Abstract
Background
Q waves and negative T waves are common electrocardiographic (ECG) abnormalities in patients with Hypertrophic Cardiomyopathy (HCM). Several studies correlated ECG findings with presence and extent of fibrosis and hypertrophy; however, their significance remains incompletely clarified. Our study aimed to explain the mechanism behind Q and negative T waves by comparing their positions on a 12-lead ECG with phenotypes observed at Late Gadolinium Enhancement (LGE) Cardiac Magnetic Resonance (CMR).
Methods
12-lead ECG and LGE-CMR were performed in 88 consecutive patients with HCM (42 SD 16 years, 65 males). Using Delta Thickness ratio (DT ratio), and “global” and “parietal” LGE at CMR, the extent and distribution of myocardial hypertrophy and fibrosis were studied in correlation with ECG abnormalities.
Results
Q waves in different leads were not associated with “parietal” LGE score. Lateral Q waves correlated with an increased DT ratio Inferior Septum/Lateral wall (p = 0.01). A similar correlation between inferior Q waves and an increased DT Ratio Anterior wall/Inferior wall was of borderline statistical significance (p = 0.06). As expected, ECG signs of LV hypertrophy related to a raised Left Ventricular Mass Index (LVMI) (p < 0.0001) and mean wall thickness (p = 0.01). Depolarization disturbances, including negative T waves in lateral (p = 0.044) and anterior (p = 0.031) leads correlated with “parietal” LGE scores while QT dispersion (p = 0.0001) was associated with “global” LGE score.
Conclusion
In HCM patients, Q waves are generated by asymmetric hypertrophy rather than by myocardial fibrosis, while negative T waves result from local LGE distribution at CMR.
Keywords
Hypertrophic Cardiomyopathy;Electrocardiography;Cardiac Magnetic Resonance
1. Background
Hypertrophic Cardiomyopathy (HCM) is a genetic disorder with extremely variable morphologic, functional, and clinical features [1]. More than 90% of HCM patients with evidence of the disease at echocardiogram show various abnormalities in the 12-lead ECG [2], including pathological Q waves, signs of LV hypertrophy, and repolarization disturbances such as negative T-wave [3]; [4] ; [5]. The pathogenesis of the ECG findings, however, remains unclear. Different studies have correlated ECG abnormalities with different imaging tests, adding important piece of information in what seems to be a pathophysiological puzzle. Authors have tried to explain the mechanism of these ECG findings using low-definition and two-dimensional imaging investigations, such echocardiography and left ventriculogram, with controversial results [3]; [4] ; [6]. Late Gadolinium Enhancement Cardiac Magnetic Resonance (LGE-CMR) however, offers high spatial resolution and 3-dimensional tomographic imaging allowing not only for a more accurate morphologic and functional definition of the left ventricle (LV), but also giving information regarding tissue composition and revealing areas of myocardial fibrosis [7]; [8]; [9]; [10] ; [11]. However: Q waves have not been unequivocally correlated yet to a specific phenotype pattern. The hypothesis that an asymmetric distribution of myocardial hypertrophy rather than myocardial fibrosis may generate Q waves has been verified in anterior leads but it has not been verified in other LV walls. In addition, negative T waves have been so far correlated with apical hypertrophy while their association with local distribution of LGE at CMR appears to be relevant and needs to be supported more. The aim of the present study is to verify the hypothesis that in patients with HCM, Q waves are generated by asymmetric distribution of myocardial hypertrophy and to demonstrate that negative T waves result from local distribution of fibrosis.
