Highlights
- NSO improves the endogenous antioxidant status and metabolic activity of liver.
- NSO protects the liver against CP generated free radical attack.
- Oral administration of NSO ameliorates the hepatotoxicity induced by CP treatment.
Abstract
Cisplatin (CP) is a potent anti-cancer drug widely used against solid tumors. However, it exhibits pronounced adverse effects including hepatotoxicity. Several strategies were attempted to prevent CP hepatotoxicity but were not found suitable for therapeutic application. Nigella sativa has been shown to prevent/reduce the progression of certain type of cardiovascular, kidney and liver diseases. Present study investigates whether N. sativa oil (NSO) can prevent CP induced hepatotoxic effects. Rats were divided into four groups viz. control, CP, NSO and CPNSO. Animals in CPNSO and NSO group were administered NSO (2 ml/kg bwt, orally) with or without single hepatotoxic dose of CP (6 mg/kg bwt, i.p.) respectively. CP hepatotoxicity was recorded by increased serum ALT and AST activities. CP treatment caused oxidant/antioxidant imbalances as reflected by increased lipid peroxidation and decreased enzymatic and non-enzymatic antioxidants. Furthermore, the activities of various carbohydrate metabolism and membrane enzymes were altered by CP treatment. In contrast, NSO administration to CP treated rats, markedly ameliorated the CP elicited deleterious alterations in liver. Histopathological observations showed extensive liver damage in CP treated animals while greatly reduced tissue injury in CPNSO group. In conclusion, NSO appears to protect CP induced hepatotoxicity by improving energy metabolism and strengthening antioxidant defense mechanism.
Abbreviations
ACPase , acid phosphatase ; ALP , alkaline phosphatise ; ALT , alanine aminotransferase ; AST , aspartate aminotransferases ; BUN , blood urea nitrogen ; BBM , brush border membrane ; BBMV , BBM vesicles ; Scr , serum creatinine ; CAT , catalase ; CP , cisplatin ; Chl , cholesterol ; FBPase , fructose 1,6 ; G6Pase , glucose 6-phosphatase ; G6PDH , glucose 6-phosphate dehydrogenase ; GGTase , γ-glutamyl transferase ; Glc , glucose ; GR , glutathione reductase ; GSH , glutathione ; GSHPx , glutathione peroxidise ; GST , glutathione S-transferase ; HK , hexokinase ; H2 O2 , hydrogen peroxide ; LAP , leucine aminopeptidase ; LDH , lactate dehydrogenase ; LPO , lipid peroxidation ; MDA , malondialdehyde ; MDH , malate dehydrogenase ; ME , malic enzyme ; μm , micrometer ; NADPH , nicotinamide adenine dinucleotide phosphate reduced ; NADP , nicotinamide adenine dinucleotide phosphate ; NSO , Nigella sativa oil ; Pi , inorganic phosphate ; PLs , phospholipids ; PUFA , polyunsaturated fatty acids ; ROS , reactive oxygen species ; SH , sulfhydryl ; SOD , superoxide dismutase ; TCA , tricarboxylic acid ; TR , thioredoxin reductase
Keywords
Cisplatin ; Nigella sativa oil ; Carbohydrate metabolism ; Antioxidant
1. Introduction
Cisplatin (cis-diamminedichloroplatinum II, CP) is one of the most effective chemotherapeutic agent used in the treatment of variety of human malignancies including those of bladder, head, neck, testis and ovary [1] and [2] . However, the use of high dose of CP is difficult in clinical practice due to the associated side effects such as nephrotoxicity, neurotoxicity and ototoxicity. Recent studies suggest that hepatotoxicity is also a major dose limiting side effect in CP based chemotherapy [3] and [4] . High dose protocol of CP chemotherapy, therefore, necessitates the investigation of ways for prevention of dose limiting side effects that inhibit CP administration at tumoricidal doses. Until now, various methods to prevent or reduce the side effects of CP, such as synthetic antioxidants and chemoprotective agents, have been tested but effective method for clinical application has not yet been established [5] and [6] .
CP induced toxicity is reported to be mediated by increased production of reactive oxygen species (ROS) and free radicals [3] and [7] . These ROS could interfere with the antioxidant defense system and can cause extensive tissue damage and cell dysfunction by reacting with macromolecules like proteins, membrane lipids and nucleic acids [7] , [8] , [9] and [10] . Several studies suggest that oxidative stress plays an important role in CP induced hepatotoxicity [11] and [12] . Naturally occurring dietary antioxidants therefore have excellent scope to protect against CP hepatotoxicity. In consequence, the search for effective, naturally occurring dietary substances with antioxidant activity, has been intensified in recent years. Plants such as Nigella sativa (NS) provide such a dietary source of biologically active components/phytochemicals which exhibit wide spectrum of biological properties such as antioxidant, anti-diabetic, anti-inflammatory, nephroprotective and hepatoprotective properties [13] and [14] . NS oil (NSO) contains fixed oil (30%) and volatile oil (0.5%), proteins, alkaloids and saponins. Thymoquinone, the principal active ingredient of NS volatile oil, possesses antioxidant potential to scavenge free radicals and to protect the cell against oxidative damage [15] , [16] and [17] . In addition, NSO is an important source of polyunsaturated fatty acids (PUFA) that contains omega (ω)-3 PUFA and ω-6 PUFA in the recommended optimal dietary intake ratio of 1:4 [18] and [19] . Consumption of ω-3 and ω-6 essential fatty acid in the right proportion has been found to suppress the pathogenesis of many diseases [20] and [21] .
Since ancient times, NS seed/oil consumption has been shown to exhibit a multitude of beneficial health effects under normal as well as in various pathological conditions [14] . NSO has been shown to downregulate CCl4 induced nitric oxide (NO) synthase mRNA and NO production in liver [22] . Thymoquinone, the major bioactive component of NSO, has been reported to improve plasma and liver antioxidant capacity and to enhance the expression of liver antioxidant genes in hypocholestrolemic rats [23] . Studies have shown that NSO/thymoquinone administration ameliorates CP induced nephrotoxic effects [24] and [25] . Thymoquinone was also found to potentiate/enhance the anti-tumor activity of CP against Ehrlich ascites carcinoma (EAC) [24] . NSO has been demonstrated to attenuate cyclosporine A and gentamicin induced renal dysfunction in experimental rats [26] and [27] . However, the protective potential of NSO against CP induced hepatotoxicity remains uninvestigated.
