Abstract
Background
Transverse aortic constriction (TAC) operation is used as an experimental model of left ventricular (LV) hypertrophy and LV failure in mice. The severity of LV remodeling or failure may depend on the degree of TAC, but is variable among operated animals. Therefore, we tried to identify the optimal diameter of TAC to create this model with ease and high reproducibility.
Methods and results
To produce TAC in C57BL/6J mice (7–9 weeks, body weight 19–26 g, n = 109), a 7–0 nylon suture ligature was tightly tied around the transverse aorta against needles with 3 different diameters (mm); 0.40, 0.385 and 0.375. LV wall thickness, end-diastolic dimension, fractional shortening were measured by echocardiography. At 4 weeks after TAC, no mouse with the 0.400 mm gauge progressed in LV failure. The 0.385 mm pin gauge mouse kept a more survival rate compared with the 0.375 mm (59% vs 48%), representing same efficient in LV failure. With the 0.385 mm pin gauge, hearts of mice remained LV hypertrophy at 1 week after TAC, followed by LV failure at 4 weeks.
Conclusion
TAC with the diameter of 0.385 mm can effectively induce the transition from LV hypertrophy to failure in mice with relatively preserved survival.
Keywords
Methodology;Transverse aortic constriction;Cardiac hypertrophy;Cardiac failure;Mice
1. Introduction
To investigate the effect of drug therapy and the molecular mechanism for heart failure (HF), researchers have used three main models of HF in mice; myocardial infarction (MI) by coronary artery ligation, chronic pressure overload model by transverse aortic constriction (TAC), and chronic volume overload by aortocaval fistula [1]. TAC operation is often used as an experimental model of left ventricular (LV) hypertrophy and HF in mice. In spite of very common model, TAC model has a diversity of the severity of LV remodeling or HF, which has been shown to depend on strain [2], sex [3] and standardizing needle size used for TAC operation [4]. Strain and sex of mice can be determined by study design. However, only the needle sizes need to be adjusted to create an appropriate model for the purpose of study.
In recent studies, needle sizes from 24 to 28-gauge, frequently 27-gauge (0.40 mm outside diameter (OD)), have been selected in TAC operation [5]; [6] ; [7]. In the study of HF, we need mice model with severe HF and obvious LV remodeling by TAC keeping high survival rate. However, we actually noticed that some mice remained hypertrophy without the manifestations of HF in surviving mice, others died quickly. The purpose of this study was which size of gauges was suitable for TAC operation from the point of view of survival and severity of HF.
Now pin gauges for industrial use are commercially available, which can vary its size used for TAC operation in 0.005 mm increments. To determine which size of gauges is most suitable for TAC operation, we studied survival and severity of HF after TAC by using three sizes of gauges; medical needle with a 0.400 mm OD, pin gauges for industrial use with a 0.385 mm OD and a 0.375 mm OD.
2. Methods
All experimental procedures and animal care were approved by our institutional animal research committee and conformed to the Animal Care Guideline for the Care and Use of Laboratory Animals of Hokkaido University Graduate School of Medicine and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
2.1. TAC operation
As previously described by Rockman et al. [8], TAC was created in male C57BL/6J mice (7 to 10 weeks old and 19 to 26 g body weight (BW), CLEA Japan, Inc., Tokyo, Japan) by a suture ligature around the transverse aorta. All procedures were performed by a single operator. Briefly, mice were anesthetized with a combination anesthetic, called MMB, via intraperitoneal administration. We mixed 3 drugs; 0.3 mg/kg of medetomidine (Dorbene® Kyoritsuseiyaku Co., Ltd., Tokyo, Japan), 4.0 mg/kg of midazolam (Dormicum®, Astellas Pharma Inc., Tokyo, Japan), and 5.0 mg/kg of butorphanol (Vetorphale®, Meiji Seika Kaisha, Ltd., Tokyo, Japan) [9]. Mice were placed on the pad in the dorsal position, and then the limbs were fixed with surgical tapes. Mice were intubated, the tube connected to experimental rodent ventilators (Shinano Co., Ltd., Tokyo, Japan) under the condition of 120 breaths per minutes and 0.3 ml tidal volume.