2. Methods
2.1. Population
The initial study population included 137 consecutive patients with Hypertrophic Cardiomyopathy (HCM) referred to our Centre between October 2004 and January 2014. The diagnosis of HCM was based on 2-dimensional echocardiographic evidence of a hypertrophic left ventricle (maximal wall thickness ≥ 15 mm in adult index patient or ≥ 13 mm in adult relatives of a HCM patient) in the absence of another cardiac or systemic disease able to justify the amount of hypertrophy observed (e.g hypertensive cardiopathy, athletes heart, storage disease). Using this imaging technique maximal left ventricular wall thickness (LVT max) was initially assessed along with left ventricular outlow tract obstruction (LVOTO), which was measured as instantaneous peak Doppler LV outflow tract pressure gradient ≥ 30 mmHg at rest or during physiological provocation such as Valsalva manoeuvre. Forty-nine patients with a history of surgical myectomy, alcohol ablation, CMR contraindications (e.g pacemakers, implantable defibrillators or atrial fibrillation), or with a poor quality ECG strip were excluded. Therefore the final study population included 88 patients.
A standard 12-lead ECG was performed before CMR in all patients; it was analysed by ECG analysis software (CALL ECG Version 2.6.5.) and systematically reviewed by a single operator. The third universal definition of myocardial infarction and modified Sokolow–Lyon criteria were used to characterize pathological Q waves [12], and signs of LV hypertrophy [13]. Depolarization disturbances, including T wave inversion, ST depression and QT dispersion, calculated as the difference between minimum and maximum QT duration, were also noted.
Within 6 months form clinical examination and 12-lead ECG, Cardiac Magnetic Resonance images were obtained using a 1.5T scanner (Siemens Synphony, Erlangen, Germany) with a phased array cardiac coil and electrocardiogram gating. Cine magnetic resonance images were acquired to assess ventricular function, using a segmented, balanced, steady-state free precession sequence (b-SSFP) in three long-axis planes and sequential 8 mm short-axis slices from the atrio-ventricular ring to apex. On the latter axis LV endocardial and epicardial borders were manually traced at end diastole and end systole respectively. Other parameters were also measured, including LV mass, end-diastolic (LVEDV), end-systolic (LVESV) volumes divided by body surface area (BSA), as well as ejection fraction (LVEF). All CMR images were analysed by validated software (Argus; Siemens Medical Solutions) and reviewed by experienced radiologist and cardiologist. A 17-segment model for the LV was used to assess extent and distribution pattern of both end-diastolic wall thickness and LGE [14]. In regards with the hypertrophic features of the LV, the short axis was used to measure end-diastolic wall thickness of 16 segments while long axis was used for the apex. All 17 thickness values were marked and then averaged to obtain an absolute value of maximal left ventricular thickness (LVT max) as well as the mean end-diastolic thickness (mean DT). A Delta Thickness ratio (DT ratio) was created to assess LV asymmetric hypertrophy. This was expressed as ratio between the end-diastolic thickness of a hypertrophied wall, including basal, mid and apical segments, and its opposite wall. In particular, a DT ratio between Inferior Septum/and Lateral wall (DT ratio Sep-lat), anterior/inferior wall (DT ratio ant-inf) and inferior/anterior wall (DT ratio inf-ant) was calculated. Then 0.2 mmol/kg of Gadolinium-DTPA (Gadovist; Bayer Schering Pharma, Berlin, Germany or Magnevist, SheringPharma, Germany) was injected, and 10–15 min later LGE images were obtained with a breath-hold 2-D segmented phase sensitive inversion-recovery sequence (inversion time between 240 and 300 ms). LGE was considered present in areas with signal intensity exceeding 5 standard deviation (SD) compared to normal myocardium signal. The amount of LGE was measured by a semi-quantitative method, using a visual score post-processed by software CMR42 (Circle Cardiovascular Imaging, Calgary, Alberta, Canada) and blindly assessed by 2 different and expert operators. Each segment was scored out of three (1 if LGE involved < 25%, 2 if between 25% and 50%, and 3 if more than 50% of the wall thickness) and a “parietal” and “global” LGE scores were calculated as the sum of the visual scores of basal, mid and apical segments of a given wall and the whole ventricle.
