), pp. 730–739
- [30] E.R. Neptune, P.A. Frischmeyer, D.E. Arking, L. Myers, T.E. Bunton, B. Gayraud, F. Ramirez, L.Y. Sakai, H.C. Dietz; Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome; Nat. Genet., 33 (3) (Mar 2003), pp. 407–411
- [31] M. Nataatmadja, J. West, M. West; Overexpression of transforming growth factor-beta is associated with increased hyaluronan content and impairment of repair in Marfan syndrome aortic aneurysm; Circulation, 114 (1 Suppl.) (Jul 4, 2006), pp. I371–I377
- [32] A.A. Ahimastos, A. Aggarwal, R. Savarirayan, A.M. Dart, B.A. Kingwell; A role for plasma transforming growth factor-beta and matrix metalloproteinases in aortic aneurysm surveillance in Marfan syndrome?; Atherosclerosis, 209 (1) (Mar 2010), pp. 211–214
- [33] F. Edelmann, G. Gelbrich, A. Duvinage, R. Stahrenberg, A. Behrens, C. Prettin, E. Kraigher-Krainer, A.G. Schmidt, H.D. Dungen, W. Kamke, C. Tschope, C. Herrmann-Lingen, M. Halle, G. Hasenfuss, R. Wachter, B. Pieske; Differential interaction of clinical characteristics with key functional parameters in heart failure with preserved ejection fraction — results of the Aldo-DHF trial; Int. J. Cardiol., 169 (6) (Nov 30, 2013), pp. 408–417
- [34] A.K. Taneja, D. Gaze, A.J. Coats, D. Dumitrascu, L. Spinarova, P. Collinson, M. Roughton, M.D. Flather; Effects of nebivolol on biomarkers in elderly patients with heart failure; Int. J. Cardiol., 175 (2) (Aug 1, 2014), pp. 253–260
- [35] F.L. Martin, P.M. McKie, A. Cataliotti, S.J. Sangaralingham, J. Korinek, B.K. Huntley, E.A. Oehler, G.E. Harders, T. Ichiki, S. Mangiafico, K.A. Nath, M.M. Redfield, H.H. Chen, J.C. Burnett Jr.; Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney–heart connection; Am. J. Physiol. Regul. Integr. Comp. Physiol., 302 (2) (2012 Jan 15), pp. R292–R299
Document information
Published on 19/07/16
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?