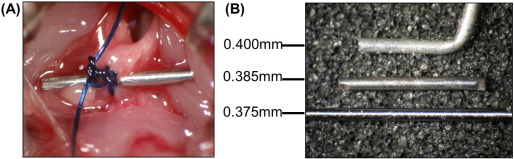
The skin from the neckline to the diaphragm was disinfected with 70% alcohol. After thoracic incision with the scissor, the second intercostal space through a small incision with the forceps, the thymus being divided into two pieces, the transverse aorta was exposed with four rib spreaders. A 7–0 nylon (Akiyama Co., Ltd., Yamanashi, Japan) suture ligature was “very tight without deformation of the nylon” tied around the transverse aorta with a thin metal rod to produce a constriction after removal of the rod (Fig. 1A). Three kinds of thin metal rods were used in the present study; the 27 gauge medical needle with a 0.400 mm OD (TERUMO Co., Ltd., Japan), the pin gauges for industrial use with a 0.385 mm OD and a 0.375 mm OD (EISEN Co., Ltd., Shiga, Japan). The picture of these 3 gauges used in the present study was shown in Fig. 1B. Their ODs were confirmed and analyzed with ImageJ software (data not shown). The intercostal muscle, the greater pectoral muscle and the skin were closed by a 4–0 suture, respectively. After spontaneous breathing appeared, mice were extubated and moved into a cage on a pad maintained at 37 °C up to next morning. Sham operation was also performed.
|
|
|
Fig. 1. Photographs of TAC operation. (A) Mice were subjected to TAC operation using the gauge. (B) Three gauges used with TAC operation: top; a 0.400 mm gauge needle, middle; a 0.385 mm pin gauge, bottom; a 0.375 mm pin gauge. |
2.2. Experiment 1
Mice were randomly divided into sham (n = 21) and 3 different TAC groups operated by metal rods with a 0.400 mm OD (n = 11), a 0.385 mm OD (n = 29), and a 0.375 mm OD (n = 22). These mice were observed for 4 weeks.
2.3. Survival
The survival analysis was performed in all groups of mice. During four weeks after operation, the cages were inspected daily for dead animals. All dead mice were examined for the presence of TAC.
2.4. Echocardiographic measurements
At four weeks after operation, echocardiographic measurements were performed in surviving mice under light anesthesia with tribromoethanol/amylene hydrate (avertin; 2.5% wt/vol, 8 μl/g ip), as described previously [10]. A two-dimensional parasternal short-axis views were obtained at the levels of the papillary muscles. In general, the best views obtained with the transducer lightly applied to the mid upper left anterior chest wall. The transducer was then gently moved cephalad or caudad and angulated until desirable images were obtained. After it had been ensured that the imaging was on axis, two-dimensional targeted M-mode tracings were recorded at a paper speed of 50 mm/s.
2.5. Organ weights
After an addition of avertin, mice were euthanized by cervical dislocation under deep anesthesia with avertin (2.5% wt/vol, total 10 μl/g ip). Hearts and lungs were then excised and weighed.
2.6. Experiment 2
Another set of mice was divided into control (n = 11), and TAC group operated by metal rods with a 0.385 mm OD and observed for one week (n = 15). These mice were compared with TAC mice operated by metal rods with a 0.385 mm OD and observed for four weeks (n = 29) in Experiment 1.
2.7. Hemodynamic measurements, and organ histology
After echocardiographic study, a 1.4-Fr micromanometer-tipped catheter (Millar Instruments, Houston, TX) was inserted into the right carotid artery, advanced to aorta and into the LV to measure pressures in the ascending aorta and the LV.
For histological analysis, tissue was fixed in 10% formaldehyde, cut into three transverse sections; apex, middle ring, and base, then stained with hematoxylin-eosin and Massons trichrome. Myocyte cross-sectional area and collagen volume fraction were determined as described previously [10].