Data were analysed using the software STATA 13.1 (STATA Corporation College Station, Tx, USA). Continuous variables were expressed as median and interquartile range and analysed with the Mann–Whitney U test; categorical variables were expressed as a percentage and compared by Fishers exact test. The association between continuous variables was analysed using Pearsons coefficient R or Spearmans coefficient R, depending on the distribution, with their associated CI at 95%. A two-tailed p value of < 0.05 was considered statistically significant. All participants provided written informed consents.
3. Results
Clinical characteristics and risk factors are detailed in Table 1. The study population consisted of 88 patients, 65 males (74%) and 23 females (26%), aged between 8 and 76 years old (mean age 42 SD 16 years). Fifty-seven patients (65%) were in New York Heart Association functional class I (NYHA I), 23 patients (26%) in NYHA II, 8 patients (16%) in NYHA III. Only 14 patients showed signs of subvalvular obstruction with an intraventricular gradient > 30 mmHg at continuous-Doppler placed on the LVTOT during the echocardiogram study. Thirty-one patients had a familial history of HCM (FHHCM), while 8 patients (11%) was found to have a LVT max > 30 mm at USS.
| Clinical features and risk factors | N (%) |
|---|---|
| Age (years) | 42 ± 16 |
| Male | 65 (74%) |
| Functional class NYHA I–II | 80 (91%) |
| Family history of SCD | 31 (25%) |
| Cardiac arrest or SVT | 3 (3%) |
| NSVT | 25 (28%) |
| Loss of consciousness | 1 (1%) |
| Abnormal BP response during exercise | 3 (3%) |
| Familial history of HCM | 9 (10%) |
| High-risk genetic mutation | 2 (2%) |
| LVT max > 30 mm | 8 (11%) |
| LVOTO | 14 (16%) |
SVT sustained ventricular tachycardia, SCD: Sudden Cardiac Death secondary to HCM, NSTV: nonsustaintained ventricular tachycardia at 24-hour tape, BP: Blood Pressure, HCM Hypertrophic Cardiomyopathy, LVT max > 30 mm: Left Ventricular Thickness > 30 mm at cardiac USS.
Only 2 patients (2%) had a normal ECG, while the other 86 (98%) showed different ECG abnormalities. The presence of negative T waves on ECG was by far the most common ECG abnormality detected (89%), while signs of LV hypertrophy and pathological Q waves [12] in different leads were found in 51% and 38% of cases respectively. Other electrocardiographic abnormalities are listed in detail in Table 2.
| ECG features | N (%) |
|---|---|
| Normal ECG | 2 (2%) |
| Abnormal Q waves | 33 (38%) |
| Inferior leads | − 18 (20%) |
| Lateral leads | − 11 (12%) |
| Anterior leads | − 5 (6%) |
| LBBB | 2 (2%) |
| Signs of LV hypertrophy | 45 (51%) |
| Negative T waves | 79 (89%) |
| Antero-lateral leads | − 21 (23%) |
| Lateral leads | − 23 (26%) |
| Anterior leads | − 5 (6%) |
| ST depression | 28 (31%) |
Pathological Q waves: latest universal definition of Myocardial Infarction [16]; LBBB: Left Bundle Branch Block; Signs of LV hypertrophy: Left ventricular Hypertrophy using modified Sokolow–Lyon criteria, ST depression: depression ST segment > 1 mm at 80 msec from J point.
Morpho-functional data were obtained from a systematic review of CMR images (Table 3). In detail, the mean wall thickness was 11.2 SD 2.9 mm, the mean value for the maximum thickness was 23 SD 7.5 mm, and the LV Mass Index (LVMI) was 94.2 SD 42.7 g/m2. Seventy-eight patients (88%) had features of asymmetric LV hypertrophy either localised in the posterior septum, anterior or posterior wall, with 13 patients (15%) had a LVT max > 30 mm at CMR. Seventy-five patients (86%) showed different levels and patterns of LGE after paramagnetic medium of contrast injection, with a mean “global” LGE score above 10 (10.4 SD 9.9). Twenty-nine patients (33%) had parietal LGE score > 1 in antero-Lateral wall (6th, 12th and 16th segments) while 51 patients (58%) had LGE score > 1 in the inferior wall (4th, 10th and 15th segments).
