- C. Pfarrer and C. Staszyk contributed equally to this work.
- C. Schröck and F. Geburek contributed equally to this work.
Abstract
Aspiration of equine sternal bone marrow is required for the cultivation of bone marrow-derived multipotent mesenchymal stromal cells (BM-MSCs) for regenerative therapies. For bone marrow aspiration as well as for MSC cultivation, there is a need to optimize techniques and protocols to enhance MSC harvest at minimized culture times. In a comparative study bone marrow aspirates from sternebra 4 and 5 were collected at two different positions within the sternebrae, either from 10 mm or from 30 mm dorsal from the ventral margin of the sternebrae. Accuracy of the puncture depth was confirmed by ultrasonography and computed tomography. Isolated MSCs were cultivated using media supplemented with three alternative sera, i.e. fetal calf serum, standardized horse serum and autologous serum. Due to morphological characteristics (spherical shape, only thin layer of hyaline cartilage at the ventral site, reliable bone marrow aspiration from only 10 mm intraosseous depth), sternebra 5 appeared most suitable for bone marrow aspiration. Cultivation and expansion of BM-MSCs was most efficient using fetal calf serum.
Introduction
Aspiration of equine sternal bone marrow is a surgical procedure conducted for various diagnostic and therapeutic purposes. For diagnostic purposes, equine bone marrow is analysed to characterize and further explain diagnose haematopoietic disorders and bone marrow-associated neoplastic conditions (Russell et al. 1994; Sellon 2006). For therapeutic purposes, sternal bone biopsies are successfully used for the production of autologous cancellous bone grafts to repair large bony defects (Richardson et al. 1986; Désévaux et al. 2000). Meanwhile, sternal bone marrow is commonly used and well recognized as a source of MSCs for potentially regenerative therapies of musculoskeletal injuries (Smith et al. 2003, 2013, 2014; Fortier & Smith 2008; Goodrich et al. 2008; Smith 2008; Garvican et al. 2014). Bone marrow-derived MSCs appear to have the potential to produce actual tendon matrix rather than scar tissue which is by contrast associated with the normal pathway of tendon healing (Frank et al. 1997; Smith et al. 2003; Kajikawa et al. 2007). Although, the aspiration of bone marrow from the equine sternum is accepted as a safe method, fatal thoracic and cardiac punctures (Jacobs et al. 1983) as well as a case of pneumopericardium (Durando et al. 2006) have been described. To avoid complications, a maximum ventro-dorsal insertion depth of the bone marrow aspiration cannula into the sternebrae of 30 mm has been recommended (Goodrich et al. 2008). Based on anatomical studies of sternebrae 4–6 the maximum tolerable insertion depth was adjusted to 10–20 mm (Kasashima et al. 2011). A recent investigation showed that sternebrae 4 and 5 are most suitable for bone marrow aspiration (Eydt et al. 2014). These sternebrae possess an almost spherical shape and a dorso-ventral extension of at least 52 mm. On the basis of these experimental data, an insertion depth in the median plane of even more than 30 mm is regarded as safe in warmblood horses (Eydt et al. 2014).
