Summary
Background
Application of minimally invasive techniques in the surgical management of distal pancreatic lesions is increasing. Despite this, numbers of laparoscopic distal pancreatectomy remain low and limited to treatment of benign and premalignant lesions.
Methods
Retrospective analysis of 31 patients who underwent distal pancreatectomy from 2005 to 2010. Patients were grouped according to mode of surgical access: open (ODP) or laparoscopic (LDP). Perioperative parameters were compared.
Results
Twenty-one (67.7%) patients underwent ODP and 10 (32.3%) LDP (median age 61; 80.0% females in LDP group, p = 0.030). Postoperative morbidity rate were comparable between the two groups. In the LDP group, there were significantly lower estimated blood loss (p < 0.001) and amount of blood transfusion (p = 0.001), smaller tumor size (p = 0.010) and fewer lymph nodes harvested (p = 0.020), shorter postoperative length of stay (p = 0.020), and shorter length of stay in surgical high dependency (p = 0.001).
Conclusion
LDP is a safe, efficient technique for resection of benign and premalignant pancreatic lesions. Indices reflecting perioperative outcomes in this study are highly competitive with those in other major centers.
Keywords
clinical outcomes;distal pancreatectomy;laparoscopic surgery
1. Introduction
Over the course of the past quarter century, the progressive advancement of surgical technologies has been accompanied by a rise in the use of minimally invasive techniques for the surgical management of several gastrointestinal conditions. With the development of advanced bioimaging techniques and their increasing popularity as diagnostic tools in the clinical approach to abdominal symptoms, more pancreatic lesions have been detected,1 especially among young women.2
The safety and feasibility of laparoscopic distal pancreatectomy (LDP) was first documented in a porcine model by Soper et al.3 This was then followed by the earliest reports on LDP carried out in humans by Cuschieri et al.4 Since then, this procedure has been further examined in a number of case series and multi-institutional studies with emphasis on its safety and efficacy.5; 6 ; 7 In general, it has been found to have comparable postoperative morbidity rates and shorter lengths of stays in hospitals when measured up against open distal pancreatectomy (ODP). Despite substantial support for LDP based on the available literature, to date there are no clear international guidelines detailing best surgical practices with regard to the various types of distal pancreatic lesions.
Although there has been a growing interest in the ability to perform LDP, the number of LDP performed by many centers remains low.8 A recent review of 72 studies revealed that centers with higher case volumes have better clinical outcomes.9 This trend has also been found to exist with regards to various gastrointestinal operations such as esophagectomies, gastrectomies and colectomies. However, it is especially true for pancreatic resections.10
This report presents the results of a retrospective review in our institution designed to compare perioperative parameters in the group of patients who underwent LDP to the group who underwent ODP. Differences in postoperative outcomes between the 2 treatment groups were identified and analysed critically.
2. Patients and methods
We performed a retrospective analysis of a prospectively maintained database of 31 patients who underwent distal pancreatectomy (DP) for both benign and malignant conditions in our institution from 2005 to 2010. The patient cohort was divided into two groups according to the mode of surgical access: laparoscopic (LDP) or open (ODP) distal pancreatic resection. The perioperative parameters were compared between the two groups.