| CMR-LGE features | |
|---|---|
| LVEDV/BSA (ml/m2) | 80.2 SD 20 |
| LVESV/BSA (ml/m2) | 48.5 SD 12 |
| LVEF (%) | 62 SD 9.3 |
| LVMI (g/m2) | 94.2 SD 42.7 |
| Mean thickness (mm) | 11.2 SD 2.9 |
| Patients with LVT max > 30 mm (n) | 13 (15%) |
| Mean max thickness (mm) | 23 SD 7.5 |
| Patients with LGE n (%) | 75 (86%) |
| “Global” LGE score | 10.4 SD 9.9 |
| “Parietal” LGE score > 1 | |
| –In the 6th,12th,16th segments | − 29 (33%) |
| –In the 4th, 10th and 14th segments | − 51 (58%) |
LVEDV: Left Ventricular End-Diastolic Volume, BSA: Body Surface Area, LVESV: Left Ventricular End Sistolic Volume, LVEF: Left Ventricular Ejection Fraction, LVMI: Left Ventricular Myocardial Index, LVT max maximal Left Ventricular Thickness LGE Late Gadolinium Enhancement.
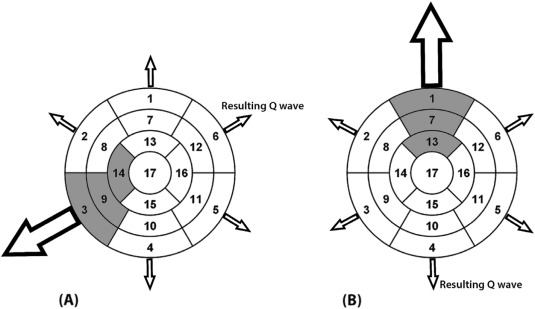
Two patients were excluded from the Q wave analysis since showing LBBB features on ECG. It was also decided not to analyse the anterior wall since only few patients showed pathological Q waves in the anterior leads (n = 5). Pathological Q waves in both lateral (1.3 SD 2.06 vs 1.13 SD 2.01, p = 0.81) and inferior leads (0.88 SD 2.31 vs 1.36 SD 2.12, p = 0.42) did not correlate with the “parietal” LGE score of lateral and inferior walls, calculated as the sum of the LGE score of the 3 correspondent segments. However, pathological Q waves in the lateral leads correlated with DT Ratio Inferior Septum–Lateral wall (1.62 SD 0.46 vs 1.34 SD 0.39, p = 0.01) and an association between Q waves in the inferior leads and a DT ratio anterior wall – inferior wall, approached statistical significance (1.5 SD 0.64 vs 1.21 SD 0.45, p = 0.06) (Fig. 1). No significant correlation was noted (p = 0.6) between the presence of LVT max > 30 mm in any of the 17 segments and the presence of Q waves on the ECG, with only 3 patients satisfying both criteria.
