Summary
Introduction
One of the great challenges in pancreas transplantation is the ischemia reperfusion injury. It is mentioned that free oxygen and/or nitrogen radicals play a prominent role in this phase. To minimize this problem, a modified histidine–tryptophan–ketoglutarate (HTK) solution that contains modified antioxidants has been developed. Our aim was to evaluate this solution in improving the viability of the pancreas in comparison with standard HTK and University of Wisconsin (UW) solutions in a porcine model of pancreas transplantation.
Materials and methods
Twenty-three Landrace pigs were divided into three identical groups. After a 10-hour preservation time at 4°C, the pancreas was implanted in the organs of the recipients in a standardized manner. Serum parameters were assessed prior to and after implantation on the 1st postoperative day, 3rd postoperative day, and 7th postoperative day. Furthermore, three biopsies were taken: prior to and after reperfusion, and on Day 7 to assess the grafts.
Results
An analysis of serum glucose among the three groups showed no significant differences. Evaluation of the insulin levels showed no significant difference between the modified and standard HTK groups; however, differences between HTK and UW were significant (p = 0.004 in favor of UW solutions). The histopathological results showed a trend of a higher grade of rejection of pancreas tissue in the UW group compared to both HTK groups.
Conclusion
The modified HTK solution could preserve the pancreas for the preservation of the graft with similar results to those observed for standard solutions without any significant difference. The trend showed that the pathological finding in the UW group was not as good as that in the modified HTK and standard HTK groups.
Keywords
histidine tryptophan ketoglutarate;ischemia reperfusion injury;pancreas transplantation;University of Wisconsin
1. Introduction
In experimental studies, a procured pancreas has been reported to tolerate cold storage for a maximum of 72 hours,1 but clinically, according to the United Network for Organ Sharing and the International Pancreas Transplant Registry reports, 75% of the grafts are preserved for <18 hours to reduce the possibility of ischemia reperfusion injury (IRI).2 As shown in pancreas transplantation (PTx), IRI is correlated with acute rejection rate, primary graft dysfunction, delayed graft function, and late graft failure.3 IRI is also the main cause of graft pancreatitis, known as the most significant complication of PTx and seen in up to 35% of recipients.4; 5 ; 6 Today, the two most commonly used preservation solutions in PTx are University of Wisconsin (UW) and histidine–tryptophan–ketoglutarate (HTK) solutions.7 ; 8 UW solution was the gold standard for perfusion and storage in clinical multiorgan procurement prior to the approval of HTK.9; 10 ; 11 Today, HTK is being used commonly for kidney,12 liver,13 cardiac,14 and PTx.15 The benefits of HTK compared to UW include better tissue perfusion because of its lower viscosity, decreased risk of reperfusion hyperkalemia, favorable cell preservation over a wider range of temperatures,7 easy handling, and lower cost.1 By contrast, some studies have noted that HTK preservation is associated with a 30% increased risk of graft loss when compared with UW,7 and a trend of poorer graft survival was shown with HTK preservation at 30 days.9 In addition, some recent experiments have reported that allografts preserved in HTK may be more edematous than those stored in UW.7; 16 ; 17
It seems that, in HTK solutions, as hypothesized by Rauen et al18 using rats, histidine in combination with hypothermia increases the formation of intracellular iron-dependent reactive oxygen radicals. Therefore, to reduce the oxidative stress, recent efforts have introduced a modified HTK solution19 supplemented by two main modifications: decreasing the production of reactive iron-dependent oxygen radicals by replacing a part of histidine with nontoxic N-acetyl-l-histidine18 and enhancing a favorable capacity of chelating-free iron components by using a combination of different chelators such as deferoxamine and LK-614.20 Deferoxamine is a traditional, well-evaluated iron chelator, which has been experienced previously in other preservation solutions.21 LK-614 as a lipophilic but weaker iron chelator19 has also been incorporated in modified HTK, considering the fact that LK-614 with better membrane permeability protects the organ from iron-dependent cell injuries.20 For evaluating this solution, we assess the modified HTK preservation solution with reduced disadvantages by better radical scavenger capacity and compare it with standard HTK and UW solutions in a porcine model of PTx.