Demographic information and blood parameters were compared between the two groups, as were the operative information and histopathology. Postoperative outcome was examined especially looking at the morbidity and mortality rates and the length-of-stay (LOS). The definitions of the common morbidity following pancreatectomy as recommended by the International Study Group for Pancreatic Surgery (ISGPS) were adopted in this study. They included postoperative pancreatic fistula (POPF), delayed gastric emptying and postpancreatectomy hemorrhage.11; 12 ; 13 All complications were graded according to the classification proposed by Clavien et al.14; 15; 16 ; 17 Operative mortality was defined as the rate of death before hospital discharge or within 30 days after the main surgical procedure.10
All patients with significant cardiac and respiratory conditions were assessed by cardiologists and respiratory physicians. Mandatory preoperative cardiac assessments were conducted on patients older than 65 years; younger patients were selected on a case-specific basis. All patients underwent preoperative chest physiotherapy and incentive spirometry, and smokers were advised to cease smoking for at least two weeks prior to the operation, to minimize postoperative respiratory morbidity.18
All 31 distal pancreatic resections were performed by either open or laparoscopic access. Types of resection included subtotal pancreatectomy, distal pancreato-splenectomy, spleen preserving distal pancreatectomy and radical antegrade modular pancreato-splenectomy (RAMP). Prior to proceeding with ODP, a thorough diagnostic laparoscopy was routinely performed to rule out obvious peritoneal and liver metastasis.19 In the absence of distant metastasis, either an upper midline or L-incision (upper midline with left subcostal extension) was created. The peritoneal cavity was then examined careful for metastasis. The lesser sac was entered through gastrocolic ligament and the pancreatic neck was dissected by creating a tunnel along superior mesenteric vein/portal vein axis. The pancreas was transected either with a surgical stapler with or without buttressing material such as Seamguard Bioabsorbable Staple Line Reinforcement (Gore Flagstaff, AZ, USA). Further reinforcement with non-absorbable continuous sutures such as polypropylene (Prolene Ethicon, Inc, Johnson & Johnson, Somerville, NJ, USA) along the transected pancreatic surface was sometimes performed. Next, the splenic artery and vein were dissected separately. The splenic artery was isolated, divided, and suture ligated, followed by ligation of splenic vein. Lymphadenectomy was performed in the medial-to-lateral direction as far as the splenic hilum. If concurrent splenectomy was required, the spleen was mobilized by dividing the lienorenal, lienocolic, lienoogastric, and lienophrenic ligaments. In bulky tumors, en bloc resection of surrounding organs such as stomach, colon, small bowel, and even a cuff of diaphragm was sometimes required. In RAMPs, the initial steps of mobilization involved complete Kocher’s maneuver as far as the left renal vein crossing the aorta, and this would mark the posterior extent of peripancreatic tissues to be removed as part of the lymphovascular clearance in this operation. The subsequent steps of dissection were largely similar to those described above. The technique of LDP was previously described in our publication on laparoscopic spleen-preserving distal pancreatectomy. 20 All patients had one or two closed drains placed in the peritoneal cavity close to the transected end of the pancreas.
A standard pancreatic surgery care pathway is used for postoperative management in the wards. Patients are kept nil-by-mouth with a Ryle’s tube for passive drainage and aspiration at 4-hourly intervals. A single dose of 200 μg of subcutaneous sandostatin is administered during pancreatic transection and this is continued until the patient resumes a solid diet postoperatively. The postoperative dose is dependant on the consistency of the pancreatic tissue assessed during operation. If the pancreas is soft or pancreatic duct is <3 mm, 200 μg at 8-hourly dosing interval is administered, otherwise 100 μg 8 hourly is given. Patients are allowed nonmilk feeds if nasogastric output is <100 ml on the first postoperative day (POD) and the Ryle’s tube is removed on the second POD if the output remains <100 ml. Feeding is graduated as tolerated. In general, patients will be taking full diet by the third POD.21 Drain fluid and serum amylase are performed on the first, third and fifth PODs. The definitions of pancreatic leak and other complications were according to ISGPS recommendations as mentioned above.
The data were analyzed using statistical software (SPSS Statistics 18.0, SPSS Inc, Chicago, IL, USA). Categorical variables were analyzed using the Chi-square test while continuous variables were compared using the Student t test (parametric distribution) or Mann-Whitney test (nonparametric distribution). All tests were two-sided and p < 0.050 was considered to be statistically significant.
3. Results
3.1. Demographics
Ten patients (32.3%) underwent LDP while 21 (67.7%) underwent ODP. Median age of the cohort was 61 (37–79) years and the majority were ethnic Chinese (93.5%). The proportion of female patients in LDP was more than twice that in ODP (80.0% vs 38.1%, p = 0.030). However, there was no difference between the two groups in terms of age (p = 0.470), racial distribution (p = 0.310) and ASA classification ( Table 1).