The process of cultivation of equine MSCs from bone marrow aspirates has been described in detail in the literature (Smith et al. 2003; Arnhold et al. 2007; Bourzac et al. 2010; Spaas et al. 2012). After bone marrow aspiration, it takes however between 3 and 6 weeks in culture to harvest a number of cultured MSCs being adequate for therapeutic purposes (Brems & Jebe 2008; Fortier & Smith 2008; Goodrich et al. 2008; Reed & Leahy 2013; Schnabel et al. 2013). Consequently, efforts were made to optimize the yield of MSCs by evaluating alternative aspiration techniques and different cell isolation protocols (Bourzac et al. 2010; Kasashima et al. 2011). In continuation of these studies, the quality of bone marrow aspirates obtained from different insertion depths were compared and the influence of different sera supplemented to the MSC culture medium was determined.
Materials and methods
Animals and bone marrow aspiration technique
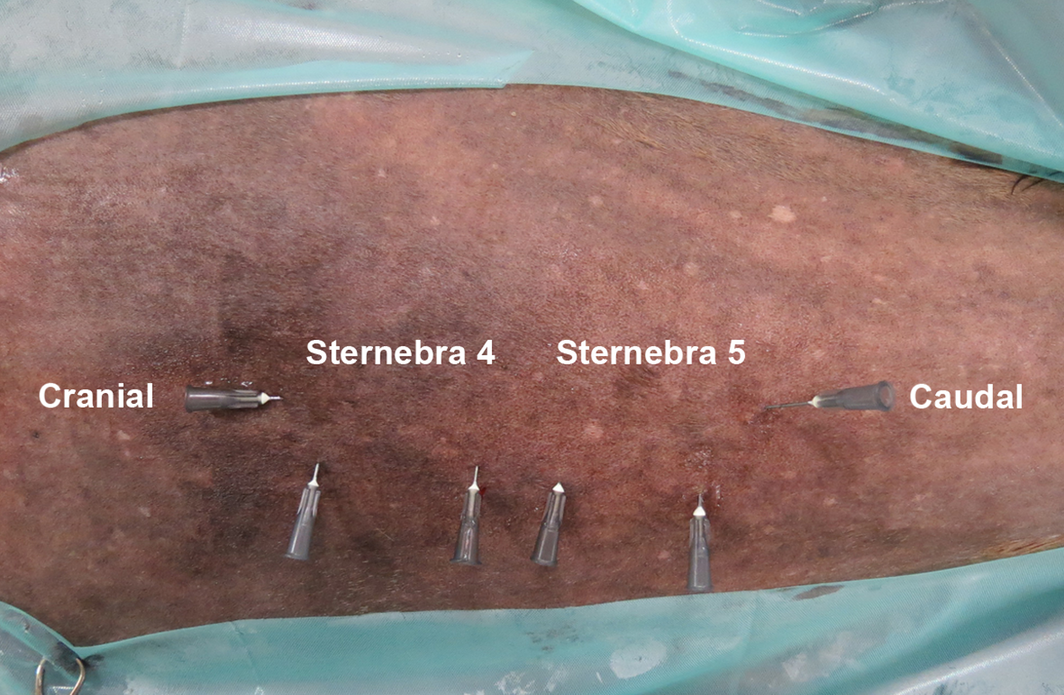
Sternal bone marrow aspirates were collected from twelve horses receiving general anaesthesia. Subsequently, the animals were euthanized for reasons unrelated to this study. The horses (five geldings, seven mares, aged 2–28 years, body mass between 480 and 640 kg) had no history of previous bone marrow aspiration. All protocols were approved by the Animal Welfare Commissioner of the University of Veterinary Medicine Hannover in accordance with the German Animal Welfare Law (Lower Saxony State Office for Costumer Protection and Food Safety, 33.9-42502-04-11/0572). Blood was taken from each horse to extract autologous serum (AS). Horses were premedicated with 0.6–0.8 mg/kg xylazine (Xylazin 2%®, CP-Pharma GmbH, Burgdorf, Germany). Anaesthesia was induced by 0.05 mg/kg midazolam (Midazolam® B. Braun 5 mg/mL, B. Braun Melsungen AG, Melsungen, Germany) and 2.2 mg/kg ketamine (Narketan®, 100 mg/mL, Vétoquinol GmbH, Ravensburg, Germany). General anaesthesia was maintained with isoflurane (Isofluran CP®, CP-Pharma GmbH, Burgdorf, Germany) in 100% oxygen. Mean arterial blood pressure was maintained at 70–80 mmHg. Horses were positioned in dorsal recumbency and the area of the sternum was clipped. After degreasing the skin with soap and alcohol, acoustic gel was applied and the sternum was examined ultrasonographically (Logiq E9, GE Healthcare, Wauwatosa, WI, USA) with a 5–9 MHz and a 9–15 MHz linear probe parallel and transverse to the longitudinal axis of the horse. In addition, longitudinal panoramic ultrasonograms of the caudal sternal region were produced, using the LOGIQview function of the ultrasound machine. The positions of the median plane of sternebrae 4 and 5 were determined using longitudinal and transverse probe positions (Fig. 1). Freeze frames of transverse B-mode ultrasonograms centred on the respective sternebra were stored (Fig. 1b). Subsequently, the distances from the skin surface to the continuous hyperechoic line representing the ventral margins of the sternebrae, as well as the distance between skin surface and hypoechoic zone (cartilage) ventral to the bony sternebrae (Fig. 1b, arrows) were