2. Materials and methods
2.1. Experimental design
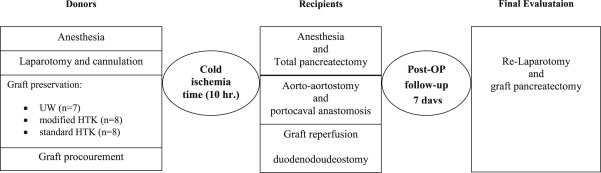
Forty-six Landrace pigs with a body weight of 30–40 kg were randomized into three groups: UW (7 donors, 7 recipients), modified HTK (8 donors, 8 recipients), and standard HTK (8 donors, 8 recipients). The simplified technique of PTx in a porcine model was produced as previously described.22 In each group, after perfusion with the respective solution and a 10-hour preservation at 4°C, the organs were implanted in the recipient pigs through a standardized technique. The recipients were observed for 7 days, and then the final evaluation was performed after relaparotomy. All animals were scarified after completing the experiments (Figure 1).
|
|
|
Figure 1. Study design from the graft procurement to final evaluation of the transplanted pancreas. |
2.2. Anesthesia
Twelve hours before the experiments, the animals food intake had been restricted, but they had free access to water. Ketamine (5 mg/kg; Ketanes; Parke-Davis/Pfizer, Karlsruhe, Germany), azaperone (8 mg/kg; Stresnil; Jansen-Cilag, Neuss, Germany), and midazolam (0.5 mg/kg; Dormicum; Hoffmann-La Roche, Grenzach-Wyhlen, Germany) were administered as premedication 15 minutes prior to anesthesia. Anesthesia was induced with ketamine [5 mg/kg, intravenously (i.v.)], midazolam (0.1 mg/kg, i.v.), and atropine (0.04 mg/kg, i.v.; Atropium sulfuricum; Eifelfango, Bad Neuenahr, Germany). The animals were intubated and ventilated with a mixture of 1.5–2.0 L/min oxygen, 0.5–1.0 L/min air, and 0.75–1.5% isoflurane (Isufloran, Baxter, Unterschleißem, Germany).
2.3. Surgical procedure
All operations were performed by the same surgical team, as in the case of experimental PTx.
2.3.1. Graft procurement
Under general anesthesia and after midline laparotomy, the pancreas was mobilized, and the main supplying vessels (abdominal aorta and portal vein) were dissected gently. The common hepatic and left gastric artery with the common bile duct were isolated. Ten minutes prior to the perfusion, 20,000 IU heparin was intravenously injected. A perfusion catheter was placed proximally into the iliac bifurcation, and the abdominal aorta was blocked proximal to the celiac trunk. Perfusing of the organs was made through the former cannula with one of the following preservation solutions: (1) UW solution (ViaSpan, Bristol Mayers Squibb & Co. KGA, München, Germany; n = 7); (2) modified HTK solution (Custodiol-N; Dr F. Köhler Chemie GmbH, Alsbach-Hahnlein, Germany; n = 8); and (3) standard HTK solution (Custodiol; Dr F. Kohler Chemie GmbH; n = 8).
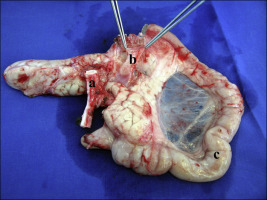
Two liters of HTK solution, either standard or modified, or 750 mL of UW solution was used for flushing out the graft with a controlled pressure of 120 mmHg at 4°C. At the end of the perfusion, the portal vein was divided. An aortic tube graft including the celiac trunk and the superior mesenteric artery was obtained for the arterial anastomosis. The proximal and distal parts of the duodenum were cut with a linear stapler (Multifire GIA 60, Covidien, MN, USA). Finally, the pancreas graft with its duodenum was removed (Figure 2). The organs were placed in a preservation solution at 4°C for 10 hours.
|
|
|
Figure 2. An explanted pancreas with the prepared vessels (a = aortic graft; b = portal vein; c = duodenum) after a 10-hour cold ischemia time. |
2.3.2. Total pancreatectomy and graft implantation
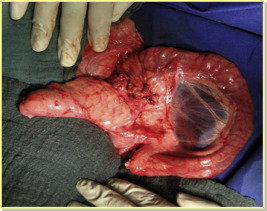
After anesthesia induction, a venous catheter was inserted from the left external jugular vein, and an arterial catheter was placed in the common carotid artery. The mean arterial pressure (MAP), heart rate, electrocardiogram, SaO2, and rectal temperature were monitored during the operation. A median abdominal incision was applied. Next, a duodenum preserving total native pancreatectomy was performed. After a 10-hour cold ischemia time, the procured pancreas graft was placed heterotopically in the right abdominal fossa for easy access to the main abdominal vessels. The aorta graft, which had been prepared previously, was used for an end-to-side anastomosis to the recipient aorta with continuous single-layer sutures (prolene 5/0). A similar end-to-side anastomosis between the graft portal vein and the recipient inferior vena cava was constructed (prolene 6/0). Prior to the pancreas graft reperfusion, heparin (200 IU/kg, i.v.) was administered to avoid coagulation in the organ vessels. The reperfusion started with removing the venous and arterial clamps, respectively. Finally, the donor duodenum was anatomized to the recipients duodenum with 5/0 polydioxanon in an end-to-side manner (Figure 3). During the operations, all recipients received 5 mL/min isotonic solution infusions (NaCl 0.9% or Ringer), 2 mL (i.v.) metamidazole (Novalgin; Sanofi-Aventis, Frankfurt, Germany), 2.5 mL (i.v.) enrofloxacin (Baytril; Bayer, Leverkusen, Germany), and 20 mg (i.v.) pantazol (Takeda, Berlin, Germany).