| Demographics | All (n = 31) | ODP (n = 21) | LDP (n = 10) | p ^ | |||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | ||
| Age | 61 | 37–79 | 62 | 37–77 | 58 | 42–79 | 0.472 |
| n | % | n | % | n | % | ||
| Number of patients | 31 | 100 | 21 | 67.7 | 10 | 32.3 | — |
| Gender | 0.029* | ||||||
| Male | 15 | 48.4 | 13 | 61.9 | 2 | 20.0 | — |
| Female | 16 | 51.6 | 8 | 38.1 | 8 | 80.0 | — |
| Race | 0.313 | ||||||
| Chinese | 29 | 93.5 | 19 | 90.5 | 10 | 100.0 | — |
| Malay | 2 | 6.5 | 2 | 9.5 | 0 | 0 | — |
| Comorbidities present | 25 | 80.6 | 17 | 81.0 | 8 | 80.0 | 0.950 |
| Hypertension | 16 | 51.6 | 12 | 57.1 | 4 | 40.0 | 0.372 |
| Diabetes mellitus | 13 | 41.9 | 8 | 38.1 | 5 | 50.0 | 0.530 |
| Hyperlipidemia | 10 | 32.3 | 5 | 23.8 | 5 | 50.0 | 0.145 |
| Ischemic heart disease | 3 | 9.7 | 1 | 4.8 | 2 | 20.0 | 0.180 |
| COLD | 1 | 3.2 | 1 | 4.8 | 0 | 0 | 0.483 |
| ASA Score | 0.240 | ||||||
| 1 | 1 | 3.2 | 0 | 0 | 1 | 10.0 | — |
| 2 | 20 | 64.5 | 15 | 71.0 | 5 | 50.0 | — |
| 3 | 10 | 32.3 | 6 | 28.0 | 4 | 40.0 | — |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | — |
^ p values for ODP vs LDP; * Statistically significant.
COLD = chronic obstructive lung disease.
3.2. Preoperative clinical information
Indices of preoperative clinical information are summarized in Tables 2 and 3. More than 80% of patients had at least one comorbid condition. The most common comorbidity in both groups was hypertension (57.1% in ODP vs. 40.0% in LDP, p = 0.370). Abdominal pain was the most common presentation in both groups. No statistically significant difference was found with regards to other preoperative clinical information between the two groups of patients.
| All (n = 31) | ODP (n = 21) | LDP (n = 10) | p ^ | |
|---|---|---|---|---|
| BMI, median (range) | 21.8 (18.7–32.5) | 21.0 (18.7–28.7) | 25.0 (21.8–32.5) | 0.112 |
| Clinical presentation, n (%) | ||||
| Abdominal pain | 11 (35.5) | 9 (42.9) | 2 (20.0) | — |
| Other presentations# | 9 (29.0) | 6 (28.6) | 3 (30.0) | — |
| Weight loss | 6 (19.4) | 6 (28.6) | 0 | — |
| Loss of appetite | 3 (9.7) | 3 (14.3) | 0 | — |
| Abdominal mass | 3 (9.7) | 3 (14.3) | 0 | — |
| Back pain | 2 (6.5) | 2 (9.5) | 0 | — |
| Diarrhea | 2 (6.5) | 1 (4.8) | 1 (10.0) | — |
| Melena | 1 (3.2) | 1 (4.8) | 0 | — |
| Vomiting | 1 (3.2) | 1 (4.8) | 0 | — |
| Acute pancreatitis | 0 | 0 | 0 | — |
| New onset DM | 0 | 0 | 0 | — |
| Steatorrhea | 0 | 0 | 0 | — |
^ p values for ODP vs LDP; # Abdominal bloatedness/distention, constipation, epigastric discomfort, dizziness, hematemesis, hypoglycemia.
BMI = body mass index; DM = diabetes mellitus.
| All (n = 31) | ODP (n = 21) | LDP (n = 10) | p ^ | |
|---|---|---|---|---|
| Preoperative hematology, median (range) | ||||
| Hemoglobin concentration (g/dL) | 13.1 (6.8–15.4) | 13.2 (6.8–15.4) | 13.0 (10.2–15.2) | 0.667 |
| PT (s) | 13.1 (11.6–17.0) | 13.2 (11.6–17.0) | 12.8 (12.1–16.4) | 0.846 |
| PTT (s) | 30.5 (24.4–37.2) | 29.8 (24.4–37.2) | 30.2 (27.5–34.3) | 0.543 |
| Preoperative biochemistry, median (range) | ||||
| Albumin (g/L) | 37.0 (19.0–44.0) | 36.0 (19.0–44.0) | 37.5 (31.0–43.0) | 0.831 |
| CA 19-9 (U/ml) | 18.0 (0.5–4702.0) | 15.5 (0.5–4702.0) | 21.0 (0.5–1619.0) | 0.846 |
^ p values for ODP vs. LDP.
CA = carbohydrate antigen; PT = prothrombin time; PTT = partial thromboplastin time.