Considering the potential clinical use of CP and numerous health benefits of NSO, we hypothesized that owing to its inherent biochemical and antioxidant properties, NSO would prevent CP induced hepatotoxicity that would lead to improved metabolism and antioxidant defense mechanism of the liver.
2. Material and methods
2.1. Chemicals and drugs
N. sativa oil was supplemented from Mohammedia Products, Red Hills, Nampally, Hyderabad, India. Cisplatin was obtained from Sigma–Aldrich Chemical Corp., St. Louis, MO, USA. All other chemicals used were of analytical grade and were purchased either from Sigma Chemical Corp., or SRL (Mumbai, India).
2.2. Diet
A nutritionally adequate laboratory pellet diet was obtained from Aashirwaad Industries, Chandigarh (1544, Sector 38-B, Chandigarh, India).
2.3. Experimental design
The animal experiments were conducted according to the guidelines of Committee for purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India. Adult male Wistar rats (6 rats/group) weighing between 150 and 200 gm were used in the following experimental protocol. Rats were acclimatized to the animal facility for a week on a standard rat laboratory pellet diet and allowed water ad libitum under controlled conditions of 25 ± 2° C temperature, 50 ± 15% relative humidity and normal photoperiod (12 h dark and light). Four groups of rats entered the study after acclimatization; Control (C), CP treated (CP), N. sativa oil treated (NSO) and N. sativa oil + CP treated (CPNSO). Rats in the group CPNSO were administered NSO (2 ml/kg bwt, orally) for 14 days prior to and 4 days following CP treatment. However, rats in NSO group were administered NSO alone without CP treatment. CP solution (2 mg/ml) was freshly prepared in 0.9% normal saline by continuous stirring at room temperature for 10 min. A single intraperitoneal injection of CP (6 mg/kg bwt.) was administered to the animals in CP and CPNSO groups, while animals in the control and NSO group received an equivalent amount of normal saline. Body weight of rats was recorded at the start and completion of the experimental protocol. Blood was collected from fasted rats on 5th day after CP administration under light ether anesthesia. Liver was removed and processed for histopathology and the preparation of homogenate as described below.
2.4. Serum parameters
Blood samples were centrifuged at 2000 × g for 10 min and sera were separated. Serum samples were deproteinated with 3% trichloroacetic acid in a ratio of 1:3, left for 10 min and then centrifuged at 2000 × g for 10 min. The precipitate obtained was used to quantitate total phospholipids (PLs). Cholesterol (Chl) levels was determined directly in serum samples by method of Zlatkis et al. [28] . Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were determined in serum by Reitman and Frankel method using kit from span diagnostics.
2.5. Preparation of homogenates
Liver was homogenized in 0.1 M Tris–HCl buffer (pH 7.5) by a glass–teflon homogenizer (Thomas PA, USA) by passing 5 pulses; at 4° C to make a 10% (w/v) homogenate. The homogenate was then subjected to high-speed Ultra-Turrex Kunkel homogenizer (Type T-25, Janke & Kunkel GMBH & Co., KG. Staufen) for 3 pulses of 30 s each with an interval of 30 s between each stroke. One part of the homogenate was saved at −20° C for estimation of GSH, total-SH and lipid peroxidation while other part was centrifuged at 2000 rpm for 10 min at 4° C in high-speed Remi centrifuge (Remi motors, Mumbai, India) to remove the cell debris. The supernatant was saved in aliquots and stored at −20° C for analyses of membrane, carbohydrate metabolism and free radical scavenging enzymes.
2.6. Assay of carbohydrate metabolism enzymes
The activities of the enzymes involving oxidation of NADH or reduction of NADP were determined spectrophotometrically on UV-1700 (Pharma Spec, Shimadzu Corp., Japan) fixed for 340 nm using 3 ml of assay buffer in a 1-cm cuvette at room temperature (28–30 °C). The enzyme activities of lactate dehydrogenase (LDH), malate dehydrogenase (MDH), malic enzyme (ME), glucose-6-phosphate dehydrogenase (G6PDH), glucose-6-phosphatase (G6Pase) and fructose-1, 6-bisphosphatase (FBPase) were assayed as described by Khundmiri et al. [29] . Hexokinase (HK) was estimated by the method of Crane and Sols [30] and the remaining glucose was measured by method of Nelson [31] .
2.7. Assay of membrane enzymes and lysosomal marker enzyme
The activities of membrane marker enzymes viz. alkaline phosphatase (ALP), γ-glutamyl transferase (GGTase), leucine amino peptidase (LAP), and lysosomal marker enzyme viz. acid phosphatase (ACPase) were determined as described by Farooq et al. [32] .
2.8. Assay of enzymes involved in free radical scavenging
Superoxide dismutase (SOD) was assayed by the method of Marklund and Marklund [33] . Catalase (CAT) and glutathione peroxidase (GSH-Px) activities were determined by the method of Giri et al. [34] and Flohe and Gunzler [35] , respectively. The activities of thioredoxin reductase (TR) and glutathione-S-transferase (GST) were determined using 5,5′-dithiobisnitrobenzoic acid and 1-chloro-2-4-dinitrobenzene as substrates, respectively [36] and [37] . Glutathione reductase (GR) was assayed from the oxidation of NADPH to NADP+ in presence of oxidized glutathione [38] .
2.9. Thiobarbituric acid reactive substances (TBARS), total −SH group and GSH estimation
TBARS (products of LPO) were measured as malondialdehyde (MDA) equivalents as described by Ohkawa et al. [39] . Total-SH and GSH were determined after reaction with 5,5′-dithiobisnitrobenzoic acid by the method of Sedlak and Lindsay [40] and Jollow et al. [41] , respectively.
2.10. Histopathology
Liver was cut into pieces and kept in Karnovsky’s fixative for one week (Immersion fixation). Small pieces of tissue samples were processed for paraffin embedding. Sections of 7 μm thickness were cut and stained with haematoxylin and eosin. Light microscopic observations were made under trinocular microscope (Olympus BX-40, Japan). Interesting findings were recorded at the initial magnification of ×400.