|
|
|
Figure 3. The implanted pancreas graft 10 minutes after reperfusion.22 |
2.4. Postoperative management and relaparotomy
The recipients were observed for 7 days and visited two times daily. They received 20 mL/kg/d (i.v.) fluid infusion (NaCl 0.9% or Ringer). Also, for immunosuppressive therapy, 250 mg/d (i.v.) methylprednisolone (Urbason; Sanofi-Avenits) and 0.05 mg/kg/d (i.v.) tacrolimus (Prograf; Fujisawa, München, Germany) were administered. In addition, pantazol (20 mg/d, i.v.), enoxaparin (40 mg/d, subcutaneously) (Clexane; Aventis, Frankfurt, Germany), and enrofloxacin (2.5 mg/kg/d, i.v.) were administered for stress ulcers, vascular thrombosis, and infection prophylaxis, respectively. Finally, a relaparotomy was performed under deep anesthesia on Day 7 for transplant pancreatectomy; subsequently, the animals were sacrificed using 2 mmol/kg (i.v.) of potassium chloride.
2.5. Measurement protocol
Five blood samples were obtained at predefined time points (after graft procurement, 10 minutes after reperfusion, and also on the 1st day, 3rd day, and 7th days after implantation) for evaluation of serum insulin (ng/mL), glucose (mg/dL), hemoglobin (g/dL), hematocrit (%), amylase (U/L), lipase (U/L), and C-reactive protein (mg/L). Insulin level was analyzed with a specific insulin kit for pigs (Porcine Insulin Elisa; Mercodia AB, Uppsala, Sweden).
2.6. Histopathological evaluation
Three biopsies were taken at the end of the cold ischemia time: prior to reperfusion, after reperfusion, and 7 days after the operation. All samples were fixed in formaldehyde 5% and evaluated by a pathologist. As reported by Drachenberg et al,23 the histomorphological criteria for rejection were classified in a semiquantitative scoring system regarding the progress of the present inflammatory infiltration, endothelitis, and necrosis, and received a score ranging from 0 to 5 (where 0 = normal; 1 = inflammation of undetermined significance; 2 = minimal rejection; 3 = mild rejection; 4 = moderate rejection; and 5 = severe rejection). According to this scoring system, all pancreas grafts received a score at each biopsy time point.
2.7. Animal rights
The study protocol was approved by the German Committee for Animal Care, Karlsruhe, Germany. During the experiment, all animals received humane care according to institutional guidelines established for the Animal Care Facility at the University of Heidelberg, Heidelberg, Germany.
2.8. Statistical analysis
The analysis was performed using SPSS version 11.5 for Windows (Stata Corp, College Station, TX, USA). After evaluating the distribution of the data with a Kolmogorov–Smirnov test, we used the Mann–Whitney U or the Kruskal–Wallis tests when needed. Repeated measurement analysis was used for comparing the five predefined time points (on the same dependent variable) in the three study groups (UW, modified HTK, and standard HTK). All data were expressed as mean ± standard deviation. A p value <0.05 was considered statistically significant.