3.3. Intraoperative outcomes
Duration of operation. The overall median length of operation was 353 (115–660) min. The median duration of operation in ODP was 330 (115–660) min and the median duration of operation in LDP was 383 (240–490) min (p = 0.500; Table 4).
| All (n = 31) | ODP (n = 21) | LDP (n = 10) | p ^ | |
|---|---|---|---|---|
| Median operative time: min (range) | 353 (115–660) | 330 (115–660) | 383 (240–490) | 0.495 |
| Median estimated blood loss: ml (range) | 300 (200–1500) | 600 (200–1500) | 275 (200–300) | <0.001* |
| Median blood transfusion: units (range) | 4 (0–29) | 4 (0–29) | 2 (0–11) | 0.001* |
| Median lymph nodes harvested (range) | 4 (0–29) | 4 (0–29) | 2 (0–11) | 0.020* |
^ p values for ODP vs LDP; * Statistically significant.
Lymph node resection. The overall median number of lymph nodes harvested was 4 (0–29). The median number of lymph nodes harvested in LDP was 2 (0–11), half that of the median number of lymph node harvested in ODP 4 (0–29; p = 0.020; Table 4).
Estimated blood loss (EBL) and blood transfusion. The median overall EBL was 300 (200–1500) ml. The median EBL for LDP was 275 (200–300) ml, which was about one-third of that in ODP (median 600, 200–1500 ml; p < 0.001). Concomitantly, the median amount of blood transfusion in ODP was 4 (0–29) units compared to the median of 2 (0–11) units in the LDP group (p = 0.001; Table 4).
Tumor size. The median overall tumor size was 45 (2–170) mm. In the ODP group, the median size of tumors removed was 50 (14–170) mm, which was significantly larger than those removed in LDP (median 25, 2–67) mm (p = 0.009; Table 5).
| All (n = 31) | ODP (n = 21) | LDP (n = 10) | p ^ | |
|---|---|---|---|---|
| Tumor size, median (range), mm | 45 (2–170) | 50 (14–170) | 24.5 (2–67) | 0.009* |
| Nature of tumor, n (%) | 0.004* | |||
| Benign | 4 (12.9) | 3 (14.3) | 1 (10.0) | — |
| Premalignant | 12 (38.7) | 4 (19.0) | 8 (80.0) | — |
| Malignant | 15 (48.4) | 14 (66.7) | 1 (10.0) | — |
| Histopathology, n (%) | 0.068 | |||
| Ductal adenocarcinoma | 9 (29.0) | 8 (38.1) | 1 (10.0) | — |
| MCN (benign) | 5 (16.1) | 2 (9.5) | 3 (30.0) | — |
| Serous cystadenoma | 3 (9.7) | 2 (9.5) | 1 (10.0) | — |
| Neuroendocrine tumor (benign) | 3 (9.7) | 0 | 3 (30.0) | — |
| Neuroendocrine tumor (malignant) | 2 (6.5) | 2 (9.5) | 0 | — |
| MCN (malignant) | 1 (3.2) | 1 (4.8) | 0 | — |
| IPMT (benign) | 1 (3.2) | 0 | 1 (10.0) | — |
| IPMT (malignant) | 1 (3.2) | 1 (4.8) | 0 | — |
| Others | 6 (19.4) | 5 (23.8) | 1 (10.0) | — |
| Location of tumor, n (%) | 0.919 | |||
| Pancreatic body | 12 (38.7) | 8 (38.1) | 4 (40.0) | — |
| Pancreatic tail | 19 (61.3) | 13 (61.9) | 6 (60.0) | — |
MCN = mucinous cystic neoplasm; IPMT = intraductal papillary mucinous tumor.
^ p values for ODP vs LDP; * Statistically significant.
Tumor pathology. The most common site of pathology in this cohort was the pancreatic tail (n = 19, 61.3%). Similar distribution of site of pathology was observed in the LDP and ODP groups (p = 0.919; Table 5). There was significantly higher proportion of malignancy in the ODP group (n = 14, 66.7%) as compared to LDP group (n = 1, 10.0%; p = 0.004). Majority of the tumors in LDP was premalignant (n = 8, 80.0%). The detailed histopathology information is listed in Table 5.