measured on the ultrasonograms (Fig. 1b). Hypodermic injection-needles with a diameter of 22G were used to mark the positions of sternebrae 4 and 5, i.e. their cranio-caudal extensions and the median plane (Fig. 2). After a stab incision of the skin above, the centre of sternebrae 4 and 5 with a No. 11 scalpel blade (Helmut Zepf Medizintechnik GmbH, Seitingen-Oberflacht, Germany), a Jamshidi needle (11 gauge × 100 mm, Fa. Angiotech, Gainesville, FL, USA) was introduced through the stab incision until a resistance (ventral margin of the sternebra) was encountered. Then the distance from the skin to the ventral margin of the sternebra was marked on the Jamshidi needle using a sterile marker pen (Kearing, Shanghai Kearing Stationary Co., Ltd.). Additionally, the Jamshidi needle was marked for an intended sternal insertion depth of 10 and 30 mm. Subsequently, the Jamishidi needle was introduced in the median plane of the respective sternebra with rotating movements. In each horse, bone marrow aspirates were taken from sternebrae 4 and 5 at an intraosseous insertion depth of either 10 mm (six horses) or 30 mm (six horses). In each group (10 mm insertion depth and 30 mm insertion depth), three horses were sampled, aspirating 5 mL bone marrow from sternebra 4 and 10 mL from sternebra 5; the remaining three horses were sampled, aspirating 10 mL bone marrow from sternebra 4 and 5 mL from sternebra 5. Then bone marrow was collected in a 20 mL syringe (filled with 0.1 mL/5 mL heparin; Heparin-calcium-12500-ratiopharm®, 12500 I.U., Ratiopharm GmbH, Ulm, Germany) and transported in a refrigerated box to the laboratory. After surgery, horses were euthanized under general anaesthesia by intravenous injection of 70 mg/kg bodyweight pentobarbital.
|
|
|
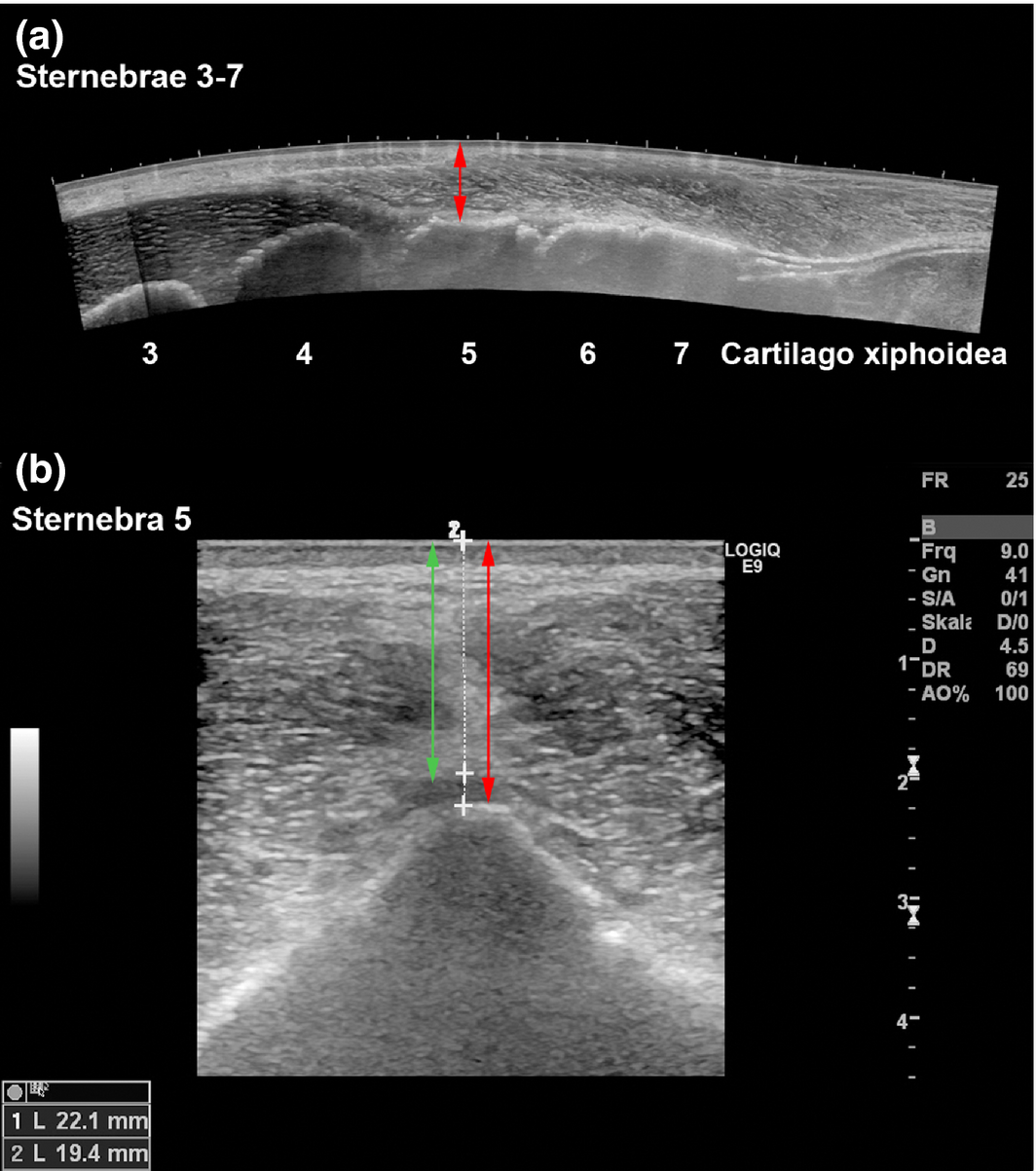
Figure 1. Ultrasonograms of the equine sternum, horse 18 (Mare, 5 years). (a) Longitudinal panoramic image (LOGIQview function). The ventral bony margins of the sternebrae 3–7 are clearly visble. The red arrow indicates the distance between sternebrae 5 (ventral bony margin) and the skin. (b): Transverse B-mode image of sternebra 5. Skin-to-cartilage distance (green double arrow, 19.4 mm) and skin-to-bone distance (red double arrow, 22.1 mm). |
|
|
|
Figure 2. Ventral view of the sternal region, horse 13 (mare, 23 years). The median plane of the sternum and the cranial and caudal borders of sternebrae 4 and 5 were identified by ultrasonography and marked with hypodermic needles. |
Clinical computed tomography (cCT) and micro computed tomography (μCT)
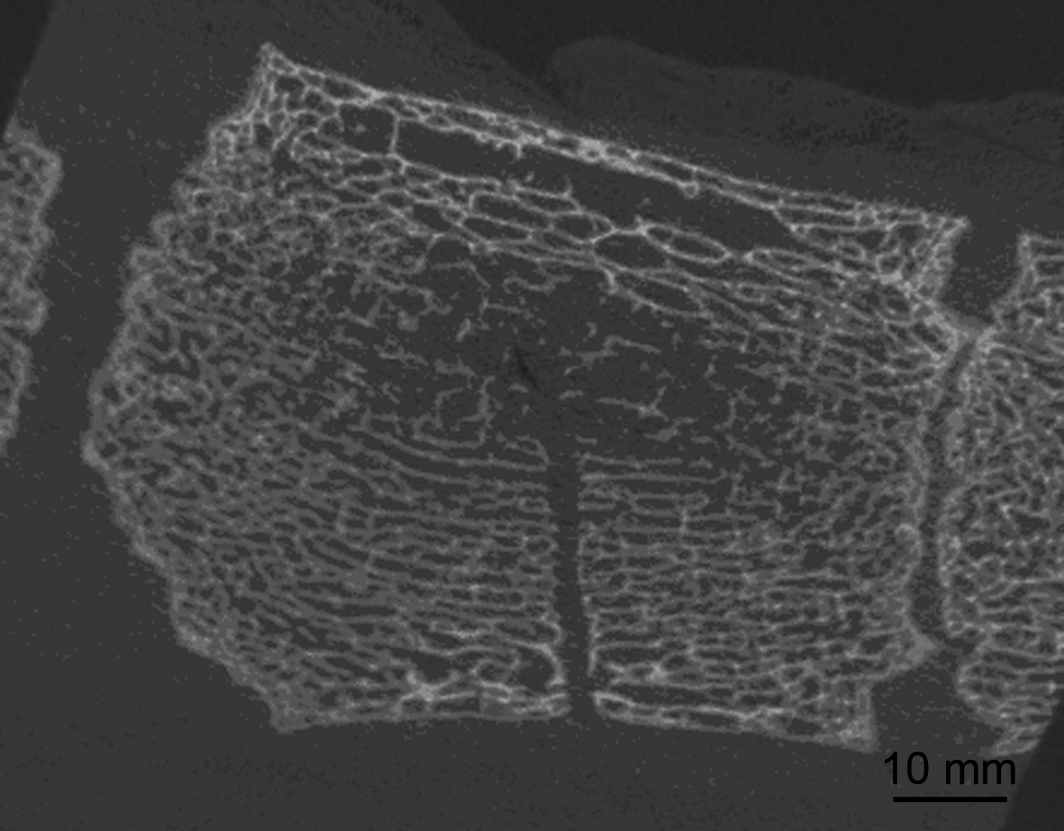
The sternum of each horse was removed after euthanasia at the ventral third of the costal cartilage using a reciprocating saw (EFA 61, EFA Schmid & Wezel GmbH & Co. KG, Maulbronn, Germany). By use of a clinical CT (BrillianceTM CT – Big Bore Oncology Scanner, Philips Medical Systems, Best, Netherlands) each sternum was scanned as described previously (Eydt et al. 2014). Additionally, μCT images (XTremeCT, Scanco Medical AG, Brüttisellen, Switzerland) were generated to visualize the bony architecture of sternebrae 4 and 5 and the effects of the puncture procedure (Fig. 3).