2.11. Statistical analysis
All data are expressed as mean ± SEM for at least 3 different experiments. Statistical evaluation was conducted by one-way ANOVA using origin 6.1 software followed by post hoc test (Student–Newman–Keuls and Dunnets multiple comparison test). A probability level of p < 0.05 was selected as indicator of statistical significance. Most of the changes between various groups were compared with control values for better understanding and clarity. However, specific differences and statistical significance between other groups were evaluated separately, e.g., CP vs. CPNSO.
3. Results
The present work was undertaken to study the detailed mechanism of CP-induced deleterious effects on liver and possible protection by N. sativa oil (NSO). The effect of CP alone and in combination with NSO was determined on various serum parameters and membrane marker enzymes, enzymes of carbohydrate metabolism and oxidative stress parameters in rat liver.
3.1. Effect of NSO on CP induced alterations in serum parameters
Hepatotoxicity induced by CP was characterized by significant increase in serum transaminases, ALT and AST. The administration of CP caused significant increase in ALT (+62%), AST (+56%), Chl (+30%) and PLs (+24%) (Table 1 ). NSO administration prior to and following CP treatment, resulted in significant reversal of various CP-elicited deleterious effects on serum parameters. CP-induced increase in ALT, AST, PLs, and Chl were prevented by NSO administration. However, NSO administration alone caused significant decrease in ALT and Chl.
| Enzymes/parameters Groups | ALT (IU/L) | AST (IU/L) | Cholesterol (mg/dl) | Phospholipid (mg/dl) |
|---|---|---|---|---|
| Control | 45.65±2.55 | 26.65±0.25 | 86.85±2.64 | 209.68±7.56 |
| CP | 74.12±2.22* (+62%) | 41.60±0.60* (+56%) | 112.64±3.06* (+30%) | 260.15±1.78* (+24%) |
| NSO | 34.65±2.08* (−24%) | 27.25±1.35 (+2%) | 61.59±1.68* (−29%) | 196.97±3.1 (−6%) |
| CPNSO | 35.35±3.05** (-22%) | 30.41±1.36**(+14%) | 81.44±0.81** (-6%) | 210.48±3.57** (+0.38%) |
CP: cisplatin treated; NSO: Nigella sativa oil administered; CPNSO: Nigella sativa oil administered + cisplatin treated; ALT: alanine aminotransferases; AST: aspartate aminotransferases. Results are mean ± SEM for three different experiments. Values in parenthesis represent percent change from control.
- . Significantly different from control.
- . Significantly different from CP at p < 0.05 by one way ANOVA.
3.2. Effect of NSO on CP induced alterations in membrane enzymes and biomarker enzymes of lysosomes
To evaluate the structural integrity of certain organelles e.g., plasma membrane and lysosomes, the effect of CP alone and in combination with NSO was determined on membrane and lysosomal marker enzymes (Table 2 ). CP treatment caused significant decrease in the specific activities of membrane enzymes viz. ALP (−32%), GGTase (−35%) and LAP (−50%) in liver homogenate. Administration of NSO to CP treated rats prevented CP induced decline in membrane enzyme activities. However, the activity of ACPase, a lysosomal enzyme, was increased (+62%) by CP treatment in liver homogenate. However, NSO administration prior to and following CP treatment, improved ACPase activity insignificantly compared to CP group.
| Enzyme Groups | ALP (μmol/mg protein/h) | GGTase (μmol/mg protein/h) | LAP (μmol/mg protein/h) | ACPase (μmol/mg protein/h) |
|---|---|---|---|---|
| Control | 8.60±0.212 | 1.17±0.087 | 0.76±0.046 | 2.75±0.214 |
| CP | 5.77±0.125* (−32%) | 0.76±0.091* (−35%) | 0.38±0.017* (−50%) | 4.48±0.240* (+62%) |
| NSO | 9.20±0.481 (+6%) | 1.22±0.044 (+4%) | 0.91±0.032* (+19%) | 2.17±0.160* (−21%) |
| CPNSO | 8.39±0.133** (−2%) | 1.09±0.043** (−6%) | 0.71±0.032** (−6%) | 3.22±0.111 (+17%) |
CP: cisplatin treated; NSO: Nigella sativa oil administered; CPNSO: Nigella sativa oil administered + cisplatin treated; ALP: alkaline phosphatase; GGTase: γ—glutamyl transferase; LAP: leucine amonipeptidase; ACPase: acid phosphatase. Results (specific activity expressed as μmoles/mg protein/h) are mean ± SEM for three different experiments. Values in parenthesis represent the percent change from control.
- . Significantly different from control.
- . Significantly different from CP at p < 0.05 by one way ANOVA.
3.3. Effect of NSO on CP induced alterations in carbohydrate metabolism
The effect of CP and NSO alone and their combination (CPNSO) was determined on the activities of various enzymes of carbohydrate metabolism. As shown in Table 3 , CP treatment significantly enhanced the activity of LDH (+37%) and decreased the activity of MDH (−61%) while activity of HK was altered insignificantly by CP treatment in the liver. In contrast, when CP was administered to NSO consuming rats, CP-induced decline in MDH activity and enhancement in LDH and HK activities was prevented (Table 3 ). CP treatment was found to cause a marked reduction in gluconeogenic enzymes viz. G6Pase and FBPase activities in the liver. In contrast, NSO administration prior to and following CP treatment, reduced CP induced decline in G6Pase and FBPase activities. The activities of G6PDH and ME, that provide NADPH needed in various anabolic reactions, were also determined. CP treatment significantly decreased G6PDH activity (−64%) while activity of ME was profoundly increased (+95%) in the liver (Table 3 ). Administration of NSO alone to the rats did not cause significant alterations in the activities of carbohydrate metabolism enzymes except for the significant reduction of G6Pase. However, CP treatment to NSO administered rats significantly reduced the CP induced alterations in the activities of G6PDH and ME.