3.4. Postoperative outcomes
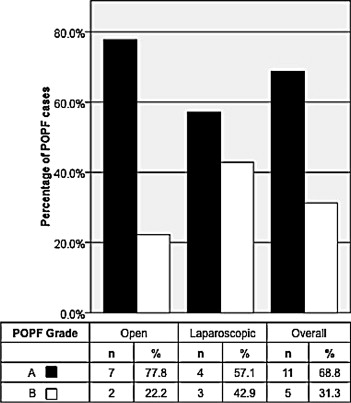
Morbidity rate. The overall postoperative morbidity rate was 61.3% in this cohort. The rate of postoperative morbidity in the LDP group (70.0%) was comparable to that in the ODP group (57.1%; p = 0.490). The rate of POPF in the LDP group was 70.0% (n = 7) as compared to 42.9% (n = 9) in ODP (p = 0.160; Table 6). However, the vast majority of them were Grade A POPF, which does not contribute to significant poor clinical outcome (ODP = 77.8%, n = 7 vs. LDP = 57.1%, n = 4; Fig. 1). This was translated into an overestimation of postoperative morbidity by approximately 30% in both the ODP and LDP groups (28.4% and 30.0%, respectively; Fig. 1). There was no Grade C POPF in either study group. There was no statistical difference between the two groups in terms of the incidence of delayed gastric emptying and postpancreatectomy hemorrhage (Table 6). The rate of wound infection was also comparable between the two groups (ODP= 14.3% vs. LDP= 10.0%, p = 0.740).
| Post-Operative Morbidity | All (n = 31) | ODP (n = 21) | LDP (n = 10) | p ^ | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Morbidity | 19 | 61.3 | 12 | 57.1 | 7 | 70.0 | 0.492 |
| Morbidity without POPF-A | 10 | 32.3 | 6 | 28.6 | 4 | 40.0 | 0.525 |
| Mortality | 0 | 0 | 0 | 0 | 0 | 0 | — |
| General complications | |||||||
| Wound infection | 4 | 12.9 | 3 | 14.3 | 1 | 10.0 | 0.739 |
| Specific complications | |||||||
| POPF | 16 | 51.6 | 9 | 42.9 | 7 | 70.0 | 0.157 |
| Intra abdominal abscess | 9 | 29.0 | 5 | 23.8 | 4 | 40.0 | 0.177 |
| DGE | 1 | 3.2 | 1 | 4.8 | 0 | 0 | 0.483 |
| PPH | 1 | 3.2 | 1 | 4.8 | 0 | 0 | 0.483 |
| Grade of POPF | |||||||
| Grade A | 11 | 35.5 | 7 | 33.3 | 4 | 40.0 | 0.237 |
| Grade B | 5 | 16.1 | 2 | 9.6 | 3 | 30.0 | 0.237 |
| Grade C | 0 | 0 | 0 | 0 | 0 | 0 | — |
^ p values for ODP vs. LDP.
POPF = post operative pancreatic fistula; DGE = delayed gastric emptying; PPH = post pancreatectomy hemorrhage.
|
|
|
Figure 1. Distribution of postoperative pancreatic fistula (POPF) according to ISGPS definition. There was no Grade C POPF in either group. |
Postoperative LOS. The median postoperative LOS in LDP group was 5 (3–8) days, while that in the ODP group was 7 (5–29) days (p = 0.020). There was also significantly longer LOS in the surgical high dependency unit for patients in the ODP group (median 2, 0–5 days) compared to the LDP group (median 0, 0–1 days; p = 0.001; Table 7).
| All (n = 31) | ODP (n = 21) | LDP (n = 10) | p ^ | |
|---|---|---|---|---|
| Median length of stay in HD: d (range) | 1 (0–5) | 2 (0–5) | 0 (0–1) | 0.001* |
| Median length of stay in ICU: d (range) | 0 (0–6) | 0 (0–6) | 0 (0–2) | 0.400 |
| Median post-operative length of stay: d (range) | 7 (3–29) | 7 (5–29) | 5 (3–8) | 0.020* |
| Median total length of stay: d (range) | 7 (4–32) | 8 (5–32) | 5 (4–15) | 0.016* |
^ p values for ODP vs. LDP. * Statistically significant.
Operative mortality. There was no postoperative or in-hospital 30-day mortality in this cohort of patients (Table 7).