|
|
|
Figure 3. Sternebra 5 after bone marrow aspiration. μCT image, longitudinal plane. The puncture canal within the bony, trabecular meshwork is clearly visible. No damage or fractures of the bony trabeculae are evident. |
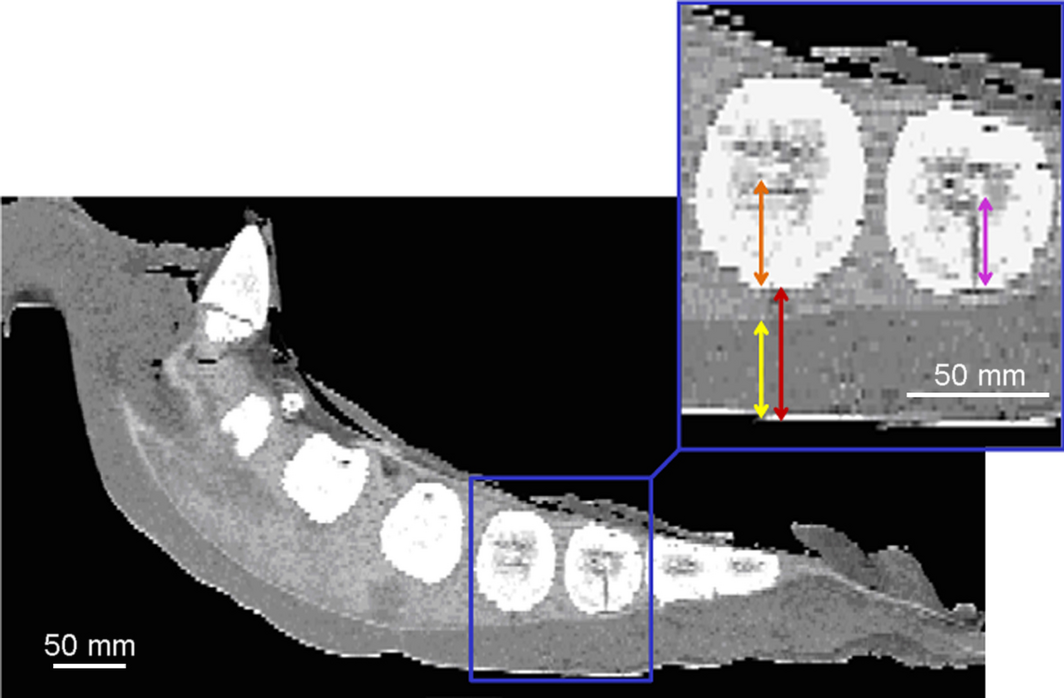
Morphometric measurements were taken by using the computer program AMIRA (version 5.2.0, Visualization Sciences Group, Merignac Cedex, Frankreich) as described previously (Eydt et al. 2014). The following measurements were recorded (Fig. 4):
|
|
|
Figure 4. Equine sternum, clinical CT images, resolution (1024 × 1024 pixel), horse 7 (gelding, 3 years). For the CT scan, the sternum was placed ventral side up to prevent impression of the skin. Sternebrae 4 and 5 are enlarged (blue inset) to visualize performed measurement procedure. Orange arrow: distance from the bony ventral margin to the centre of the sternebra. Red arrow: Distance from skin to the bony margin of the sternebra. Yellow arrow: ventral-to-dorsal thickness of the cartilage at the ventral aspect of the sternebra. Purple arrow: length of the intraosseous puncture canal. |
- ventro-dorsal distance from the surface of the skin to the bony sternebra
- thickness of the cartilage at the ventral margin of the sternebra
- distance from the bony ventral margin to the centre of the sternebra
- length of the puncture canal.
Isolation and culture of mononuclear cells (MNCs)
Bone marrow aspirates (sternebrae 4 or 5, 5 or 10 mL, from 10 or 30 mm sternal insertion depth) were transferred from the collecting syringes to sterile 50 mL plastic tubes (Sarstedt AG & Co, Nümbrecht, Germany) and stored at 4°C for 24 h to allow sedimentation. After centrifugation (1000g, 15 min) the plasma layer was removed. Subsequently, the remaining cell-rich layer was divided into two portions and mixed with 10 mL culture medium (CM) containing 88% DMEM (Dulbeccos Modified Eagle Medium, gibco®, life technologies GmbH, Darmstadt, Germany), 1% penicillin-streptomycin (10 000 U/mL, gibco®, life technologies GmbH, Darmstadt, Germany) and 1% MEM NEAA (MEM Non-Essential Amino Acids Solution, 100x, gibco®, life technologies GmbH, Darmstadt, Germany). The CM was supplemented with either 10% fetal calf serum (FCS, PAA Laboratories GmbH, Pasching, Austria) or with 10% autologous serum (AS). Erythrocytes were removed using a cell strainer (70 μm, BD Falcon™, BD Bioscience, Durham, NC, USA). MNCs were isolated by density-gradient centrifugation using Easycoll® (Biochrom AG, Berlin, Germany, 1.086 g/mL) in a mixture of 1:1 by volume. After washing with phosphate-buffered saline (PBS, gibco®, life technologies GmbH, Darmstadt, Germany) and centrifugation at 250g for 5 min, cells were resuspended in CM. The cells were plated in 2500 mm2 cell culture flasks and incubated at 37°C and 5% CO2. Media were changed 24 h after seeding and then every second day until the cells achieved 80% confluence. Culture characteristics (size, shape and alignment of cells) were assessed by inverted light microscopy. The time until 80% confluence was reached was recorded and cells were trypsinized (0.05% Trypsin-EDTA (1x), gibco®, life technologies GmbH, Darmstadt, Germany) and reseeded at different densities according to experiments described in the following. Therefore, FCS cultivated cells were divided into two parts. Cells were either further cultivated using 10% FCS or were cultivated using 10% standardized horse serum (SHS, PAA Laboratories GmbH, Pasching, Austria). The content of SHS was gradually increased over two steps of medium change. Cells that were initially expanded using CM supplemented with 10% AS were further cultivated, using 10% AS.
Colony-forming unit (CFU) assay
CFU assays were performed at passage 2 in a 12 well cell culture plate (Greiner Bio-One GmbH, Frickenhausen, Germany). MSCs (supplemented with FCS, SHS or AS) were plated at 1, 2, 4 and 8 cells/100 mm2. Culture medium was replaced every second day. After 14 days of culture, adherent cells were washed in PBS, fixed and stained with 1% cresyl violet in 100% methanol. Cell colonies containing more than 20 cells were counted. Calculation of the CFU efficiency was performed according to the formula: CFU efficiency (%) = (counted CFU /cells originally seeded) × 100.
Scratch assay (Wound healing assay)
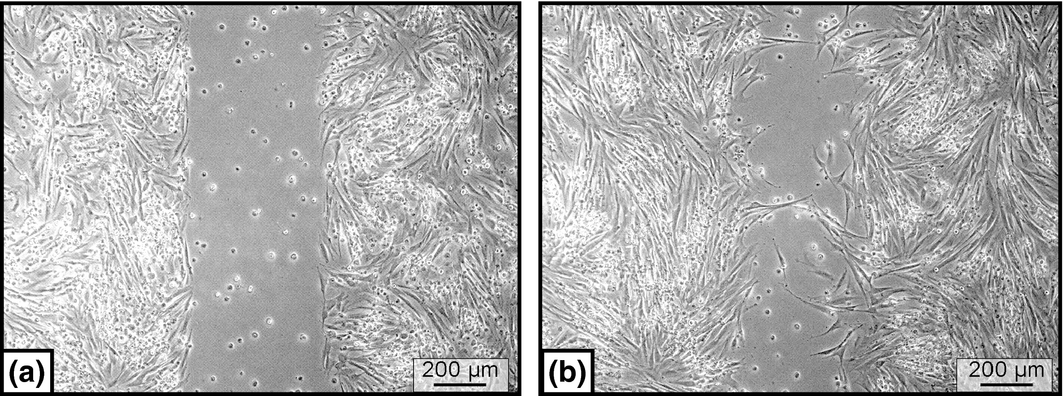
Scratch assays were performed at passage 2 in a 6-well cell culture plate (Greiner Bio-One GmbH, Frickenhausen, Germany) to which a sterile culture insert (ibidi GmbH, Martinsried, Germany) was added. A 500-μm thick wall separated each culture into two 70 μL cell culture reservoirs. MSCs (supplemented with FCS, SHS or AS) were plated at a density of 30 000 cells/70 μL well and cells were cultivated for 24 h at 37°C and 5% CO2. Subsequently, the culture insert was removed creating a cell-free gap. Cells were further cultivated in a life cell imaging system (Cell Observer® Systems, Zeiss MicroImaging). Cell-migration and cell-proliferation was continuously recorded for 24 h with the computer program Axio Vision. Finally, the gap area overgrown by cells was determined using the picture processing software Photoshop (Adobe Photoshop, CS3; Fig. 5).