3. Results
3.1. General findings
Twenty-three successfully transplanted animals with a cold ischemia time of 10 hours were evaluated in three groups with different preservation solutions: UW (n = 7), modified HTK (n = 8), and standard HTK (n = 8). All pigs were followed-up for 7 days without mortality. The weights of all animals in the mentioned groups on the implantation day were 29 ± 2.6 kg, 29.5 ± 2.7 kg, and 31.3 ± 4 kg in UW, modified HTK, and standard HTK, respectively. At the end of the follow-up period, the mean body weights were 29 ± 2.3 kg in the UW group, 29.2 ± 2.6 kg in the modified HTK group, and 29.2 ± 5 kg in the standard HTK group without any significant differences at two time points or in the studied groups. The anastomosing time (warm ischemia time) was 62 ± 5 minutes in the UW group, 55 ± 8 minutes in the modified HTK group, and 57 ± 7 minutes in the standard HTK group. Cold and warm ischemic times were not considerably different among the experimental groups. The mean values of all perioperative measurements of animals are summarized in Table 1. There were no significant changes in the cardiovascular parameters including MAP and heart rate during graft implantation in the experimental groups. Because of the use of warm blankets during the operation, even the body temperature did not differ significantly among the experimental groups. After removing the venous and arterial clamps, all implanted pancreata showed, macroscopically, a good homogenous reperfusion. On Day 7 during the reoperation, no signs of fluid collection or macroscopic infection were noticed. All grafts showed a homogenous color without any evidence of vascular thrombosis or leakage from the duodenal anastomosis.
| Mean arterial pressure (mmHg) | Heart rate (beat/min) | Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| Before reperfusion | After reperfusion | Before reperfusion | After reperfusion | Before reperfusion | After reperfusion | |
| UW | 99.1 ± 22.7 | 102.0 ± 22.7 | 89.1 ± 8.8 | 86.7 ± 16.8 | 36.0 ± 0.6 | 35.3 ± 0.5 |
| Modified HTK | 104.2 ± 15.6 | 88.8 ± 10.5 | 80.0 ± 11.6 | 84.2 ± 10.0 | 36.6 ± 0.8 | 36.0 ± 1.1 |
| Standard HTK | 90.7 ± 15.2 | 86.2 ± 15.5 | 88.1 ± 25.7 | 87.7 ± 20.8 | 36.8 ± 0.6 | 36.9 ± 0.7 |
HTK = histidine tryptophan ketoglutarate; UW = University of Wisconsin.
3.2. Hematology and biochemistry findings
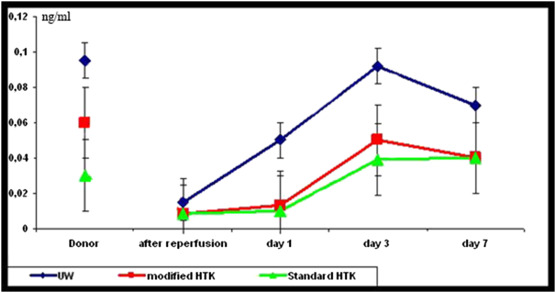
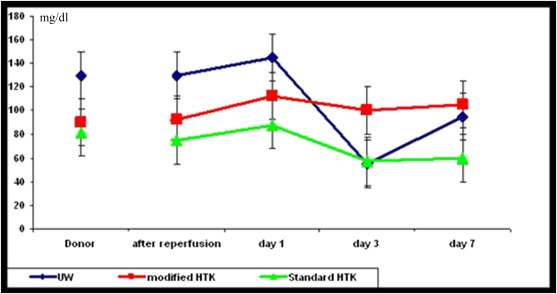
In donors, the blood samples were obtained to assess any probable graft problems. The mean values of laboratory results during organ procurement, perioperation, and postoperation in different groups are reported in Table 2. There were inconsiderable fluctuations in hemoglobin, hematocrit, and C-reactive protein after implantation in all experimental groups. Plasma amylase and lipase levels did not differ significantly among the groups at all the predefined time points. The insulin and glucose levels in different time points are shown in Figure 4 ; Figure 5. Although no significant difference in insulin level was seen between the groups of modified HTK and standard HTK (p = 0.65), the pairwise comparisons showed that the insulin level in the UW group during the five predefined time point follow-ups is significantly higher compared with that of the modified HTK (p = 0.006) and standard HTK groups (p = 0.001). There were no significant differences in the glucose level between the UW and modified HTK groups (p = 0.935), UW and standard HTK groups (p = 0.131), as well as modified HTK and standard HTK groups (p = 0.138).