4. Discussion
Approaches to pancreatic surgery are fast evolving and expanding continuously. Minimally invasive techniques have now been developed to the extent that they allow for surgical resection of pancreatic lesions, debridement, and drainage of tumors.22; 23 ; 24 Considering the retroperitoneal location of the pancreas, a laparoscopic approach presents itself as a procedure with: 1) minimal incisions for entry25; 2) good accessibility to the pancreatic lesion; and 3) closer and more definitive views.2 ; 26 At the same time, laparoscopic approaches have been shown in studies to produce shorter/similar operation times and LOS in hospital, less blood loss, and fewer complications when compared to open access methods, without any compromise on safety of the procedure.20 ; 27 As such, the trend of using laparoscopic distal pancreatectomy to treat premalignant and benign pancreatic body and tail lesions has gained increasing popularity over the years as they are less technically challenging compared to laparoscopic pancreaticoduodenectomy.28 ; 29 Many of the studies comparing clinical outcomes between LDP and ODP originated from Europe and the USA.5; 6 ; 8 Only in recent years have we started reading more reports coming from Asian centers.2; 7; 30; 31 ; 32 Nonetheless, such data remain limited from our part of the world. To our knowledge, this series is the only single-institutional study in our nation comparing clinical outcomes between LDP and ODP.
Our study yielded several important findings. Firstly, the preoperative variables such as demographics, comorbid conditions and blood investigations were comparable between the two groups, signifying that patients were relatively alike in terms of their preoperative status and subsequent comparisons are more meaningful.
Secondly, although some studies showed that LDP had shorter duration of operation, we found no significant difference between the two groups in terms of operative time. We can rationalize this difference as follows: LDP was technically more challenging compared to ODP, especially when laparoscopic spleen preservation was required.33; 34 ; 35 However, as the tumor size in the ODP was much larger comparatively, this balanced out the difficulties during the operation resulting in comparable operative time. The significantly greater EBL and amount of blood transfusion in the ODP group supported this explanation. The feasibility of LDP for the surgical treatment of pancreatic malignancies depends significantly on its ability to offer robust lymphadenectomies in comparison to ODP,36 which was not the case in this study. A similar pattern was also observed in other studies where the number of nodes resected in laparoscopic procedures was less than half of that in open procedures.37 While this may be reflective of an inherent drawback of LDP surgeries, the impact of such a finding on the appraisal of LDP surgeries for the treatment of pancreatic malignancies is so pivotal that we recommend that more studies on this subject be carried out before we come to definite conclusions.8; 38; 39 ; 40
The third observation in our study was that the rates of postoperative morbidities were comparable between the LDP and ODP groups, suggesting similar safety profiles of the two surgical modalities. In addition, we also found that the new ISGPS definition inflated the postoperative morbidity by approximately 30% in both groups when clinically insignificant Grade A POPF was included in the analysis.This is consistent with the experience of other centers that adopted this new definition.5 As expected, we observed a significantly shorter postoperative LOS by 2 days in the LDP group compared to the ODP group. That was the advantage that was intended for minimally invasive procedure. A recent collective review of studies on laparoscopic distal pancreatectomy found that the mean LOS across major centers in the USA was 5.3 ± 1.1 days, while mean LOS outside the USA was 7.4 ± 5.0 days.8 In our center, the median LOS for LDP was 5 (4–15) days. This highlights the competitiveness of our institution’s health care system and the effectiveness of a standardized post-operative care and discharge pathway.
There are a few important limitations in our study. One of the major aspects is the small sample size. Other retrospective studies on LDP reported sample sizes of up to 90 patients.2; 5 ; 7 Other than that, there was a selection bias in this study as there was a tendency to offer LDP for patients with benign or premalignant conditions. These lesions tend to be smaller in size compared to those in the ODP. Elimination of this selection bias would require a prospective randomized controlled study. This is especially important if LDP were to be implemented as standard of treatment for malignancies requiring distal pancreatic resection.