| Enzyme Groups | HK (μmol/mg protein/h) | LDH (μmol/mg protein/h) | MDH (μmol/mg protein/h) | G6Pase (μmol/mg protein/h) | FBPase (μmol/mg protein/h) | G6PDH (μmol/mg protein/h) | ME (μmol/mg protein/h) |
|---|---|---|---|---|---|---|---|
| Control | 1.36±0.073 | 0.43±0.161 | 2.21±0.03 | 0.20±0.018 | 0.746±0.081 | 0.034±0.003 | 0.049±0.006 |
| CP | 1.73±0.079 (+27%) | 0.59±0.019* (+37%) | 0.85±0.035* (−61%) | 0.133±0.008* (−33%) | 0.64±0.019* (−14%) | 0.012±0* (−64%) | 0.096±0.003* (+95%) |
| NSO | 1.25±0.032 (−8%) | 0.41±0.020 (−5%) | 1.82±0.158 (−17%) | 0.178±0.007* (−11%) | 0.78±0.037 (+4%) | 0.035±0.003 (+2%) | 0.039±0.003 (−20%) |
| CPNSO | 1.35±0.072** (−1%) | 0.50±0.019 (+16%) | 1.25±0.064** (−43%) | 0.155±0.005 (−22%) | 0.75±0.058** (+0.536%) | 0.020±0.001** (−41%) | 0.041±0.002** (−16%) |
CP: cisplatin treated; NSO: Nigella sativa oil administered; CPNSO: Nigella sativa oil administered + cisplatin treated; HK: hexokinase; LDH: lactate dehydrogenase; MDH: malalte dehydrogenase; G6Pase: glucose-6-phosphatase; FBPase: fructose-1, 6-bisphosphatase; G6PDH: glucose-6-phosphatase dehydrgenase; ME: malic enzyme. Results (specific activity expressed as μmoles/mg protein/hr) are mean ± SEM for three different experiments. Values in parenthesis represent percent change from control.
- . Significantly different from control.
- . Significantly different from CP at p < 0.05 by one way ANOVA.
3.4. Effect of NSO on CP induced alterations in antioxidant defense parameters
The effect of CP-induced toxicity was assessed on the antioxidant system and MDA. CP treatment resulted in profound increase in the production of malondialdehyde (MDA), an end-product of lipid peroxidation. Total-SH and GSH were found to decrease significantly in CP treated rats (Table 4 ). CP treatment caused significant decline in the activities of SOD (−30%), CAT (−43%), GSH-Px (−35%), TR (−62%), GR (−44%) and GST (−41%) (Table 5 ). Administration of NSO alone had no significant effect on the activities of antioxidant enzymes. However, administration of NSO to CP treated rats significantly prevented CP induced increase in MDA and decrease in GSH in the liver homogenate. NSO treatment significantly reduced the CP induced decrease in the activities of antioxidant enzymes viz. SOD, CAT, GSH-Px, GR and GST, while the activity of TR was not effected significantly by NSO administration. In general, the present results demonstrate marked prevention of CP induced alterations in oxidative stress parameters by NSO administration.
| Parameters Groups | Lipid peroxidation (nmol/g tissue) | Total SH (μmol/g tissue) | GSH (μmol/g tissue) |
|---|---|---|---|
| Control | 258.72±2.00 | 19.21±0.744 | 0.98±0.035 |
| CP | 335.46±5.31* (+229%) | 15.55±0.824* (−19%) | 0.55±0.039* (−44%) |
| NSO | 210.97±8.03* (−18%) | 20.88±0.270 (+8%) | 0.87±0.035 (−11%) |
| CP NSO | 274.03±3.28** (+5%) | 17.94±0.603 (−6%) | 0.79±0.054** (−19%) |
CP: cisplatin treated; NSO: Nigella sativa oil administered; CPNSO: Nigella sativa oil administered + cisplatin treated; GSH: glutathione. Results are mean ± SEM for three different experiments. Values in parenthesis represent percent change from control.
- . Significantly different from control.
- . Significantly different from CP at p < 0.05 by one way ANOVA.
| Enzyme Groups | SOD (units/mg protein) | CAT (μmol/mg protein/min) | GSH-Px (μmol/mg protein/min) | TR (μmol/mg protein/min) | GR (μmol/mg protein/min) | GST (μmol/mg protein/min) |
|---|---|---|---|---|---|---|
| Control | 39.07±1.55 | 24.39±1.056 | 0.054±0.003 | 0.066±0.004 | 0.036±0.003 | 0.58±0.041 |
| CP | 27.32±0.88* (−30%) | 13.71±0.266* (−43%) | 0.035±0.001* (−35%) | 0.025±0.002* (−62%) | 0.020±0.003* (−44%) | 0.34±0.01* (−41%) |
| NSO | 45.06±2.94 (+15%) | 24.77±0.543 (+1%) | 0.052±0 (−4%) | 0.044±0.002 (−33%) | 0.033±0.003 (−8%) | 0.576±0.026 (−1%) |
| CPNSO | 37.45±1.05** (−4%) | 21.39±0.652** (−12%) | 0.046±0.001** (−14%) | 0.038±0.005 (−42%) | 0.032±0.003** (−22%) | 0.52±0.013** (−2%) |
CP: cisplatin treated; NSO: Nigella sativa oil administered; CPNSO: Nigella sativa oil administered + cisplatin treated; SOD: superoxide dismutase; CAT: catalase; GSH-Px: glutathione peroxidase; TR: theoredoxin reductase; GR: glutathione reductase; GST: glutathione- S- transferase. Results are mean ± SEM for three different experiments. Values in parenthesis represent percent change from control.
* Significantly different from control.
** Significantly different from CP at p < 0.05 by one way ANOVA.
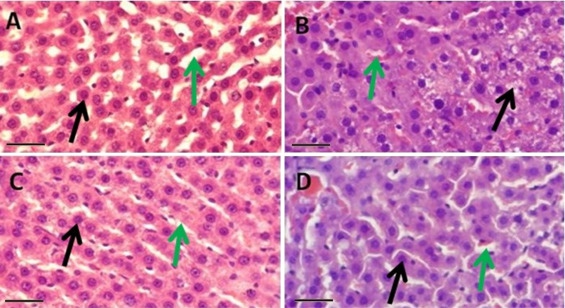
3.5. Histopathological observations
Histopathological observations (Fig. 1 ) are suggestive of obvious and extensive damage to the liver by CP treatment. CP treatment (Fig. 1 B) lead to loss of normal histoarchitecture, disorganised hepatic cords, necrosis of hepatocytes and congested sinusoids. NSO treatment does not show such kind of toxic effects (Fig. 1 C). The combination group (CPNSO) shows promising protective potential of NSO against the damaging effects of CP on liver (Fig. 1 D).