2.8. Statistical analysis
Data are expressed as mean ± SEM. Survival analysis was performed by the Kaplan–Meier method, and between-group differences in survival were tested by the log-rank test. A between-group comparison of means was performed by one-way ANOVA, followed by t-test. The Tukey correction was applied for multiple comparisons of means. P < 0.05 was considered statistically significant.
3. Results
3.1. Experiment 1
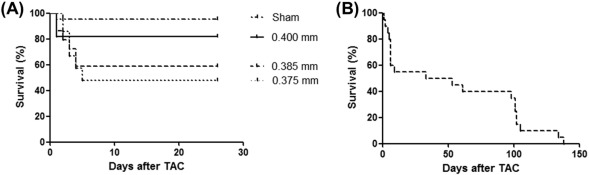
During the perisurgical period from the beginning of administrating anesthetic to 24 h after TAC, no mice died without accidental events such as bleeding around aortic arch or pneumothorax (usually less than 5%). Up to seven days after TAC operation, about 20–50% of TAC mice died of acute HF due to pressure overload by TAC, which was confirmed by pulmonary edema. From seven days after TAC to one month, TAC mice seldom died. Survival curve analysis showed the lower survival rate as the thinner metal rods were used for TAC operation. Survival rate of the 0.400 mm, the 0.385 mm, and the 0.375 mm groups was 82%, 59%, and 48%, respectively (Fig. 2A). All TAC mice with 0.385 mm gauge died within five months after TAC operation, suggesting that all of mice by TAC with 0.385 mm gauge, even if having varying degrees, suffered from HF. (Fig. 2B).
|
|
|
Fig. 2. Survival curves in TAC operation. (A) Mice were subjected to TAC operation with three kinds of gauges and followed for 4 weeks. Sham, n = 21; 0.400 mm, n = 11; 0.385 mm, n = 29; 0.375 mm, n = 22. (B) Mice were subjected to TAC operation with the 0.385 mm pin gauge and followed for 5 months. n = 20. |
Organ weight and echocardiographic data were shown in Table 1. At four weeks after TAC, while heart weight/BW, LV weight/BW and wall thickness were higher in the 0.400 mm group than sham group, lung weight/BW, LV dimension, and fractional shortening were similar, suggesting the 0.400 mm group were in the state of hypertrophy without LV dysfunction and HF. Both in the 0.385 mm group and the 0.375 mm group, heart weight/BW, LV weight/BW, lung weight/BW, LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD) were significantly increased and fractional shortening was decreased compared to sham group. There were no significant differences in all parameters in both groups. These results suggested that both groups of mice were in the state of HF with LV systolic dysfunction.
| Sham | 0.400 | 0.385 | 0.375 | |
|---|---|---|---|---|
| N | 21 | 9 | 15 | 9 |
| Organ weights | ||||
| BW (g) | 26.0 ± 0.3 | 24.8 ± 0.4 | 24.4 ± 0.3⁎ | 23.7 ± 0.7⁎ |
| HW/BW (mg/g) | 4.4 ± 0.0 | 6.6 ± 0.3⁎ | 9.7 ± 0.5⁎,† | 10.2 ± 0.8⁎,† |
| LVW/BW (mg/g) | 3.1 ± 0.0 | 4.9 ± 0.2⁎ | 6.7 ± 0.3⁎,† | 7.1 ± 0.5⁎,† |
| Lung/BW (mg/g) | 5.8 ± 0.1 | 6.4 ± 0.4 | 14.1 ± 1.5⁎,† | 16.1 ± 2.2⁎,† |
| ⟨Echocardiographic data⟩ | ||||
| N | 21 | 9 | 14 | 9 |
| HR (bpm) | 532 ± 10 | 505 ± 17 | 520 ± 19 | 496 ± 16 |
| LV EDD (mm) | 3.2 ± 0.1 | 3.2 ± 0.2 | 3.8 ± 0.2⁎ | 3.8 ± 0.2⁎ |
| LV ESD (mm) | 1.7 ± 0.1 | 2.0 ± 0.2 | 2.6 ± 0.2⁎ | 2.6 ± 0.2⁎ |
| %FS | 47 ± 2 | 38 ± 5 | 32 ± 3⁎ | 32 ± 3⁎ |
| Anterior wall thickness (mm) | 0.8 ± 0.0 | 1.2 ± 0.1⁎ | 1.0 ± 0.1⁎ | 1.2 ± 0.1⁎ |
| Posterior wall thickness (mm) | 0.8 ± 0.0 | 1.2 ± 0.1⁎ | 1.0 ± 0.1⁎ | 1.2 ± 0.1⁎ |
Data are shown as mean ± SE. N, number; BW, body weight; HW, heart weight; LVW, left ventricle weight; HR, heart rate; EDD, end-diastolic diameter; ESD, end-systolic diameter; %FS, percent fractional shortening.