5. Conclusion
The findings in our study validated the safety and efficacy of LDP for the management of benign and premalignant pancreatic lesions.41 ; 42 Further studies on the oncologic quality for LDP should be performed before applying this to the management of pancreatic malignancies.43 ; 44
References
- 1 J.M. Winter, J.L. Cameron, K.D. Lillemoe, et al.; Periampullary and pancreatic incidentaloma: a single institutions experience with an increasingly common diagnosis; Ann Surg, 243 (2006), pp. 673–683
- 2 S.C. Kim, K.T. Park, J.W. Hwang, et al.; Comparative analysis of clinical outcomes for laparoscopic distal pancreatic resection and open distal pancreatic resection at a single institution; Surg Endosc, 22 (2008), pp. 2261–2268
- 3 N.J. Soper, L.M. Brunt, D.L. Dunnegan, T.A. Meininger; Laparoscopic distal pancreatectomy in the porcine model; Surg Endosc, 8 (1994), pp. 57–60
- 4 A. Cuschieri; Laparoscopic surgery of the pancreas; J R Coll Surg Edinb, 39 (1994), pp. 178–184
- 5 J.Y. Mabrut, L. Fernandez-Cruz, J.S. Azagra, et al.; Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients; Surgery, 137 (2005), pp. 597–605
- 6 G. Melotti, G. Butturini, M. Piccoli, et al.; Laparoscopic distal pancreatectomy. Results on a consecutive series of 58 patients; Ann Surg, 246 (2007), pp. 77–82
- 7 B.W. Eom, J.Y. Jang, S.E. Lee, H.S. Han, Y.S. Yoon, S.W. Kim; Clinical outcomes compared between laparoscopic and open distal pancreatectomy; Surg Endosc, 22 (2008), pp. 1334–1338
- 8 D. Borja-Cacho, W.B. Al-Refaie, S.M. Vickers, T.M. Tuttle, E.H. Jensen; Laparoscopic distal pancreatectomy; J Am Coll Surg, 209 (2009), pp. 758–765
- 9 R.A. Dudley, K.L. Johansen, R. Brand, D.J. Rennie, A. Milstein; Selective referral to high-volume hospitals: estimating potentially avoidable deaths; JAMA, 283 (2000), pp. 1159–1166
- 10 J.D. Birkmeyer, A.E. Siewers, E.V. Finlayson, et al.; Hospital volume and surgical mortality in the United States; N Engl J Med, 346 (2002), pp. 1128–1137
- 11 M.N. Wente, C. Bassi, C. Dervenis, et al.; Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISPGS); Surgery, 142 (2007), pp. 761–768
- 12 C. Bassi, Butturini G. Dervenis, et al.; Post operative pancreatic fistula: an International Study Group (ISGPF) definition; Surgery, 138 (2005), pp. 8–13
- 13 M.N. Wente, A.V. Johannes, C. Bassi, et al.; Postpancreatectomy hemorrhage (PPH) – An International Study Group of Pancreatic Surgery (ISGPS) definition; Surgery, 142 (2007), pp. 20–25
- 14 P. Clavien, J. Sanabria, S. Strasberg; Proposed classification of complication of surgery with examples of utility in cholecystectomy; Surgery, 111 (1992), pp. 518–526
- 15 P. Clavien, J. Sanabria, G. Mentha, et al.; Recent results of elective open cholecystectomy in a North American and a European center. Comparison of complications and risk factors; Ann Surg, 216 (1992), pp. 618–626
- 16 P. Clavien, C. Camargo, R. Croxford, B. Langer, G.A. Levy, P.D. Greig; Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation; Ann Surg, 220 (1994), pp. 109–120
- 17 D. Dindo, N. Demartines, P. Clavien; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey; Ann Surg, 240 (2004), pp. 205–213
- 18 A.W.C. Kow, S.P. Chan, A. Earnest, et al.; Striving for a better operative outcome: 101 pancreaticoduodenectomies; HPB, 10 (2008), pp. 464–471
- 19 T. Mori, N. Abe, M. Sugiyama, Y. Atomi; Laparoscopic pancreatic surgery; J Hepatobiliary Pancreat Surg, 12 (2005), pp. 451–455
- 20 S. Das De, A.W. Kow, K.H. Liau, K.H. Lim, C.K. Ho; Novel approach to laparoscopic resection of tumors of the distal pancreas; ANZ J Surg, 79 (2009), pp. 288–293
- 21 H. Friess, C.K. Ho, J. Kleeff, M. Albertsmeier, K.W. Jauch, C.J. Bruns; Pancreaticoduodenectomy, distal pancreatectomy, segmental pancreatectomy, total pancreatectomy, and transduodenal resection of the papilla of Vater; L.H. Blumgart (Ed.), Surgery of the Liver, Biliary Tract, and Pancreas (4th ed.), Saunders, Philadelphia (2007)