| Organ procurement | Recipient | |||||
|---|---|---|---|---|---|---|
| After reperfusion | Day 1 | Day 3 | Day 7 | |||
| Hb (g/dL) | UW | 10.0 ± 0.7 | 9.0 ± 0.4 | 9.0 ± 0.8 | 10.0 ± 1.9 | 9.0 ± 0.7 |
| Modified HTK | 10.0 ± 0.8 | 10.0 ± 0.8 | 13.0 ± 2.7 | 12.0 ± 3.2 | 11.0 ± 2.5 | |
| Standard HTK | 11.0 ± 0.8 | 10.0 ± 1.7 | 12.0 ± 1.5 | 11.0 ± 1.4 | 11.0 ± 1.7 | |
| Hct (%) | UW | 32.7 ± 0.1 | 28.0 ± 0.1 | 26.5 ± 3.3 | 28.0 ± 9.0 | 28.5 ± 1.0 |
| Modified HTK | 31.0 ± 2.0 | 32.0 ± 4.0 | 37.0 ± 7.0 | 34.0 ± 8.0 | 33.0 ± 7.0 | |
| Standard HTK | 32.0 ± 2.0 | 31.0 ± 5.0 | 36.0 ± 5.0 | 32.0 ± 5.0 | 32.5 ± 6.0 | |
| CRP (mg/L) | UW | <0.5 | <0.5 | <0.5 | 4.0 ± 0.3 | 4.0 ± 0.6 |
| Modified HTK | <0.5 | <0.5 | <0.5 | 4.0 ± 1.1 | 4.0 ± 0.7 | |
| Standard HTK | <0.5 | <0.5 | <0.5 | 4.0 ± 0.8 | 4.0 ± 0.8 | |
CRP = C-reactive protein; Hb = hemoglobin; Hct = hematocrit; HTK = histidine–tryptophan–ketoglutarate; UW = University of Wisconsin.
|
|
|
Figure 4. Mean values and standard deviations of serum insulin in three groups of UW, modified HTK, and standard HTK during the experimental study (donor, after reperfusion, and 1st day, 3rd day, and 7th day after transplantation). HTK = histidine–tryptophan–ketoglutarate; UW = University of Wisconsin. |
|
|
|
Figure 5. Mean values and standard deviations of serum glucose in three groups (UW, modified HTK, and standard HTK) during the experimental study (donor, after reperfusion, and 1st day, 3rd day, and 7th day after transplantation). |
3.3. Pathological findings
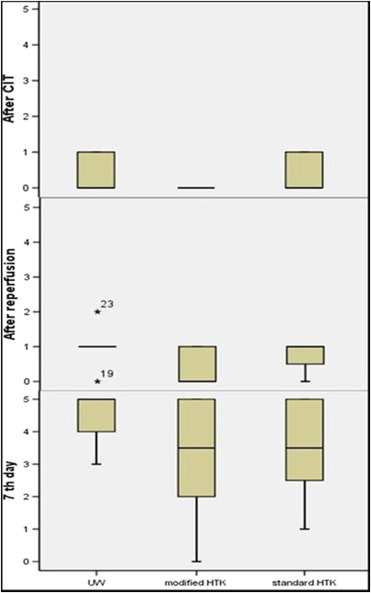
Biopsies taken after the cold ischemia time and directly after reperfusion generally showed normal pancreatic tissue or sticking of neutrophils, irrespective of the various groups. Beyond this, one pig each in the UW and standard HTK groups displayed infiltration of the acinar parenchyma by neutrophils, and one pig from the standard HTK group showed focal fatty necrosis. After 7 days, more severe changes with partially marked inflammatory infiltration and necrosis were observed. Furthermore, varying extent of fatty necrosis with accompanying inflammation was found in the majority of samples. Based on histomorphological findings, seven of eight (87.5%) pigs in the UW group displayed criteria of moderate rejection or more, as did four of eight (50%) pigs in the modified HTK group, and four of eight pigs (50%) in the standard HTK group. Although pathological findings have reported superior results in the HTK groups, these improvements in the histopathological structure showed no significant difference between the solutions in different biopsy phases (after cold ischemia phase, p = 0.12; after reperfusion, p = 0.09; 7th day, p = 0.35; Figure 6).
|
|
|
Figure 6. Comparison of the histopathological results based on the semiquantitative scores through Kruskal–Wallis test. The vertical line is the calculated score regarding the semiquantitative scoring system. The rectangles depict the range between the lower (25%) and upper (75%) quarter of the evaluation. The horizontal line in the middle of the rectangles represents the median (CIT = cold ischemia time). |
4. Discussion
Reducing the damage induced in cold ischemia is an important factor for successful solid organ transplantation. Over the past decade, the transplant centers have increasingly used HTK solutions for abdominal organ procurement as their primary solid organ preservation solution.24; 25 ; 26 This trend is attributable to the lower cost, reduced risk of reperfusion hyperkalemia, and improved microvasculature perfusion because of lower viscosity and better cell preservation over a wider range of temperatures.27 ; 28 In the modified HTK solution, histidine was partially substituted with N-acetyl-histidine, and chloride was replaced with aspartate, glycine, alanine, and arginine; LK 614, which is highly membrane-permeable because it is small and lipophilic, is incorporated. 20; 29 ; 30 To assess the efficacy of the mentioned modifications, this study has been planned to compare the modified HTK solution with the two currently clinically established preservation solutions: UW (Viaspan) and HTK (Custodiol).