⁎. P < 0.05 vs. Sham.
†. P < 0.05 vs. 0.400 mm.
3.2. Experiment 2
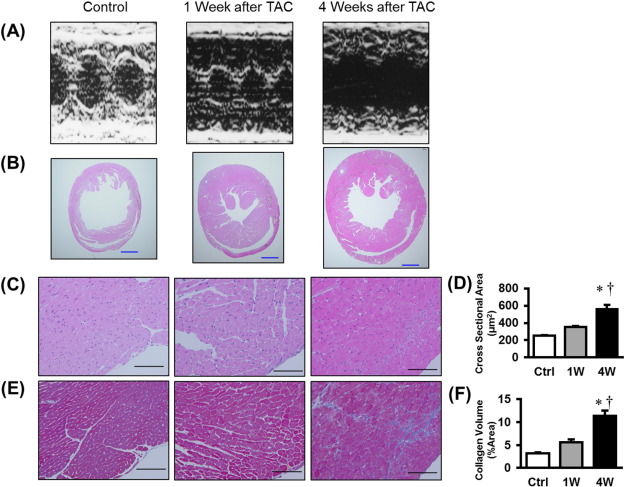
Given the survival rate and severity of HF, TAC operation with a 0.385 mm OD was suitable for the experimental model of the transition from compensated cardiac hypertrophy to HF. We next examined the time course in this group. Heart weight/BW and LV weight/BW were significantly increased in TAC 1 week group compared with control group and further increased in TAC 4 weeks group (Table 2). Lung weight/BW was not altered in TAC 1 week group compared with control group, and was significantly increased in TAC 4 weeks group (Table 2). In parallel with these results, echocardiography showed LV hypertrophy without LV dilation and LV systolic dysfunction in TAC 1 week group (Table 2, Fig. 3A and B). On the contrary, it showed LV dilation and LV systolic dysfunction in TAC 4 weeks group (Table 2, Fig. 3A and B). Myocyte cross-sectional area and collagen volume tended to be increased in TAC 1 week group compared with sham group and significantly increased in TAC 4 weeks group (Fig. 3C–F). Systolic blood pressure in proximal of aortic constriction was significantly increased in TAC 1 week compared with sham group, and further increased in TAC 4 weeks group (Table 2). LV end-diastolic pressures were significantly increased in TAC 4 weeks group to sham group (Table 2).