|
|
|
Fig. 1. Histopathology of rat liver showing hepatocytes ( ) and sinusoids ( ) in control (A) with normal histoarchitecture of hepatocytes and intact hepatic cord whereas in CP treated group (B) disorganised hepatic cords, necrosis of hepatocytes and congested sinusoids were observed. NSO administered group (C) shows well preserved conditions of both, hepatocytes and sinusoids very similar to control. CPNSO group (D) shows fairly intact histoarchitecture, hepatic cords and no obvious necrosis. However, sinusoids appear moderately congested as compared to control. H & E stain, initial magnification ×400 scale bar = 50 μm. |
4. Discussion
Liver is an important organ to be considered when the effect of CP are investigated, since it plays a central role in the body’s metabolism of drugs and other xenobiotics. Liver is known to accumulate significant amount of CP and therefore, high dose protocol of CP chemotherapy causes hepatotoxicity. Although CP induced nephrotoxicity has been very well studied in both clinical and animal researches, hepatotoxicity has been rarely characterized and is less studied [3] , [42] and [43] . Several studies have demonstrated that CP induces pronounced histopathological abnormalities viz. inflammatory infiltration, dissolution of hepatic cord, dilated sinusoids and extensive disorganisation of hepatocytes [44] and [45] . The toxic effects of CP are attributed to its ability to induce formation of ROS that lead to oxidative stress, which is one of the major pathogenic mechanism involved in CP induced damage to kidney and liver [8] , [10] , [11] and [12] .
In the past few years, much interest has been focussed on the role of naturally occurring dietary substances for their ability to confer health and physiological benefits. N. sativa has been revered throughout history for its medicinal properties. N. sativa seed and its oil have been shown to provide potential therapeutic benefits in a variety of diseases [14] and [16] . Several studies have shown that NSO protects against drug and chemical induced toxicities [27] and [46] . However, the ameliorative potential of NSO in CP induced hepatotoxicity has not yet been explored.
The present investigation was carried out to study the possible role of NSO in preventing CP induced deleterious effects in rat liver. Single intraperitoneal injection of CP caused marked alterations in serum parameters. Results (Table 1 ) show that CP administration caused hepatotoxicity, as indicated by elevated serum levels of transaminases viz. ALT and AST. Serum cholesterol and PLs were also increased significantly in CP treated rats. However, NSO administration prior to and following CP treatment, resulted in significant lowering of CP elicited increase in serum transaminases. Furthermore, NSO consumption either alone or in combination with CP treatment, caused significant decline in serum cholesterol and that might be attributed to the hypocholesterolemic effect of NSO as reported previously [47] .
The effect of CP and NSO alone and in combination, on the hepatocyte membrane, was assessed by the status of its biomarker enzymes viz. ALP, GGTase and LAP. CP treatment caused significant decline in the activities of ALP, GGTase and LAP in the liver homogenate (Table 2 ). The decrease in the membrane enzyme activities could be due to the oxidative modification and consequent inactivation of enzymes by CP generated free radicals and ROS. Initiation of self-propagating LPO reactions due to interaction of CP generated ROS with membrane PUFA, could have impaired membrane structure and functions, resulting in the loss of membrane enzymes. In contrast, NSO administration alone, caused significant increase in the activities of membrane enzymes while, NSO administration to CP treated rats prevented/retarded CP-induced decrease in membrane enzyme activities in the liver. Reduction in LPO and oxidative modification of membrane enzymes by NSO, owing to the intrinsic antioxidant and free radical scavenging properties of its bioactive constituents might have caused the observed increase of membrane enzyme activities in CP-treated NSO-fed rats [23] and [48] . NSO, being a good source of essential fatty acids, might have replaced the PUFA components of cellular membranes that had been attacked by oxygen free radicals [49] and [50] , thereby accelerating the repair process. Hence, NSO seems to prevent the membrane perturbing effects of CP, by lessening the damage caused and/or by accelerating the repair or regeneration process, resulting in improved membrane integrity. The restoration of membrane integrity might have caused decreased leakage of enzymes from hepatocytes into the blood, as evidenced by the observed decline in serum ALT and AST activities in CPNSO group. In agreement to earlier studies, CP treatment caused significant increase in ACPase activity in liver [51] . Morphologically, increase in the size and number of lysosomes after CP treatment has been reported previously [52] . However, NSO administration prior to and following CP treatment, ameliorated the CP induced increase in ACPase activity.
Since energy depletion of cells perpetuated by disruption of metabolic pathways is a crucial factor in CP induced toxicity, hence it is imperative to evaluate the possible amelioration of CP induced alterations in hepatic metabolism by oral NSO administration. To assess the effect of CP alone and in combination with NSO, on hepatic metabolic functions, the activities of various enzymes involved in glycolysis, tricarboxylic acid (TCA) cycle, gluconeogenesis and hexose monophosphate-shunt (HMP) pathway were examined in liver homogenates prepared from animals in different experimental groups (Table 3 ). The enzyme activities were differentially altered by CP treatment and/or by NSO consumption. CP administration led to significant increase in the activities of HK and LDH (glycolysis) but decrease in the activity of MDH (TCA cycle). Although the actual rates of glycolysis and other pathways were not determined, however the increased activity of LDH along with simultaneously reduced activity of TCA cycle enzyme, suggest a shift in energy production from aerobic to anaerobic mode, apparently due to CP induced mitochondrial dysfunction [53] and [54] . The activities of FBPase and G6Pase (gluconeogenesis) also declined significantly in CP treated rats. The decreased activities of gluconeogenic enzymes might be a consequence of decreased MDH activity. Thus, in CP treated rats the reduced production of oxaloacetate from malate due to decreased MDH activity, seems to affect both TCA cycle and gluconeogenesis. The NADPH producing enzymes viz. G6PDH and ME were differentially altered by CP treatment. The oxidative conversion of glucose or glucose 6 phosphate to 6 phosphogluconate by G6PDH via HMP shunt pathway, was lowered in CP treated rats and that might be a consequence of CP induced damage to mitochondria [54] . The decreased generation of NAPDH, essential for reductive biosynthetic processes, due to lowered G6PDH activity, seems to be compensated by the increased activity of NADP malic enzyme. However, NSO consumption by CP treated rats, resulted in an overall improvement of carbohydrate metabolism as evident by higher activities of MDH, G6PDH and gluconeogenic enzymes in CPNSO in comparison to CP group. These biochemical findings were further confirmed by histopathological studies that showed loss of normal histoarchitecture, disorganised hepatic cords, congested sinusoids and necrosis of hepatocytes in the liver of CP treated rats (Fig. 1 B). However, administration of NSO to CP treated rats, prevented CP induced histopathological alterations and maintained the architecture of liver almost similar to that of control (Fig. 1 D).