Regarding the pig characteristics, owing to similarities in the immune system, physiology, and technical approach from the surgical points of view, the pig model is considered a well-established model for experimental studies of PTx.31 Furthermore, both exocrine and endocrine aspects of the pig pancreas are closely similar to those of the human pancreas.32 Because the operating team had a long-term experience in PTx on pigs (and because the anesthesia and surgical techniques were standardized), there were no technique-related complications. The result of monitoring during operation also showed a stable situation without significant differences among the groups in terms of MAP, heart rate, and temperature. Additionally, the operation duration, laboratory tests (hemoglobin and hematocrit), and cold and warm ischemia time were approximately identical among groups. All transplanted animals survived for 7 days, regardless of the preservation solution used.
In our study, for an adequate monitoring of the transplanted pancreases, the native pancreata were removed prior to the implantation of the graft. The level of serum insulin compared to serum glucose is essentially a sign of pancreas function. The insulin levels markedly decreased, whereas the levels of glucose increased, when the function of the pancreas decreased, such as during the early postoperative days following transplantation. As we have shown, the level of insulin was extremely low in all groups after reperfusion. In the 1st posttransplantation day, there was a rise in insulin levels in all groups; this trend continued until the 3rd postoperative day. The rise of insulin was significantly better in the UW group compared to the modified HTK and standard HTK groups. Subsequently, a slight reduction occurred during the next 4 days. On the 7th day, the level of insulin in the UW group was significantly higher compared to that in both HTK groups. The serum glucose level during procurement was in the normal range. After reperfusion, a slight increase occurred in the glucose level in all groups. On the 3rd posttransplantation day, there was a significant decrease in the glucose level in the UW group, which increased on Day 7 to the normal range. Meanwhile, we did not notice a significant difference in glucose levels between the three studied groups. Therefore, regarding insulin and glucose levels in the studied groups, it seems that pancreas grafts had acceptable endocrine function.
Pancreas graft inflammation might be evaluated by valuable markers such as the general condition of recipients and the levels of C-reactive protein.33 Although all the recipients in these three groups had received intravascular antibiotics, the C-reactive protein increased inconsiderably on Day 3. Regarding the good general condition of recipients and reported insulin and glucose levels during the five predefined time points, the increased level of CRP could be an indication of a mild inflammation in the implanted pancreas.
We have evaluated the histopathological changes in the transplanted organs [based on a scoring system that has been used by Drachenberg et al23 in each group during three time point biopsies—(1) at the end of the cold ischemia time (prior to reperfusion), (2) after reperfusion, and (3) on the 7th day after the operation]. All samples were fixed in formaldehyde 5% and evaluated by a pathologist. The results showed moderate to severe rejection in four out of eight samples of each of the HTK groups and six out of seven samples in the UW group. The same duration of cold ischemia time and inconsiderable differences in all other parameters among the three groups may be attributable to a potent buffer system in HTK groups and LK614 playing its role as a protective factor on the surface of endothelial cells19; 20 ; 30 in modified HTK. The rate of moderate or severe rejection detected in pathological evaluation well reflected the rate of the respective clinically detected organ rejection. In addition to the inflammation and necrosis related to organ rejection, fatty necrosis was observed to a variable degree in the majority of the samples of the analyzed subgroups. Based on our pathological findings, a varying extent of fatty necrosis with accompanying inflammation was found in different grades in histopathological examination. Most likely, this fatty necrosis could occur as a result of the surgical intervention. Therefore, in the context of organ rejection, fatty necrosis, eventually being associated with reactive-inflammatory changes, should be evaluated irrespective of acinar necrosis and inflammation—the latter two represent important criteria for estimating the organ rejection grade.
As we have shown, the level of insulin in the UW group during the five predefined time point follow-ups was significantly higher compared to that in both HTK groups, although there were no significant differences in the glucose level between the groups. By contrast, the histopathological results showed a trend of a higher grade of rejection of pancreas tissue in the UW group compared to both HTK groups. This discrepancy might be explained by a nonlinear relationship between insulin levels and the percentage of β cell, which has been previously described and can be maintained despite a significant loss of β cells.34 Pancreatic β cell mass can reach pathological conditions in which the mass is below a critical threshold. It has been shown that a loss of ∼80–90% of β cell mass prior to the onset of hypoinsulinemia is considerable.35 Theoretically, this could explain why more pathological cell injury in the UW group—which was not significant—does not cause a substantial reduction in insulin values, although the insulin levels were in the normal range among all groups.