|
|
|
Figure 5. Cultured equine bone marrow MSCs, scratch-assay. (a) A standardized gap was created. (b) After 24 h, a certain amount of the gap area was re-occupied by cells. The movement of the cells was recorded by a life cell imaging microscopy. |
Statistics
Differences between FCS, AS and SHS were calculated by one-way analysis of variance with repeated measurements and post hoc Tukey test both in scratch-assay as well as in CFU assay. Resulting P-values of P < 0.05 were considered as statistically significant. All analyses were performed with the statistics program SAS (version 9.3; SAS Institute, Cary, NC, USA).
Results
Bone marrow aspiration
The ventral contour of each sternebra could be clearly detected during ultrasonography (Fig. 1), which allowed determination of the distance between the skin surface and the ventral bony margin of the sternebrae. The mean distance between skin and sternebra measured 26.4 ± 6.4 mm for sternebra 4 and 29.6 ± 7.8 mm for sternebra 5. The use of injection needles to indicate the positions of sternebrae 4 and 5 (Fig. 2) resulted in a correct positioning of the Jamshidi needle to aspirate bone marrow from a sternal insertion depth of 10 mm or 30 mm.
In both, sternebra 4 and sternebra 5, bone marrow aspiration was always feasible at a calculated sternal insertion depth of 30 mm. In sternebra 5, bone marrow aspiration was also feasible at a calculated sternal insertion depth of 10 mm. In sternebra 4, bone marrow aspiration from 10 mm insertion depth was successful in only 2 out of 12 cases.
Depth of the puncture canal
The skin-sternebra distances determined by measurements on cCT images were compared to ultrasonographic measurements. Distances determined by ultrasonography were constantly shorter, for sternebra 4 (6.3 ± 5.8 mm) and for sternebra 5 (8.9 ± 4.5 mm). The sternal insertion depth calculated during bone marrow aspiration and the actual insertion depth measured on cCT images differed constantly. For sternebra 4, the calculated insertion depth was in almost all cases underestimated by approximately 9.0 ± 5.5 mm. For sternebra 5, the calculated insertion depth was also constantly underestimated, but by approximately 2.9 ± 2.0 mm. The puncture canal deviated from an exact ventro-dorsal orientation in the median plane but was always placed within the sternebrae.
Measurements of sternebrae 4 and 5
The geometrical centres of the sternebrae were placed at 32.2 ± 3.3 mm (sternebra 4) and 27.3 ± 3.7 mm (sternebra 5) dorsal to the ventral bony margin of the sternebrae. In each sternebra, a centre zone composed of thin and loosely arranged bony trabeculae was visible in μCT images. This centre zone was always reached with the bone marrow aspiration canula when a 30 mm sternal insertion depth was intended (Fig. 3 and Fig. 4). The thickness of the ventral cartilage, measured on cCT images was 12.0 ± 5.0 mm (sternebra 4) and 6.6 ± 2.3 mm (sternebra 5). The μCT images always showed a clearly defined puncture canal (Fig. 3). The bony trabeculae lining the puncture canal did not present any signs of fractures or damage. The same applied for the area surrounding the dorsal end of the puncture canal, which was the supposed area of bone marrow aspiration (Fig. 3).
Cultivation of MNCs obtained from sternal bone marrow aspirates
Regardless of the sampling site (sternebra 4 or 5), of the sampling technique (aspiration from 10 mm or 30 mm intraosseous depth) and of the aspirated volume of bone marrow (5 mL or 10 mL), from all bone marrow aspirates MNCs were successfully isolated by density gradient centrifugation. Subsequently cultured MNCs gave rise to plastic adherent cell colonies, with self-renewal capacities and fibroblast morphology (spindle shaped, homogenous size and parallel cell alignment). Mean culture time until 80% subconfluence for cell cultures supplemented with AS was 14 ± 5 days, cell cultures supplemented with FCS reached 80% subconfluence after 16 ± 6 days. In two cases, cultivation of cells in the presence of AS was not successful.
Colony-forming unit assays
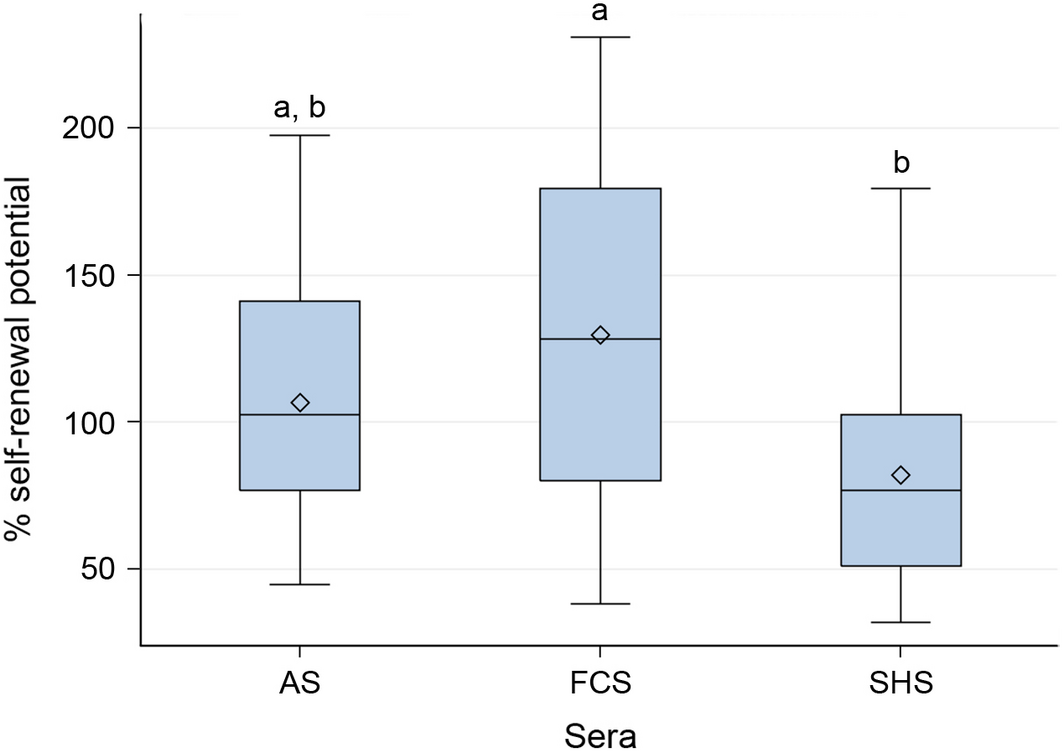
Self-renewal capacity and therefore formation of new cell colonies was obtained in all experimental cell cultures independent from the supplemented serum (FCS, AS or SHS). Cells cultured in the presence of SHS possessed a significant lower self-renewal capacity (66.3% ± 17.1%) compared to cells cultured in the presence of FCS (99.4% ± 40.2%, P < 0.0217). There was no difference in self-renewal capacity between cells cultured with FCS and AS and between cells cultured with AS and SHS (Fig. 6).