Various studies have revealed that CP exerts its toxic effect by generation of ROS and free radicals [7] , [55] and [56] . The role of ROS in CP toxicity is strengthened by the fact that many free radical scavengers, such as green tea, vitamin E and ascorbic acid were able to provide marked protection against in vivo CP toxicities [56] and [57] . In agreement with the previous studies, CP significantly depleted GSH and protein thiols in liver and enhanced LPO, as evidenced by increased malondialdehyde levels, suggesting the induction of oxidative stress [42] and [58] . However, oxidative stress could be a consequence of increased ROS generation and/or decreased antioxidant defense. SOD and CAT provide major cellular defense against ROS and together they convert superoxide radicals first to H2 O2 and then to molecular oxygen and water. Another enzyme viz. GSH-Px uses thiol reducing power of GSH to reduce oxidised lipid and protein targets of ROS. Activities of SOD, CAT and GSH-Px declined significantly in hepatic tissue of CP treated rats (Table 5 ). The observed decrease in the activity of SOD, CAT and GSH-Px might be due to the inhibition of functional
SH group of the enzymes by CP, as suggested earlier [59] . Reduced activities of these antioxidant enzymes would have caused decreased scavenging of ROS, thus increasing the susceptibility of hepatic tissue to oxidative damage. In contrast, marked increase in the activities was seen when rats were orally administered NSO. Furthermore, NSO administration to CP treated rats, prevented CP induced augmentation of LPO and suppression of SOD, CAT and GSH-Px activities. This is in agreement with previously reported studies that have shown NSO or its constituents viz. p-cymene, carvacrol and in particular thymoquinone to protect against oxidative stress related pathologies by directly scavenging free radicals and/or by enhancing the activities of antioxidant enzymes [16] , [22] and [60] . GSH, plays a major role in protecting cells against oxidative stress. CP is reported to alter cellular GSH levels either by directly binding with it or by oxidizing GSH via CP induced free radical generation [58] , [59] and [61] . Activity of GR, the enzyme responsible for recycling of GSH from oxidized form (GSSG) to the reduced form (GSH), was significantly decreased by CP treatment (Table 5 ). This would have further lowered the levels of GSH which is necessary cofactor for the detoxification of H2 O2 and hyderoperoxides. TR, a part of the thioredoxin system, is the major cellular disulfide reductase that also functions in defense against oxidative stress [62] .
Activity of TR was also found to decline significantly in CP treated rats (Table 5 ). Reduced G6PDH activity in CP treated group could have resulted in reduced activities of GR and TR, as both the enzymes essentially require NADPH for their activities. Depressed activities of GR and TR along with suppressed SOD, CAT and GSH-Px activities indicate that CP has an inhibitory effect on the endogenous antioxidant defense system and that confirms the involvement of ROS in CP hepatotoxicity as reported earlier [3] and [11] . However, oral NSO administration attenuated the CP induced decline in GSH levels and antioxidant enzyme activities, thus empowering the endogenous antioxidant defense against CP induced oxidative damage to liver.
5. Conclusions
CP treatment elicited deleterious hepatotoxic effects by causing major damage to mitochondria, lysosomes and plasma membrane as reflected by significant decrease in the activities of specific marker enzymes. CP treatment seems to increase cellular energy dependence on anaerobic glycolysis and that might be due to the CP induced mitochondrial damage. CP also inhibited the endogenous physiological antioxidant defense system and induced oxidative stress which at least in part may have mediated its hepatotoxic effects. In contrast, NSO seems to accelerate repair/regeneration of injured organelles viz. mitochondria and cell membrane as evident by increased activities of TCA cycle and membrane enzymes and as observed by histopathological examination. Most importantly, by improving the endogenous antioxidant status and metabolic activity, NSO protects the liver against CP generated free radical attack. The study put forth a good basis for NSO as new adjuvant of CP, to abrogate the hepatotoxicity induced upon cancer chemotherapy.