| Control | 1 Week | 4 Weeks | |
|---|---|---|---|
| N | 11 | 9 | 15 |
| BW(g) | 21.4 ± 0.3 | 20.6 ± 0.5 | 24.4 ± 0.3⁎,† |
| Organ weights | |||
| HW/BW(mg/g) | 4.7 ± 0.0 | 7.3 ± 0.2⁎ | 9.7 ± 0.5⁎,† |
| LVW/BW(mg/g) | 3.3 ± 0.1 | 5.4 ± 0.2⁎ | 6.7 ± 0.3⁎,† |
| Lung/BW(mg/g) | 6.4 ± 0.1 | 9.5 ± 1.1 | 14.1 ± 1.5⁎,† |
| ⟨Echocardiographic data⟩ | |||
| N | 8 | 7 | 14 |
| HR(bpm) | 541 ± 11 | 557 ± 14 | 520 ± 19 |
| LV EDD(mm) | 3.2 ± 0.1 | 2.7 ± 0.1 | 3.8 ± 0.2⁎,† |
| LV ESD(mm) | 1.6 ± 0.1 | 1.5 ± 0.2 | 2.6 ± 0.2⁎,† |
| %FS | 51 ± 3 | 46 ± 5 | 32 ± 3⁎,† |
| Anterior wall thickness(mm) | 0.8 ± 0.0 | 1.2 ± 0.1⁎ | 1.0 ± 0.1⁎ |
| Posterior wall thickness(mm) | 0.8 ± 0.0 | 1.2 ± 0.1⁎ | 1.0 ± 0.1⁎ |
| ⟨Hemodynamic data⟩ | |||
| N | 8 | 7 | 7 |
| HR(bpm) | 543 ± 20 | 521 ± 15 | 536 ± 23 |
| Ao SBP(mmHg) | 130 ± 3 | 179 ± 3⁎ | 243 ± 6⁎,† |
| Ao DBP(mmHg) | 91 ± 4 | 77 ± 3⁎ | 85 ± 4 |
| LV EDP(mmHg) | 1.8 ± 0.5 | 7.0 ± 2.4 | 8.2 ± 1.6⁎ |
Data are shown as mean ± SE. N, number; BW, body weight; HW, heart weight; LVW, left ventricle weight; HR, heart rate; EDD, end-diastolic diameter; ESD, end-systolic diameter; %FS, percent fractional shortening; Ao, aorta; SBP, systolic blood pressure; DBP, diastolic blood pressure; EDD, end-diastolic pressure.
⁎. P < 0.05 vs. Control.
†. P < 0.05 vs. 1 Week.
|
|
|
Fig. 3. Representative echocardiographic and histological images of control, and 1 and 4 weeks after TAC operation with the 0.385 mm pin gauge. (A) Representative echocardiograph and histological images from the various groups. (B) Representative histological images from the various groups. Scale Bar, 500 μm. (C) Hematoxylin and eosin (HE) stains from the various groups. (D) Masson trichrome (MT) stains from the various groups. (E) Summary data for cross sectional area. n = 3 for each. Scale Bar, 100 μm. (F) Summary data for collagen volume. n = 3 for each. Scale Bar, 100 μm. ⁎P < 0.05 vs. Control, †P < 0.05 vs. 1 Week, Ctrl, Control; 1 Week, 1 Week; 4 Weeks, 4 Weeks. |
We measured echocardiographic data before, 1 week and 4 weeks after TAC in each mouse to confirm that the mice operated with 0.385 mm gauge caused the transition from compensated LV hypertrophy to LV dysfunction and HF. They showed LV hypertrophy at 1 week and LV dysfunction at 4 weeks (data not shown).
4. Discussion
The main finding of present study was that the use of 0.385 mm OD gauge was suitable for TAC operation from the point of view of survival and severity of HF. The use of thicker gauge (0.400 mm OD) led to LV hypertrophy and showed higher survival rate than 0.385 mm OD gauge, but did not lead to HF. The use of thinner gauge (0.375 mm OD) induced HF, but showed low survival rate. Furthermore, the use of 0.385 mm OD gauge caused the transition from LV hypertrophy in early phase to LV dysfunction and HF in late phase.
TAC model has been commonly used, but has been thought to have a diversity of the severity of LV remodeling or HF. Some factors affecting the diversity have been known. Most important factor is the pressure gradient via trans-constriction with TAC, which is determined by aortic diameter, needle size, and tightness of ligature. To control the tightness of ligature is the most difficult task and is easily influenced by artificial factors during procedure of TAC operation. Therefore, we uniformed it by very tightly ligating without deformation of the nylon suture to avoid artificial factors. To minimize the variation of aortic diameter at operation, we used mice with a narrow range of BW. We further stratified mice by BW. Mice operated with 0.385 mm were divided into two groups; 19–22.5 g (lower BW group) and 22.5–26 g (higher BW group). The survival rates and the severity of LV remodeling or HF of lower BW group and higher BW group in 0.385 mm mice were similar (data not shown). Another problem is an increase in aortic diameter with growth after TAC operation. Aortic diameter may be affected by growth during long-term experiment. Therefore, we performed relatively short-term experiment, and created mice model of HF by TAC for four weeks. Our model had more severe phenotype than other report, but no mice died within 24 h after TAC operation and mortality rate was comparable during 4 weeks [11]. These results would be due to our technical advantage. We could evaluate the effects of needle size by regulating these factors affecting the diversity of TAC operation.