|
|
|
Fig. 1. Pathogenesis of Q waves in HCM patients. Based on our results, abnormal Q waves may be generated by an increased electrical force generated by hypertrophied LV wall overpowering the electrical vector by its opposite wall nearest to the exploring lead. 1: basal anterior, 2: basal anteroseptal, 3: basal inferoseptal, 4: basal inferior, 5: basal inferolateral, 6: basal anterolateral, 7:mid anterior, 8: mid anteroseptal, 9: mid inferoseptal, 10: mid inferior, 11: mid inferolateral, 12: mid anterolateral, 13: apical anterior, 14: apical septal, 15:apical inferior, 16: apical lateral, 17: apex. The shaded segments: hypertrophied segments. The arrow size is proportionate to electrical forces generated by the corresponding ventricular walls. A: increased DT ratio Inferior Septum/Lateral wall, B: Increased DT ratio Anterior/Inferior wall. |
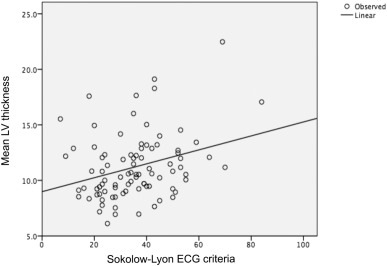
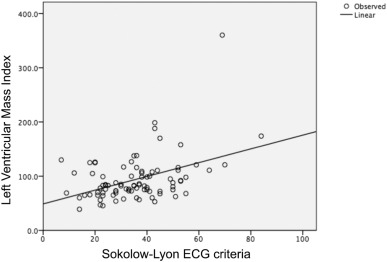
Using the modified Sokolow–Lyon criteria, half of the patients (51%) showed ECG signs of LV hypertrophy, which, as expected, shared a linear relationship with both LVMI (r = 0.38, p < 0.0001) (Fig. 2) and mean LV thickness (10.4 SD 2.5 n = 43 vs 12 SD 3.1 n = 45 p = 0.01) (Fig. 3). Conversely, there was no correlation between ECG signs of LV hypertrophy and LVT max thickness (p = 0.58).
|
|
|
Fig. 2. Correlation between ECG signs of LV hypertrophy and LVMI. |
|
|
|
Fig. 3. Correlation between ECG signs of LV hypertrophy and mean left ventricular thickness. |
Various repolarization disturbances were detected. First, negatives T waves were the most common abnormality (89%) among HCM patients. The “parietal“LGE score for the anterior wall was significantly higher in patients with negative T waves in the anterior leads if compared with patients without such ECG abnormality (3.08 SD 3.04 vs 1.82 SD 2.31, p = 0.044). Similarly, negative T waves in the lateral leads were more common in patients with increased LGE score of the Lateral wall (1.33 SD 2.13 vs 0.46 SD 1.31, p = 0031). However correlation between inferior negative T waves and increased LGE of the inferior wall was also suggested, without attaining statistical significance (p = 0.089). Second, ST segment depression was not correlated neither with LGE score (11.14 SD 10.5 vs 10.1 SD 9.8, p = 0.6) or LVMI (103.2 SD 60.3 vs 89.8 SD 30.6, p = 0.2), but it was more common in patients showing ECG signs of LV hypertrophy. Last, the QT dispersion was predicted by the LGE score (β = 0.381, p = 0.0001), but was not related with LVMI.
4. Discussion
The main result of the present study is the contribution to a more precise understanding of the exact pathogenesis of ECG abnormalities in HCM patients. In particular our data strongly support the hypothesis that abnormal Q waves result from imbalanced electrical forces generated by the asymmetric distribution of myocardial hypertrophy in opposite left ventricular walls.
A direct correlation between increasing LVMI, LGE prevalence/extent at CMR and number/severity of ECG abnormalities has been previously observed and a simple score has been proposed for the quantification of the abnormal ECG findings in HCM patients, including Q and negative T waves and other repolarisation disturbances [15]. The pathophysiological mechanism causing abnormal Q waves is still object of controversy. Some Authors previously suggested that abnormal Q waves in HCM patients are caused by the presence of fibrotic tissue similarly to transmural myocardial infarction [16] ; [17]. Others, by correlating ECG findings with echocardiogram first and LGE-CMR later suggested that lateral Q waves might be secondary to an abnormal activation of the hypertrophied septum rather than fibrosis [6]; [13] ; [18]. This hypothesis was also recently supported by a French study of 42 patients [19]. Negative T waves have been correlated with a specific pattern of apical hypertrophy extremely common in Asia (25% of cases) called “Japanese variant” ascribing the pathogenesis of such ECG abnormalities to a chronic ischaemia and fibrosis replacement [20].
With a sensible sample size, the present study aimed to confirm the hypothesis that focal distribution of myocardial hypertrophy and fibrosis on LGE-CMR correlated with ECG abnormalities in the leads exploring the very same LV walls.