|
|
|
Figure 6. Efficiency for self-renewal assessed by rate of colony formation in Colony-forming unit (CFU)-F assays (culture time 14 days, cresyl violet staining). Significant differences were denoted by a and b (P < 0.0217). Differences between cells cultured in SHS and FCS were statistically significant. Box: interquartile range (25%, 75%), horizontal line: median, rectangle: mean, whiskers: range (5%, 95%). |
Scratch-assay
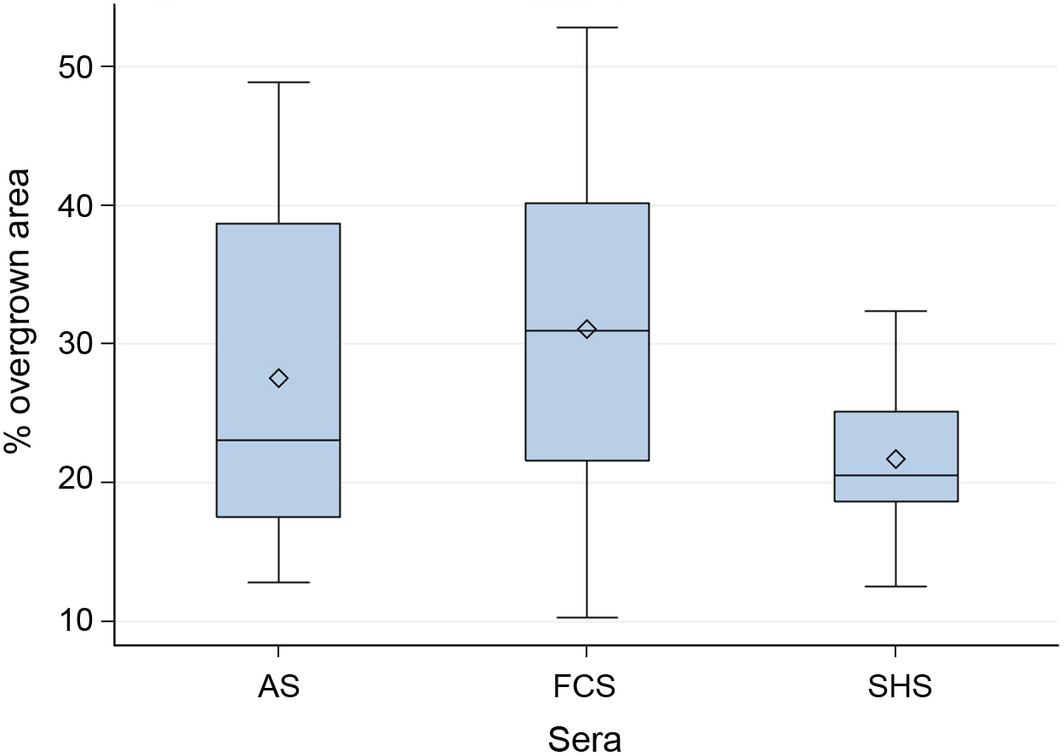
All cultures contained cells exhibiting the capacity for cell-migration, independent from the supplemented serum (FCS, AS or SHS). Migrating cells showed typical morphological characteristics, e.g. asymmetric cell shape with protrusion of lamellipodia. Actual cellular movement was clearly visualized by life cell imaging. Created gaps in the culture dishes became re-occupied by cells. This process was mainly facilitated by cell proliferation rather than by cell migration. 24 h after gap formation, cells cultured in FCS re-occupied 31.1% ± 12.8% of the gap area. Cells cultured in AS re-occupied 27.5% ± 13.2% and cultures supplemented with AS re-occupied 21.7% ± 5.7%. Statistical analyses did not reveal any significant difference, all P > 0.05 (Fig. 7).
|
|
|
Figure 7. Efficiency of cell migration and cell proliferation assessed by 24 h scratch assays (width of scratch: 500 μm). No statistical differences between cells cultured in autologous serum (AS), FCS and SHS were detected, all P > 0.05. Box: interquartile range (25%, 75%), horizontal line: median, rectangle: mean, whiskers: range (5%, 95%). |
Discussion
The anatomical characteristics of the equine sternum were evaluated with special regard to the procedure of sternal bone marrow aspiration by use of a Jamshidi needle set. Reported cases of failed sternal punctures with fatal consequences demonstrated the potential risks that are associated with this method (Jacobs et al. 1983; Durando et al. 2006). Fatal inadvertent thoracocentesis results from a complete penetration of the sternum due to overestimated sizes of the sternebrae or due to improper direction of the instruments. In order to provide a morphological basis for a safe and efficient bone marrow aspiration technique, the dimensions, shapes and internal structures of the sternum and its sternebrae were examined in previous studies (Kasashima et al. 2011; Eydt et al. 2014). The data presented here provide additional information concerning the maximum harvest of MNCs by use of an optimal and safe puncture technique. Previous recommendations for a maximal puncture depth of 10–30 mm for sternebra 5 were not confirmed by our data. As, the ventro-dorsal dimension of sternebra 5 measures 52 ± 0.8 mm a deeper puncture than 30 mm into this sternebra does not increase the risk of thoracic puncture if performed perpendicularly to the ventral margin of the sternebra. However, there is no need to aspirate bone marrow from areas deeper than 10 mm. Moreover, bone marrow from sternebra 5 from 10 mm depth always contained a sufficient number of vital MNCs to produce proliferative MSC cultures in vitro. In contrast, bone marrow yield from sternebra 4 from 10 mm depth appeared to be insufficient to produce MSC cultures. These differing results between sternebra 4 and 5 are likely due to a thicker coverage of cartilage at the ventral margin of sternebra 4 compared to a thin cartilage coverage at the ventral margin of sternebra 5. The thick cartilage layer at sternebra 4 may impede the correct determination of the distance between the skin and the bony part of the sternebra during ultrasonography. Moreover, it is more difficult to predict in advance, using ultrasonography, the point at which the needle encounters resistance and may occure before the bone of the sternebra. Nevertheless, bone marrow aspiration from 30 mm insertion depth appeared always as safe and successful in terms of MSC cultivation in both, sternebra 4 and 5.