In conclusion, the modified HTK solution could preserve the pancreas graft with similar outcomes to those of standard HTK and UW solutions without any significant differences. Although the pathological finding in the UW group was not as good as that in the modified HTK and standard HTK solutions, the insulin levels were significantly better in comparison with that in the HTK groups. However, further prospective experimental and clinical trials will be required with extended criteria (e.g., long cold ischemia time) to compare modified HTK with standard HTK and UW solutions.
References
- 1 A. Agarwal, J.A. Powelson, W.C. Goggins; Organ preservation with histidine–tryptophan ketogluatarate solution in clinical pancreas transplantation: an update of the Indiana university experience; Transplant Proc, 40 (2008), pp. 498–501
- 2 A.C. Gruessner, D.E. Sutherland; Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004; Clin Transplant, 19 (2005), pp. 433–455
- 3 A. Mehrabi, Mood ZhA, M. Sadeghi; Thymoglobulin and ischemia reperfusion injury in kidney and liver transplantation; Nephrol Dial Transplant, 22 (2007), pp. viii54–viii60
- 4 M. Maglione, R.J. Ploeg, P.J. Friend; Donor risk factors, retrieval technique, preservation and ischemia/reperfusion injury in pancreas transplantation; Curr Opin Organ Transplant, 18 (2013), pp. 83–88
- 5 M. Maglione, R. Oberhuber, B. Cardini, et al.; Donor pretreatment with tetrahydrobiopterin saves pancreatic isografts from ischemia reperfusion injury in a mouse model; Am J Transplant, 10 (2010), pp. 2231–2240
- 6 H. Mayer, J. Schmidt, J. Thies; Characterization and reduction of ischemia/reperfusion injury after experimental pancreas transplantation; J Gastrointest Surg, 3 (1999), pp. 162–166
- 7 Z.A. Stewart, A.M. Cameron, A.L. Singer; Histidine–tryptophan–ketoglutarate (HTK) is associated with reduced graft survival in pancreas transplantation; Am J Transplant, 9 (2009), pp. 217–221
- 8 U.J. Hesse, R. Troisi, B. Jacobs; Cold preservation of the porcine pancreas with histidine–tryptophan–ketoglutarate solution; Transplantation, 66 (1998), pp. 1137–1141
- 9 M.J. Englesbe, A. Moyer, D.Y. Kim; Early pancreas transplant outcomes with histidine–tryptophan–ketoglutarate preservation: a multicenter study; Transplantation, 82 (2006), pp. 136–139
- 10 F.A. García-Gil, C.D. Albendea, J. Escartín, et al.; Melatonin prolongs graft survival of pancreas allotransplants in pigs; J Pineal Res, 51 (2011), pp. 445–453
- 11 H.J. Bretschneider; Myocardial protection; Thorac Cardiovasc Surg, 28 (1980), pp. 295–302
- 12 J.A. Fridell, R.S. Mangus, J.A. Powelson; Organ preservation solutions for whole organ pancreas transplantation; Curr Opin Organ Transplant, 16 (2011), pp. 116–122
- 13 R.S. Mangus, A.J. Tector, A. Agarwal; Comparison of histidine–tryptophan ketoglutarate solution (HTK) and University of Wisconsin solution (UW) in adult liver transplantation; Liver Transpl, 12 (2006), pp. 226–230
- 14 C.H. Wilson, J.F. Asher, A. Gupta; Comparison of HTK and hypertonic citrate to intraarterial cooling in human non-heartbeating kidney donors; Transplant Proc, 39 (2007), pp. 351–352
- 15 J.A. Fridell, A. Agarwal, M.L. Milgrom; Comparison of histidine–tryptophan–ketoglutarate solution and University of Wisconsin solution for organ preservation in clinical pancreas transplantation; Transplantation, 77 (2004), pp. 1304–1306
- 16 R. Troisi, D. Meester, C. Van Den Broecke; Functional and structural integrity of porcine pancreatic grafts subjected to a period of warm ischemia and cold preservation with histidine–tryptophan ketoglutarate (Custodiol) or University of Wisconsin solution; Transplantation, 75 (2003), pp. 1793–1799
- 17 R. Troisi, D. Meester, B. Regaert; Tolerance of the porcine pancreas to warm and cold ischemia: comparison between University of Wisconsin and histidine–tryptophan–ketoglutarate solution; Transplant Proc, 34 (2002), pp. 820–822