Acknowledgments
The authors gratefully acknowledge University Grants Commission (UGC), New Delhi for the award of Junior Research Fellowship to M.A and Senior Research Fellowship (under MANF scheme) to Z.F and S.R. Authors acknowledge the help extended by Ms. Faaiza Shahid during completion of work.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- [1] D. Lebwohl, R. Canetta; Clinical development of platinum complexes in cancer therapy: an historical perspective and an update; Eur. J. Cancer, 34 (1998), pp. 1522–1534
- [2] R.Y. Tsang, T. Al-Fayea, H.J. Au; Cisplatin overdose: toxicities and management; Drug Saf., 32 (2009), pp. 1109–1122
- [3] A. Naqshbandi, W. Khan, S. Rizwan, F. Khan; Studies on the protective effect of flaxseed oil on cisplatin induced hepatotoxicity; Hum. Exp. Toxicol., 31 (2012), pp. 364–375
- [4] R. Bentli, H. Parlakpinar, A. Polat, E. Samdanci, M.E. Sarihan, M. Sagir; Molsidomine prevents cisplatin-induced hepatotoxicity; Arch. Med. Res, 44 (2013), pp. 521–528
- [5] B.H. Ali, M.S. Al Moundhri; Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research; Food. Chem. Toxicol., 44 (2006), pp. 1173–1183
- [6] Y. Liao, X. Lu, C. Lu, G. Li, Y. Jin, H. Tang; Selection of agents for prevention of cisplatin-induced hepatotoxicity; Pharmacol. Res., 57 (2008), pp. 125–131
- [7] R. Pratibha, R. Sameer, P.V. Rataboli, D.A. Bhiwgade, C.Y. Dhume; Enzymatic studies of cisplatin-induced oxidative stress in hepatic tissue of rats; Eur. J. Pharmacol., 532 (2006), pp. 290–293
- [8] Y.I. Chirino, J. Pedraza-Chaverri; Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity; Exp. Toxicol. Pathol., 61 (2009), pp. 223–242
- [9] A.M. Florea, D. Büsselberg; Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects; Cancers, 3 (2011), pp. 1351–1371
- [10] N.A. Arivarasu, S. Priyamvada, R. Mahmood; Oral administration of caffeic acid ameliorates the effect of cisplatin on brush border membrane enzymes and antioxidant system in rat intestine; Exp. Toxicol. Pathol., 65 (2013), pp. 21–25
- [11] H.H. Mansour, H.F. Hafez, N.M. Fahmy; Silymarin modulates cisplatin induced oxidative stress and hepatotoxicity in rats; J. Biochem. Mol. Biol., 39 (2006), pp. 656–661
- [12] A. Kart, Y. Cigremis, M. Karaman, H. Ozen; Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit; Exp. Toxicol. Pathol., 62 (2010), pp. 45–52
- [13] B.H. Ali, G. Blunden; Pharmacological and toxicological properties of Nigella sativa; Phytother. Res., 17 (2003), pp. 299–305
- [14] A. Ahmad, A. Husain, M. Mujeeb, S.A. Khan, A.K. Najmi, N.A. Siddique, Z.A. Damanhouri, F. Anwar; A review on therapeutic potential of Nigella sativa : A miracle herb ; Asian Pac. J. Trop. Biomed., 3 (2013), pp. 337–352
- [15] M.A. Mansour, M.N. Nagi, A.S. El‐Khatib, A.M. Al‐Bekairi; Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT‐diaphorase in different tissues of mice: a possible mechanism of action; Cell Biochem. Funct., 20 (2002), pp. 143–151
- [16] M.L. Salem; Immunomodulatory and therapeutic properties of the Nigella sativa L. seed ; Int. Immunopharmacol., 5 (2005), pp. 1749–1770
- [17] O.A. Badary, R.A. Taha, A.M. Gamal el-Din, M.H. Abdel-Wahab; Thymoquinone is a potent superoxide anion scavenger; Drug Chem. Toxicol., 26 (2010), pp. 87–98
- [18] S. Yehuda, R.L. Carasso; Modulation of learning, pain thresholds, and thermoregulation in the rat by preparations of free purified alpha-linolenic and linoleic acids: determination of the optimal omega 3-to-omega 6 ratio; Proc. Natl. Acad. Sci. U. S. A., 90 (1993), pp. 10345–10349
- [19] P. Laakso, P. Voutilainen; Analysis of triacylglycerols by silver-ion high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry; Lipids, 31 (1996), pp. 1311–1322
- [20] A.M. El-Badry, R. Graf, P.A. Clavien; Omega 3-omega 6: what is right for the liver?; J. Hepatol., 47 (2007), pp. 718–725
- [21] G.L. Russo; Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention; Biochem. Pharmacol., 77 (2009), pp. 937–946
- [22] Z.S. Ibrahim, M. Ishizuka, M. Solman, K. El Bohi, W. Sobhy, K. Muzandu, A.M. Elkattawy, K.Q. Sakamoto, S. Fujita; Protection by Nigella sativa against carbon tetrachloride-induced downregulation of hepatic cytochrome P450 isozymes in rats ; Jpn. J. Vet. Res., 56 (2008), pp. 119–128
- [23] M. Ismail, G. Al-Naqeep, K.W. Chan; Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats ; Free Radic. Biol. Med., 48 (2010), pp. 664–672
- [24] O.A. Badary, M.N. Nagi, O.A. Al-Shabanah, H.A. Al-Sawaf, M.O. Al-Sohaibani, A.M. Al-Bekairi; Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity; Can. J. Physiol. Pharmacol., 75 (1997), pp. 1356–1361
- [25] R.H.M. Salama, N.A. Abd-El-Hameed, S.K.H. Abd-El-Ghaffar, Z.T. Mohammed, N.M.A. Ghandour; Nephroprotective effect of Nigella sativa and Matricaria chamomilla in cisplatin induced renal injury—supportive treatments in cisplatin nephrotoxicity ; Int. J. Clin. Med., 2 (2011), pp. 185–195
- [26] E. Uz, O. Bayrak, E. Uz, A. Kaya, R. Bayrak, B. Uz, F.H. Turgut, N. Bavbek, M. Kanbay, A. Akcay; Nigella sativa oil for prevention of chronic cyclosporine nephrotoxicity: an experimental model ; Am. J. Nephrol., 28 (2008), pp. 517–522
- [27] L. Yaman, C. Balikci; Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats ; Exp. Toxicol. Pathol., 22 (2010), pp. 119–128
- [28] A. Zlatkis, B. Zak, A.J. Boyle; A new method for the direct determination of serum cholesterol; J. Lab. Clin. Med., 41 (1953), pp. 486–492
- [29] S.J. Khundmiri, M. Asghar, F. Khan, S. Salim, A.N. Yusufi; Effect of ischemia and reperfusion on enzymes of carbohydrate metabolism in rat kidney; J. Nephrol., 17 (2004), pp. 1–7
- [30] R.K. Crane, A. Sols; The association of particulate fractions of brain and other tissue homogenates; J. Biol. Chem., 203 (1953), pp. 273–292
- [31] N.A. Nelson; Photometric adaptation of the Somogyi method for the determination of glucose; J. Biol. Chem., 153 (1944), pp. 375–381