The analysed μCT data demonstrated that the insertion of the Jamshidi needle (10 or 30 mm) causes no significant destruction of the bony trabecular meshwork. This observation is consistent with results that demonstrated only minimal histological destruction of bony trabeculae after sternal bone marrow aspiration (Kasashima et al. 2011). However, although the bony structures remain largely intact after bone marrow aspiration it is unknown whether the microenvironment, relevant for the re-migration and homing of bone marrow stem cells, regenerates sufficiently.
The identification of the optimal puncture site for bone marrow aspiration differs considerably among various clinical descriptions. Some authors located the insertion site for the Jamshidi needle using particular anatomical landmarks: cartilage of the xyphoid (Richardson et al. 1986; Kisiday et al. 2013) or the olecranon tuber (Sellon 2006; Goodrich et al. 2008; Adams et al. 2012; Delling et al. 2012) in combination with given distances, such as 90–100 mm cranial the cartilago xyphoidea or a hand caudal the olecranon tuber. In contrast, other authors recommend the identification of the sternebrae by ultrasonography as the safest method to determine the insertion site (Smith et al. 2003; Arnhold et al. 2007; Kasashima et al. 2011; Godwin et al. 2012). The latter recommendation has been supported by our results. Due to the spherical shape of sternebra 4 and 5 it is important to determine the exact position of the central aspect of the sternebrae to ensure an optimal insertion of the aspiration needle. Significant deviations of the needle position from the centre of the sternebra increase the risk of fatal thoraco- and pericardiocentesis and hamper a sufficient harvest of bone marrow. In this investigation, ultrasonography was a reliable technique to identify the individual sternebrae and to determine distances between skin and ventral margin of the sternebrae with an acceptable accuracy. Differences between ultrasonographic and cCT measurements of the skin to bone distance may be explained by the fact that ultrasonography was preformed after the sternebrae with overlying tissues had been isolated and freezed. Consequently potential explanations for the increased thickness during cCT may be (1) slight inevitable indention of the body surface by the ultrasound probe, (2) increase in tissue thickness by contraction of muscles and skin after isolation of sterna, (3) distension of soft tissue by the process of freezing.
In terms of efficiency of in vitro cultivation and expansion of bone marrow derived MSCs, no difference was detected for bone marrow aspirates of 5 and 10 mL. This finding is in line with results presented by Kisiday et al. 2013. These authors detected similar numbers of MSCs between the first 5 mL of bone marrow aspirate and the second sequential 5 mL of aspirate, but both were significantly greater than the 50 mL fraction. As expected, bone marrow aspirates from sternebra 4 and sternebra 5 did not show differences concerning the efficiency of MSC cultivation. However, due to morphological characteristics sternebra 5 appears most suitable for bone marrow aspiration (as described above).
The cultivation of MSCs was successful with all used media (Cultivation medium supplemented with either FCS, AS or SHS). However, in two cases, cultivation of MSCs in presence of autologous serum failed. This unwanted outcome indicates the risks and disadvantages that are associated with the use of autologous serum. The quality of autologous serum in terms of the content of beneficial substances, e.g. growth factors, and in terms of the presence of potential pathogens is highly variable amongst individuals (Erickson et al. 1991; Nimura et al. 2008; Tekkatte et al. 2011). These general disadvantageous of autologous serum are reflected by several cell culture experiments that demonstrated superior culture outcomes in the presence of FCS compared to AS (Kuznetsov et al. 2000; Nimura et al. 2008). Those observations are consistent with the results from our study. Although a generalization from our experimental setting is debatable, FCS appeared to be the most suitable serum for the in vitro culture and expansion of equine bone marrow derived MSCs. FCS was even superior compared to SHS, which was suggested to combine the advantageous of a standardized serum and of an allogenic serum (derived from same species). MSCs that were cultured in SHS for the experiments were initially isolated and expanded in the presence of FCS. Thus, the cells were gradually accustomed to SHS, a procedure which might have caused suboptimal culture conditions. Nevertheless, our results are confirmed by investigations on primary equine bronchial fibroblasts (Franke et al. 2014). Equine bronchial fibroblasts cultured in SHS had a longer confluence and doubling time and a limited proliferation rate compared to cells cultured in the presence of FCS .Although the cells investigated by Franke et al. (2014, equine bronchial fibroblasts) and the cells we used (equine bone marrow derived MSCs) are of different origin, both cell types are fibroblastic cells. While it has been shown that FCS promotes in vitro proliferation of equine fibroblastic cells (Franke et al. 2014), other cell types, such as neuronal cells, proliferate preferentially in media containing SHS (Franke et al. 2014). These findings suggest a complex interaction between particular serum components, independent from the donor species, and cell type specific requirements. To establish an optimal serum supplementation the cultured cell type and the intended cellular processes should be considered.
Conclusion
Sternebra 5 appears to be the most suitable site for the harvest of equine bone marrow. Localization of the puncture site by use of ultrasonography is recommended to minimize risks and to optimize bone marrow aspiration. An intraosseous puncture depth of only 10 mm guarantees harvest of bone marrow containing vital MNCs for in vitro cultivation and expansion of equine bone marrow MSCs. Best MSCs culture characteristics were observed in the presence of culture medium supplemented with FCS.
Acknowledgements
The authors thank Dr. Klaus Hopster, Dipl. ECVA, for performing general anaesthesia and Dr. Maren Hellige for her support during cCT imaging, Melanie Kielhorn for her assistance during μCT imaging, Oliver Stünkel for his technical assistance, Dr. Patricia Schrock for her support with AMIRA and Dr. Anja Lang for her support with Photoshop. This work was supported by a grant from the Federal Ministry for Economic Affairs and Energy, AiF Project GmbH. The authors thank Mrs. F. Sherwood-Brock for proofreading the manuscript.
Source of funding
This work was supported by a grant from the Federal Ministry for Economic Affairs and Energy, AiF Project GmbH, KF2994201AJ2.
Conflicts of interest
The authors declare that they have no con?icts of interest.
Contributions
CE designed the study, collected and processed the specimens, assembled and analyzed the data, drafted and wrote the manuscript. FG contributed to the study design, helped to collect the specimens and helped to evaluate the data. CSch contributed to the study design, helped to collect and process the specimens, contributed to data analysis and interpretation, helped with editing and revision of the manuscript. NH helped to process the specimens, contributed to data analysis and interpretation. KR contributed to data analysis. CP contributed to the study design, helped with the assembling and analysis of data, helped with editing and revision of the manuscript and obtained the funding. CSt contributed to the study design, helped with the collection and processing of the specimens, helped with the assembling and analysis of data, helped with editing and revision of the manuscript and obtained the funding. All authors read and approved the final manuscript.