First, a statistically significant correlation between lateral Q waves and DT ratio Inferior Septum–Lateral wall was noted, while Q waves in inferior leads and DT ratio anterior-inferior wall had p-value just above the significance level (p = 0.06). This observation supports the hypothesis that pathological Q waves observed in a given lead might be the result of an unbalanced electrical-force generated by an asymmetric hypertrophy of the ventricular wall opposite to the ECG exploring lead, rather than by myocardial fibrosis.
Second, as expected, signs of hypertrophy on ECG correlated with both with LVMI and mean thickness, but not with maximum thickness > 30 mm.
Third, depolarization disturbances were found extremely common and correlated with LGE. In fact, almost 90% of our patients showed negative T waves. When present in anterior and Lateral walls negative T waves significantly correlated with the “parietal” LGE score of the correspondent walls. In regards with the inferior wall a correlation between ECG and CMR findings was suggested, yet not statistically significant. Also, many Authors demonstrated QT dispersion is increased in patients with HCM [21]; [22] ; [23]. In our study, LGE score predicted the QT dispersion, most likely due to the local difference of the repolarization duration, and increasing the risk of malignant ventricular arrhythmias [22]; [23] ; [24]. It is likely that myocardial disarray and fibrosis may result in heterogeneity of the ventricular refractory period and intra-ventricular conduction, causing negative T waves and QT dispersion.
We concluded that Q waves might be generated by asymmetric distribution of myocardial hypertrophy rather than by myocardial fibrosis, while negative T waves may result from local distribution of LGE at CMR.
5. Study limitations
The main limitation of the present study is the relatively small sample size of our population. Hence, our study and analysis was based on small sub-groups of patients, making difficult reaching a definite conclusion. Also, 90% of our patients were in functional class NYHA I
II, therefore ECG abnormalities might have different implications in advanced patients.
6. Conclusion
Our study suggests pathological Q waves in patients with HCM are not be caused by fibrosis, as in ischemic heart disease, but result from unbalanced electrical forces generated by asymmetric myocardial hypertrophy. Our study also proposes that fibrosis is the cause of local repolarization disturbances, including negative T waves and QT dispersion.
Conflicts of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgements
Not applicable.
References
- [1] M.S. Maron, B.J. Maron, C. Harrigan, et al.; Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance; J. Am. Coll. Cardiol., 54 (2009), pp. 220–228 (16)
- [2] C.J. McLeod, M.J. Ackerman, R.A. Nishimura, A.J. Tajik, B.J. Gersh, S.R. Ommen; Outcome of patients with hypertrophic cardiomyopathy and a normal electrocardiogram; J. Am. Coll. Cardiol., 54 (2009), pp. 229–233
- [3] M. Usui, H. Inoue, J. Susuki, et al.; Relationship between distribution of hypertrophy and electrocardiographic changes in hypertrophic cardiomyopathy; Am. Heart J., 126 (1993), pp. 177–183
- [4] F. Alfonso, P. Nihoyannopoulos, J. Stewart, et al.; Clinical significance of giant negative T waves in hypertrophic cardiomyopathy; J. Am. Coll. Cardiol., 15 (1990), pp. 965–971
- [5] R. Lemery, A. Kleinebenne, P. Nihoyannopoulos, et al.; Q waves in hypertrophic cardiomyopathy in relation to the distribution and severity of right and left ventricular hypertrophy; J. Am. Coll. Cardiol., 16 (1990), pp. 368–374