- [32] N. Farooq, A.N.K. Yusufi, R. Mahmood; Effect of fasting on enzymes of carbohydrate metabolism and brush border membrane in rat intestine; Nutr. Res., 24 (2004), pp. 407–416
- [33] S. Marklund, G. Marklund; Involvement of the superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase; Eur. J. Biochem., 47 (1974), pp. 469–474
- [34] U. Giri, M. Iqbal, M. Athar; Porphyrin mediated photosensitization has a weak tumor promoting activity in mouse skin: possible role of in-situ generated reactive oxygen species; Carcinogenes, 17 (1996), pp. 2023–2028
- [35] L. Flohe, W. Gunzler; Assays of glutathione peroxidase; S.P. Colowick, N.O. Kaplan (Eds.), Methods Enzymology, Academic Press, New York (1984), pp. 114–121
- [36] T. Tamura, T.C. Stadtman; A new selenoprotein from human lung adenocarcinoma cells: purification, properties and thioredoxin reductase activity; Proc. Natl. Acad. Sci. U. S. A., 93 (1996), pp. 1006–1011
- [37] W.H. Habig, M.J. Pabst, W.B. Jakoby; Glutathione S-transferases: the first enzymatic step in mercapturic acid formation; J. Biol. Chem., 249 (1974), pp. 7130–7139
- [38] I. Carlberg, B. Mannervik; Glutathione reductase; Method Enzymol., 113 (1985), pp. 484–490
- [39] H. Ohkawa, N. Ohishi, K. Yagi; Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction; Ann. Biochem., 95 (1979), pp. 351–358
- [40] J. Sedlak, R.H. Lindsay; Estimation of total protein bound and nonprotein bound SH groups in tissue with Ellman’s reagent; Ann. Biochem., 2 (1968), pp. 192–205
- [41] D.J. Jollow, J.R. Mitchell, N. Zampaglione, J.R. Gillette; Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite; Pharmacology, 11 (1974), pp. 151–169
- [42] A. Naqshbandi, S. Rizwan, F. Khan; Dietary supplementation of flaxseed oil ameliorates the effect of cisplatin on rat kidney; J. Funct. Foods, 5 (2013), pp. 316–326
- [43] A.Y. Nasr; Protective effect of aged garlic extract against the oxidative stress induced by cisplatin on blood cells parameters and hepatic antioxidant enzymes in rats; Toxicol. Rep., 1 (2014), pp. 682–691
- [44] A. Avci, R. Cetin, I.B. Erguder, E. Devrim, B. Kilicoglu, O. Candir, H.S. Ozturk, I. Durak; Cisplatin causes oxidation in rat liver tissues: possible protective effects of antioxidant food supplementation; Turk. J. Med. Sci., 38 (2008), pp. 117–120
- [45] H.I. El-Sayyad, M.F. Ismail, F.M. Shalaby, R.F. Abou-El-Magd, R.L. Gaur, A. Fernando, M.H.G. Raj, A. Ouhtit; Histopathological effects of cisplatin: doxorubicin and 5-flourouracil (5-FU) on the liver of male albino rats; Int. J. Biol. Sci., 5 (2009), pp. 466–473
- [46] A.M. Mohamadin, B. Sheikh, A.A. Abd El-Aal, A.A. Elberry, F.A. Al-Abbasi; Protective effects of Nigella sativa oil on propoxur-induced toxicity and oxidative stress in rat brain regions ; Pest. Biochem. Physiol., 98 (2010), pp. 128–134
- [47] M.A. Randhawa, M.S. Al-Ghamdi; A review of the pharmaco-therapeutic effects of Nigella sativa; J. Med. Res., 41 (2002), pp. 40–50
- [48] M.N. Nagi, M.A. Mansour; Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection; Pharmacol. Res., 41 (2000), pp. 283–289
- [49] K.A. Youdim, A. Martin, J.A. Joseph; Essential fatty acids and the brain: possible health implications; Int. J. Dev. Neurosci., 18 (2000), pp. 383–399
- [50] M. Carrillo-Tripp, S.E. Feller; Evidence for a mechanism by which omega-3 polyunsaturated lipids may affect membrane protein function; Biochemistry, 44 (2005), pp. 10164–10169
- [51] A. Naqshbandi, M.W. Khan, S. Rizwan, A.N.K. Yusufi, F. Khan; Studies on the protective effect of fish oil against cisplatin induced hepatotoxicity; Biol. Med., 3 (2011), pp. 86–97
- [52] S.K. Aggarwal; A histochemical approach to the mechanism of action of cisplatin and its analogues; J. Histochem. Cytochem., 41 (1993), pp. 1053–1073
- [53] M.K. Kuhlmann, G. Burkhardt, H. Kohler; Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application; Nephrol. Dial. Transplant., 12 (1997), pp. 2478–2480
- [54] N.M. Martins, N.A.G. Santos, C. Curti, M.L.P. Bianchi, A.C. Santos; Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver; J. Appl. Toxicol., 28 (2008), pp. 337–344
- [55] T. Xiao, S. Choudhary, W. Zhang, N.H. Ansari, A. Salahudeen; Possible involvement of oxidative stress in cisplatin induced apoptosis in LLC-PK1 cells; J. Toxicol. Environ. Health A, 66 (2003), pp. 469–479
- [56] S.A. Khan, S. Priyamvada, W. Khan, S. Khan, N. Farooq, A.N. Yusufi; Studies on the protective effect of green tea against cisplatin induced nephrotoxicity; Pharmacol. Res., 60 (2009), pp. 382–391
- [57] S. Palipoch, C. Punsawad, P. Koomhin, P. Suwannalert; Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatin-induced oxidative stress; BMC Complement. Altern. Med., 14 (2014), p. 111
- [58] M.I. Yousef, A.A. Saad, L.K. El-Shennawy; Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats; Food Chem. Toxicol., 47 (2009), pp. 1176–1183
- [59] M.A. Valentovic, J.G. Ball, J.M. Brown, M.V. Terneus, E. McQuade, S.V. Meter, H.M. Hedrick, A.A. Roy, T. Williams; Resveratrol attenuates cisplatin renal cortical cytotoxicity by modifying oxidative stress; Toxicol. In Vitro, 28 (2014), pp. 248–257
- [60] H. Kaatabi, A.O. Bamosa, A. Badar, A. Al-Elq, B. Abou-Hozaifa, F. Lebda, A. Al-Khadra, S. Al-Almaie; Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trials ; PLoS One, 10 (2015), p. 0113486
- [61] N.A. Santos, C.S. Catao, N.M. Martins, C. Curti, M.L. Bianchi, A.C. Santos; Cisplatin induced nephrotoxicity is associated with oxidative stress redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria; Arch. Toxicol., 81 (2007), pp. 495–504
- [62] E.S.J. Arnér, A. Holmgren; Physiological functions of thioredoxin and thioredoxin reductase; Eur. J. Biochem., 267 (2000), pp. 6102–6109
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?