References
- Adams M.K., Goodrich L.R., Rao S., Olea-Popelka F., Phillips N., Kisiday J.D. & McIlwraith C.W. (2012) Equine bone marrow-derived mesenchymal stromal cells (BMDMSCs) from the ilium and sternum: are there differences?. Equine Veterinary Journal45, 372–375.
- Arnhold S.J., Goletz I., Klein H., Stumpf G., Beluche L.A., Rohde C.et al. (2007) Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. American Journal of Veterinary Research68, 1095–1105.
- Bourzac C., Smith L.C., Vincent P., Beauchamp G., Lavoie J.-P. & Laverty S. (2010) Isolation of equine bone marrow-derived mesenchymal stem cells: a comparison between three protocols. Equine Veterinary Journal42, 519–527.
- Brems R., Jebe E.C. (2008) Comparison of treatments with autolog; cultured stem cells from adipose tissue or bone marrow. WEVA Proceedings523.
- Delling U., Lindner K., Ribitsch I., Jülke H. & Brehm W. (2012) Comparison of bone marrow aspiration at the sternum and the tuber coxae in middle-aged horses. Canadian Journal of Veterinary Research= Revue Canadienne de Recherche Veterinaire76, 52–56.
- Désévaux C., Laverty S. & Doizé B. (2000) Sternal bone biopsy in standing horses. Veterinary Surgery29, 303–308.
- Durando M.M., Zarucco L., Schaer T.P., Ross M. & Reef V.B. (2006) Pneumopericardium in a horse secondary to sternal bone marrow aspiration. Equine Veterinary Education18, 75–79.
- Erickson G.A., Bolin S.R. & Landgraf J.G. (1991) Viral contamination of fetal bovine serum used for tissue culture: risks and concerns. Developments in Biological Standardization75, 173–175.
- Eydt C., Schröck C., Geburek F., Rohn K., Staszyk C. & Pfarrer C. (2014) Three-dimensional anatomy of the equine sternum. Anatomia Histologia and Embryologia44, 99–106.
- Fortier L.A. & Smith R.K. (2008) Regenerative medicine for tendinous and ligamentous injuries of sport horses. The Veterinary Clinics of North America Equine Practice24, 191–201.
- Frank C., McDonald D. & Shrive N. (1997) Collagen fibril diameters in the rabbit medial collateral ligament scar: a longer term assessment. Connective Tissue Research36, 261–269.
- Franke J., Abs V., Zizzadoro C. & Abraham G. (2014) Comparative study of the effects of fetal bovine serum versus horse serum on growth and differentiation of primary equine bronchial fibroblasts. BMC Vet Res 10, 119. doi:10.1186/1746-6148-10-119.
- Garvican E.R., Dudhia J., Alves A.-L., Clements L.E., Du Plessis F. & Smith R.K.W. (2014) Mesenchymal stem cells modulate release of matrix proteins from tendon surfaces in vitro: a potential beneficial therapeutic effect. Regenerative Medicine5, 295–308.
- Godwin E.E., Young N.J., Dudhia J., Beamish C. & Smith R.K.W. (2012) Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Veterinary Journal44, 25–32.
- Goodrich L.R., Frisbie D.D. & Kisiday J.D. (2008) How to harvest bone marrow derived mesenchymal stem cells for expansion and injection. AAEP Proceedings54, 252–257.
- Jacobs R.M., Kociba G.J. & Ruoff W.W. (1983) Monoclonal gammopathy in a horse with defective hemostasis. Veterinary Pathology20, 643–647.
- Kajikawa Y., Morihara T., Watanabe N., Sakamoto H., Matsuda K., Konayashi M.et al. (2007) GFP chimeric models exhibited a biphasic pattern of mesenchymal cell invasion in tendon healing. Journal of Cellular Physiology210, 684–691.
- Kasashima Y., Ueno T., Tomita A., Goodship A.E. & Smith R.K.W. (2011) Optimisation of bone marrow aspiration from the equine sternum for the safe recovery of mesenchymal stem cells. Equine Veterinary Journal43, 288–294.
- Kisiday J.D., Goodrich L.R., McIlwraith C.W. & Frisbie D.D. (2013) Effects of equine bone marrow aspirate volume on isolation, proliferation, and differentiation potential of mesenchymal stem cells. American Journal of Veterinary Research74, 801–807.
- Kuznetsov S.A., Mankani M.H. & Robey P.G. (2000) Effect of serum on human bone marrow stromal cells: ex vivo expansion and in vivo bone formation. Transplantation70, 1780–1787.
- Nimura A., Muneta T., Koga H., Mochizuki T., Suzuki K., Makino H.et al. (2008) Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis and Rheumatism58, 501–510.
- Reed S. & Leahy E.R. (2013) Stem cell therapy in equine tendon injury. Journal of Animal Science91, 59–65.
- Richardson G.L., Pool R.R., Pascoeand J.R. & Wheat J.D. (1986) Autogenous cancellous bone grafts from the sternum in horses. Veterinary Surgery15, 9–15.
- Russell K., Sellon D.C. & Grindem C.B. (1994) Bone marrow in horses: indications, sample handling, and complications. The Compendium on Continuing Education for the. Practicing Veterinarian (USA)16, 1359–1365.
- Schnabel L., Fortier L.A., McIlwraith C.W. & Nobert K.M. (2013) Therapeutic use of stem cells in horses: which type, how, and when?Veterinary Journal197, 570–577.
- Sellon D.C. (2006) 2006: how to sample a diagnostic bone marrow sample from the sternum of an adult horse. AAEP Proceedings52, 621–625.
- Smith R.K. (2008) Mesenchymal stem cell therapy for equine tendinopathy. Disability and Rehabilitation30, 1752–1758.
- Smith R.K., Korda M., Blunn G.W. & Goodship A.E. (2003) Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Veterinary Journal35, 99–102.
- Smith R.K., Werling N.J., Dakin G., Alam R., Goodship A.E. & Dudhia J. (2013) Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS ONE8(9), e75697.
- Smith R.K., Garvican E.R. & Fortier L.A. (2014) The current “state of play” of regenerative medicine in horses: what the horse can tell the human. Regenerative Medicine9, 673–685.
- Spaas J.H., De Schauwer C., Cornillie P., Meyer E., Van Soom A., Van de Walle G.R. (2012) Culture and characterization of equine peripheral blood mesenchymal stromal cells. The Veterinary Journal195, 107–113.
- Tekkatte C., Gunasingh G.P., Cherian K.M., Sankaranarayanan K. (2011) Humanized stem cell culture techniques: the animal serum controversy. Stem Cells International2011, 504723.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?