- [6] H. Yamaguchi, T. Ishimura, S. Nishiyama, et al.; Hypertrophic non obstructive cardiomyopathy with giant negative T waves (apical hypertrophy): ventriculographic and echocardiographic features in 30 patients; Am. J. Cardiol., 44 (1979), pp. 401–412
- [7] C. Rickers, N.M. Wilke, M. Jerosch-Herold, et al.; Utility of cardiac 22. Magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy; Circulation, 112 (2005), pp. 855–861
- [8] R.R. Edelman, M.R. Contrast-enhanced; imaging of the heart: 23. Overview of the literature; Radiology, 232 (2004), pp. 568–653
- [9] M.W. Hansen, N. Merchant; MRI of hypertrophic cardiomyopathy: part I, MRI appearances; AJR Am. J. Roentgenol., 189 (2007), pp. 1335–1343
- [10] M.W. Hansen, N. Merchant; MRI of hypertrophic cardiomyopathy: part 2, Differential diagnosis, risk stratification, and post treatment MRI appearances; AJR Am. J. Roentgenol., 189 (2007), pp. 1344–1352
- [11] R.J. Kim, R.M. Judd; Gadolinium-enhanced magnetic resonance 25. Imaging in hypertrophic cardiomyopathy; J. Am. Coll. Cardiol., 41 (2003), pp. 1568–1572
- [12] K. Thygesen, J.S. Alpert, A.S. Jaffe, M.L. Simoons, B.R. Chaitman, H.D. White; Third universal definition of myocardial infarction. Joint ESC/ACCF/AHA/WHF Task Force for Universal Dedefinition of Myocardial Infarction; J. Am. Coll. Cardiol., 60 (2012), pp. 1581–1598
- [13] C.A. Dumont, L. Monserrat, R. Soler, et al.; Interpretation of electrocardiographic abnormalities in hypertrophic cardiomyopathy with cardiac magnetic resonance; Eur. Heart J., 27 (2006), pp. 1725–1731
- [14] M.D. Cerqueira, N.J. Weissman, V. Dilsizian, et al.; Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging; Circulation, 105 (2002), pp. 539–542
- [15] S.D. Delcrè, P. Di Donna, S. Leuzzi, et al.; Relationship of ECG findings to phenotypic expression in patients with hypertrophic cardiomyopathy: a cardiac magnetic resonance study; Int. J. Cardiol. (2012) https://doi.org/10.1016/j.ijcard.2012.03.074
- [16] O.P. Bahl, T.J. Walsh, E. Massie; Elettrocardiography and vectorcardiography in idiopathic hypertrophic subaortic stenosis; Am. J. Med. Sci., 259 (1970), pp. 262–271
- [17] B.J. Maron, S.E. Epstein, W.C. Roberts; Hypertrophic cardiomyopathy and transmural myocardial infarction without significant atherosclerosis of extramural coronary arteries; Am. J. Cardiol., 43 (1979), pp. 1086–1102
- [18] B.J. Maron; Q waves in hypertrophic cardiomyopathy: a reassessment; J. Am. Coll. Cardiol. (1990), pp. 375–376
- [19] S. Grall, L. Biere, G. Clerfond, et al.; ECG characteristics according to the presence of late gadolinium enhancement on cardiac MRI in hypertrophic cardiomyopathy; Open Heart, 1 (2014) https://doi.org/10.1136/openhrt-2014-000101
- [20] B.G. Song, H.S. Yang, H.K. Hwang, et al.; Correlation of electrocardiographic changes and myocardial fibrosis in patients with hypertrophic cardiomyopathy detected by cardiac magnetic resonance imaging; Clin. Cardiol., 36 (2013), pp. 31–35
- [21] J.C. Cowan, K. Yusoff, M. Moore, et al.; Importance of lead selection in QT interval measurement; Am. J. Cardiol., 61 (1988), pp. 83–87
- [22] G. Buja, M. Miorelli, P. Turrini, P. Melacini, A. Nava; Comparison of QT dispersion in hypertrophic cardiomyopathy between patients with and without ventricular arrhythmias and sudden death; Am. J. Cardiol. (1993)
- [23] A.T. Yetman, R.M. Hamilton, L.N. Benson, B.W. McCrindle; Long-term outcome and prognostic determinants in children with hypertrophic cardiomyopathy; J. Am. Coll. Cardiol. (1998)
- [24] M. Malik, V.N. Batchvarov; Measurement, interpretation and clinical potential of QT dispersion; J. Am. Coll. Cardiol., 36 (2000), pp. 1749–1766
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?