- 18 U. Rauen, S. Klempt, H. de Groot; Histidine-induced injury to cultured liver cells, effects of histidine derivatives and of iron chelators; Cell Mol Life Sci, 64 (2007), pp. 192–205
- 19 T. Radovits, L.N. Lin, J. Zotkina; Endothelial dysfunction after long-term cold storage in HTK organ preservation solutions: effects of iron chelators and N-alpha-acetyl-l-histidine; J Heart Lung Transplant, 27 (2008), pp. 208–216
- 20 J. Stegemann, A. Hirner, U. Rauen, T. Minor; Use of a new modified HTK solution for machine preservation of marginal liver grafts; J Surg Res, 160 (2010), pp. 155–162
- 21 K. Park, K.Y. Chung, S.H. Sung; Protective effect of desferrioxamine during canine liver transplantation: significance of peritransplant liver biopsy; Transplant Proc, 35 (2003), pp. 117–119
- 22 H. Fonouni, M. Tahmasbi Rad, M. Esmaeilzadeh, M. Golriz, A. Majlesara, A. Mehrabi; A simplified technique of pancreas transplantation in a porcine model; Eur Surg Res, 54 (2015), pp. 24–33
- 23 C.B. Drachenberg, J.C. Papadimitriou, D.K. Klassen, et al.; Evaluation of pancreas transplant needle biopsy: reproducibility and revision of histologic grading system; Transplantation, 63 (1997), pp. 1579–1586
- 24 R.S. Mangus, A.J. Tector, J.A. Fridell, M. Kazimi, E. Hollinger, R.M. Vianna; Comparison of histidine–tryptophan–ketoglutarate solution and University of Wisconsin solution in intestinal and multivisceral transplantation; Transplantation, 86 (2008), pp. 298–302
- 25 S. Schneeberger, M. Biebl, W. Steurer, et al.; A prospective randomized multicenter trial comparing histidine–tryptophane–ketoglutarate versus University of Wisconsin perfusion solution in clinical pancreas transplantation; Transpl Int, 22 (2009), pp. 217–224
- 26 Z.A. Stewart, A.M. Cameron, A.L. Singer, N.N. Dagher, R.A. Montgomery, D.L. Segev; Histidine–tryptophan–ketoglutarate (HTK) associated with reduced graft survival in pancreas transplantation; Am J Transplant, 9 (2009), pp. 217–221
- 27 Z.A. Stewart, A.M. Cameron, A.L. Singer, R.A. Montgomery, D.L. Segev; Histidine–tryptophan–ketoglutarate (HTK) associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death; Am J Transplant, 9 (2009), pp. 286–293
- 28 Z.A. Stewart, B.E. Lonze, D.S. Warren, et al.; Histidine–tryptophan–ketoglutarate (HTK) is associated with reduced graft survival of deceased donor kidney transplants; Am J Transplant, 9 (2009), pp. 1048–1054
- 29 T. Radovits, L.N. Lin, J. Zotkina, A. Koch, U. Rauen, G. Köhler, et al.; Endothelial dysfunction after long-term cold storage in HTK organ preservation solutions: effects of iron chelators and N-alpha-acetyl-l-histidine; J Heart Lung Transplant, 27 (2008), p. 208216
- 30 S. Wu, J. Wohlschlaeger, H. de Groot, U. Rauen; Evaluation of a modified HTK solution containing the new iron chelator LK 614 in an isolated rat liver perfusion model; J Invest Surg, 22 (2009), pp. 340–347
- 31 J.T. Yen; Digestive system; W.G. Pond, H.J. Mersmann (Eds.), Biology of the Domestic Pig, Cornell University Press, Ithaca, NY (2001) 399–345
- 32 E. Niebergall-Roth, S. Teyssen, M.V. Singer; Pancreatic exocrine studies in intact animals: historic and current methods; Lab Anim Sci, 47 (1997), pp. 606–616
- 33 C. Wullstein, O. Drognitz, G. Woeste, et al.; High levels of C-reactive protein after simultaneous pancreas–kidney transplantation predict pancreas graft-related complications and graft survival; Transplantation, 77 (2004), pp. 60–64
- 34 B. Topp, K. Promislow, G. deVries, R.M. Miura, D.T. Finegood; A model of beta-cell mass, insulin, and glucose kinetics: pathways to diabetes; J Theor Biol, 206 (2000), pp. 605–619
- 35 M. Cnop, N. Welsh, J.C. Jonas, A. Jörns, S. Lenzen, D.L. Eizirik; Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities; Diabetes, 54 (2005), pp. S97–S107
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?