- 22 R.A. Underwood, N.J. Soper; Current status of laparoscopic surgery of the pancreas; J Hepatobiliary Pancreat Surg, 6 (1999), pp. 154–164
- 23 P.W. Pisters, J.E. Lee, J.N. Vauthey, C. Charnsangavej, D.B. Evans; Laparoscopy in the staging of pancreatic cancer; Br J Surg, 88 (2001), pp. 325–337
- 24 K. Spanknebel, K.C. Conlon; Advances in the surgical management of pancreatic cancer; Cancer J., 7 (2001), pp. 312–333
- 25 L. Fernandez-Cruz, I. Martinez, R. Gilabert, G. Cesar-Borges, E. Astudillo, S. Navarro; Laparoscopic distal pancreatectomy combined with preservation of the spleen for cystic neoplasms of the pancreas; J Gastrointest Surg, 8 (2004), pp. 493–501
- 26 V. Velanovich; Case-control comparison of laparoscopic versus open distal pancreatectomy; J Gastrointest Surg, 10 (2006), pp. 95–98
- 27 N.B. Merchant, A.A. Parikh, D.A. Kooby; Should all distal pancreatectomies be performed laparoscopically?; Adv Surg, 43 (2009), pp. 283–300
- 28 E. Orsenigo, P. Baccari, G. Bissolotti, C. Staudacher; Laparoscopic central pancreatectomy; Am J Surg, 191 (2006), pp. 549–552
- 29 M. D’Angelica, C. Are, W. Jarnagin, et al.; Initial experience with hand-assisted laparoscopic distal pancreatectomy; Surg Endosc, 20 (2006), pp. 142–148
- 30 N. Kano, H. Kusanagi, S. Yamada, K. Kasama, A. Ota; Laparoscopic pancreatic surgery: its indications and techniques: from the viewpoint of limiting the indications; J Hepatobiliary Pancreat Surg, 9 (2002), pp. 555–558
- 31 H. Kaneko, S. Takagi, N. Joubara, et al.; Laparoscopy-assisted spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein; J Hepatobiliary Pancreat Surg, 11 (2004), pp. 397–401
- 32 N. Tagaya, K. Kasama, N. Suzuki, et al.; Laparoscopic resection of the pancreas and review of the literature; Surg Endosc, 17 (2003), pp. 201–206
- 33 M. Gagner, A. Pomp; Laparoscopic pancreatic resection: is it worthwhile?; J Gastrointest Surg, 1 (1997), pp. 20–25
- 34 H.S. Han, S.K. Min, H.K. Lee, S.W. Kim, Y.H. Park; Laparoscopic distal pancreatectomy with preservation of the spleen and splenic vessels for benign pancreas neoplasm; Surg Endosc, 19 (2005), pp. 1367–1369
- 35 A. Assalia, M. Gagner; Laparoscopic pancreatic surgery for islet cell tumors of the pancreas; World J Surg, 28 (2004), pp. 1239–1247
- 36 J.M. Fabre, J.L. Dulucq, C. Vacher, et al.; Is laparoscopic left pancreatic resection justified; Surg Endosc, 16 (2002), pp. 1358–1361
- 37 M.S. Baker, D.J. Bentrem, M.B. Ujiki, S. Stocker, M.S. Talamonti; A prospective single institution comparison of peri-operative outcomes for laparoscopic and open distal pancreatectomy; Surgery, 146 (2009), pp. 635–645
- 38 D.K. Chang, A.L. Johns, N.D. Merrett, et al.; Margin clearance and outcome in resected pancreatic cancer; J Clin Oncol, 27 (2009), pp. 2855–2862
- 39 M.B. Slidell, D.C. Chang, J.L. Cameron, et al.; Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis; Ann Surg Oncol, 15 (2008), pp. 165–174
- 40 M.G. House, M. Gonen, W.R. Jarnagin, et al.; Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer; J Gastrointest Surg, 11 (2007), pp. 1549–1555
- 41 T.A. Sohn, C.J. Yeo, J.L. Cameron, et al.; Resected adenocarcinoma of the pancreas-616 patients: results, outcomes and prognostic indicators; J Gastrointest Surg, 4 (2000), pp. 567–579
- 42 S.A. Ahrendt, R.A. Komorowski, M.J. Demeure, S.D. Wilson, H.A. Pitt; Cystic pancreatic neuroendocrine tumors: is preoperative diagnosis possible; J Gastrointest Surg, 6 (2002), pp. 66–74
- 43 A. Lebedyev, O. Zmora, J. Kuriansky, et al.; Laparoscopic distal pancreatectomy; Surg Endosc, 18 (2004), pp. 1427–1430
- 44 A. Khanna, L.G. Koniaris, A. Nakeeb, L.O. Schoeniger; Laparoscopic spleen-preserving distal pancreatectomy; J Gastrointest Surg, 9 (2005), pp. 733–738
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?