There are some limitations that should be acknowledged in the present study. First, we used inbred strain mice, which is suggested to have inherited individual variation. Indeed, it has been reported that parent of origin may affect the response to cardiac stress after TAC [2]. We did not control the parent of origin, which, however, did not have any contribution to our results. Second, we did not measure the pressure gradient via trans-constriction with TAC. Therefore, we could not prove that the pressure gradient was finely regulated by our procedure. However, our results of cardiac phenotype and mortality rate after TAC showed that it was the case.
This is the first report showing how to steadily create hypertrophy and HF by TAC. This method is important for decreasing the number of mice used for experiments, and also contributes obviously to animal protection.
5. Conclusions
TAC with the diameter of 0.385 mm can effectively induce the transition from LV hypertrophy to HF in mice with relatively preserved survival.
Disclosures
The authors have no conflicts of interest to disclose.
References
- [1] M.A. Haidara, A.S. Assiri, H.Z. Yassin, H.I. Ammar, M.M. Obradovic, E.R. Isenovic; Heart failure models: traditional and novel therapy; Curr. Vasc. Pharmacol., 13 (2015), pp. 658–669
- [2] C.J. Barrick, A. Dong, R. Waikel, D. Corn, F. Yang, D.W. Threadgill, et al.; Parent-of-origin effects on cardiac response to pressure overload in mice; Am. J. Physiol. Heart Circ. Physiol., 297 (2009), pp. H1003–H1009
- [3] M. Skavdahl, C. Steenbergen, J. Clark, P. Myers, T. Demianenko, L. Mao, et al.; Estrogen receptor-beta mediates male–female differences in the development of pressure overload hypertrophy; Am. J. Physiol. Heart Circ. Physiol., 288 (2005), pp. H469–H476
- [4] B.A. Rothermel, K. Berenji, P. Tannous, W. Kutschke, A. Dey, B. Nolan, et al.; Differential activation of stress-response signaling in load-induced cardiac hypertrophy and failure; Physiol. Genomics, 23 (2005), pp. 18–27
- [5] M. Sano, T. Minamino, H. Toko, H. Miyauchi, M. Orimo, Y. Qin, et al.; p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload; Nature, 446 (2007), pp. 444–448
- [6] S. Heymans, M.F. Corsten, W. Verhesen, P. Carai, R.E. van Leeuwen, K. Custers, et al.; Macrophage microRNA-155 promotes cardiac hypertrophy and failure; Circulation, 128 (2013), pp. 1420–1432
- [7] S. Szardien, H.M. Nef, S. Voss, C. Troidl, C. Liebetrau, J. Hoffmann, et al.; Regression of cardiac hypertrophy by granulocyte colony-stimulating factor-stimulated interleukin-1beta synthesis; Eur. Heart J., 33 (2012), pp. 595–605
- [8] H.A. Rockman, R.S. Ross, A.N. Harris, K.U. Knowlton, M.E. Steinhelper, L.J. Field, et al.; Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy; Proc. Natl. Acad. Sci. U. S. A., 88 (1991), pp. 8277–8281
- [9] S. Kawai, Y. Takagi, S. Kaneko, T. Kurosawa; Effect of three types of mixed anesthetic agents alternate to ketamine in mice; Exp. Anim., 60 (2011), pp. 481–487
- [10] S. Kinugawa, H. Tsutsui, S. Hayashidani, T. Ide, N. Suematsu, S. Satoh, et al.; Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress; Circ. Res., 87 (2000), pp. 392–398
- [11] E.D. van Deel, M. de Boer, D.W. Kuster, N.M. Boontje, P. Holemans, K.R. Sipido, et al.; Exercise training does not improve cardiac function in compensated or decompensated left ventricular hypertrophy induced by aortic stenosis; J. Mol. Cell. Cardiol., 50 (2011), pp. 1017–1025
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?