Abstract
Background
Obstructive Sleep Apnea (OSA) results in intermittent hypoxia leading to atrial remodeling, which, among other things, facilitates development of atrial fibrillation. While much data exists on the macrostructural changes in cardiac physiology induced by OSA, there is a lack of studies looking for histologic changes in human atrial tissue induced by OSA which might lead to the observed macrostructural changes.
Methods
A case control study was performed. Patients undergoing coronary artery bypass grafting (CABG) were evaluated for OSA and categorized as high-risk or low-risk. The right atrial tissue samples were obtained during CABG and both microscopic histological analysis and Sirius Red staining were performed.
Results
18 patients undergoing CABG were included; 10 high-risk OSA and 8 low-risk OSA in evenly matched populations. No statistically significant difference between the two groups was observed in amount of myocytolysis (p = 0.181), nuclear hypertrophy (p = 0.671), myocardial inflammation (p = n/a), amyloid deposition (p = n/a), or presence of thrombi (p = n/a), as measured through routine H&E staining. As well, no statistically significant difference in interstitial and epicardial collagen was observed, as measured by Sirius Red staining (for total tissue: p = 0.619: for myocardium: p = 0.776).
Conclusions
In this pilot study there were no observable histological differences in human right atrial tissue from individuals at high- and low-risk for OSA. Further investigation would be required for more definitive results.
Keywords
Obstructive Sleep Apnea;Human atrial tissue;Histological analysis;Myocardial fibrosis
1. Introduction
Obstructive Sleep Apnea (OSA) is a largely underdiagnosed but common breathing disorder that affects approximately 5–15% of North Americans [1] ; [2]. The prevalence of atrial fibrillation (AF) among patients with OSA has been reported at anywhere between 32 and 49% [3]; [4]; [5] ; [6]. OSA is known to be associated with significant atrial structural remodeling, including atrial enlargement and significant conduction abnormalities [7]; [8] ; [9]. It is thought that the electrical substrate for AF resulting from OSA is due to these structural changes, which result in increased sympathetic tone, systemic and pulmonary hypertension, intermittent hypoxia, and inflammation [10]; [11]; [12]; [13]; [14]; [15]; [16] ; [17].
No previous work has microscopically compared human atrial tissue between patients with and without OSA in order to characterize histological or pathological changes that might further explain the predisposition for AF. Given previously described histological findings known to be associated with AF [18]; [19]; [20]; [21]; [22]; [23]; [24]; [25]; [26]; [27] ; [28], we postulate that similar findings will be present in the atrial tissue of patients with OSA, which might offer further insight as to why these patients are predisposed to developing this arrhythmia. Here we present a pilot study to investigate microscopic differences in human atrial tissue induced by the presence of OSA.
2. Methods
2.1. Design
Patients presenting for elective Coronary Artery Bypass Grafting (CABG) were prospectively screened for OSA using a modified Berlin questionnaire (BQ), after obtaining appropriate consent. Patients were followed until their discharge following CABG. The primary endpoint was to perform histological analysis of atrial tissue in patients with high- and low-risk OSA profiles. In particular, hematoxylin and eosin (H&E) staining and Sirius Red staining were used to look at myocytolysis, nuclear hypertrophy, myocardial inflammation and collagen deposition/fibrosis. Pre-, peri-, and post-operative data were also collected. To maintain blinding, results of the questionnaire were not shared with the patient or study personnel, nor correlated with surgical outcomes until study completion. Internal Review Board approval from our institution was obtained.
2.2. Population
We included a total of 18 patients presenting for CABG surgery at a tertiary care hospital. Patients were grouped into two categories: 1. High risk for OSA; 2. Low risk for OSA. The study population included only stable patients undergoing elective CABG. Patients were excluded if they had a history of atrial fibrillation. Tissue samples were subsequently obtained from the right atrial appendage at the site of cannulation during standard CABG procedure.
2.3. Assessment of OSA
Patients were screened for OSA and classified as high-risk or low-risk using the modified BQ, as originally described by Netzer et al. [29], and modified by our group to account for individuals already diagnosed with OSA via polysomnography, as previously described [30], (addendum 1 in supplemental information). The BQ has been previously validated as a suitable correlate for polysomnography [29] ; [31].
2.4. Atrial tissue collection
The right atrial tissue that is normally discarded during a standard CABG was obtained by the operating cardiothoracic surgeon and the sample was prepared as indicated below.
2.5. Histological analysis
After a 24 hour fixation in 10% neutral buffered formalin the atrial tissue was embedded in paraffin, sectioned at 4 μm thickness and stained with H&E and Sirius red stains [32]. Histologic features were assessed using variations of previously reported methods [18]; [33]; [34] ; [35]. By consensus review of H&E sections, three pathologists graded cases for myocytolysis (defined as clearing of at least 10% of cell cytoplasm) and nuclear hypertrophy (defined as nuclear diameter twice that of background myocytes) as absent/mild (10% or fewer cells), moderate (11–20% of cells) and marked (> 20% of cells). Using a variation of the World Heart Federation system [36], interstitial lymphocytic inflammation was graded in the 5 most involved high power fields as absent/mild (less than moderate), moderate (> 14 cells/mm2 without myocyte necrosis), or marked (> 14 cells/mm2 with myocyte necrosis). Amyloid and endocardial thrombus were recorded as present or absent.
To assess interstitial fibrosis Sirius Red stained sections were scanned using an Aperio CS slide scanner (Leica Biosystems, Buffalo Grove, IL, USA) at 20 × magnification. The images were viewed in Aperio ImageScope software and annotated to include only myocardium for analysis and exclude epicardium (which is normally Sirius red positive). Analysis was performed using the Aperio Positive Pixel Count Algorithm with default settings to determine the proportion of positive pixels in the selected areas.
2.6. Statistical Analysis
2.6.1. For demographic information
Data were collected in Excel and imported into IBM SPSS (version 21.0 for Windows) for statistical analysis. Data were initially described using means and standard deviations for continuous data, and frequencies and percentages for categorical data. This was followed by a univariate analysis to assess the association of the collected data with the outcomes, using one-way ANOVA and independent samples t-tests for the continuous data and chi-square tests (Pearson or Fishers Exact as appropriate) for the categorical data. Length of stay was analyzed using Spearman correlation (continuous data) and t-tests or one-way ANOVA (categorical data). The Kruskal–Wallis Test and Mann–Whitney U were used in the event of non-normal distributions. Variables were considered statistically significant if they attained a p-value of < 0.05, although those close to significance were retained if they were deemed clinically relevant.
2.6.2. For histological data
All results are expressed as the mean +/− the standard deviation. Independent samples t-tests were used to determine statistical significance between the low-risk and the high-risk OSA groups. A p-value of 0.05 or less was considered significant.
3. Results
Demographic and clinical characteristics are shown in Table 1. We included right atrial tissue samples from 18 patients, 10 with a high-risk profile for OSA (two of which were previously confirmed cases via formal polysomnography) and 8 with a low-risk profile for OSA. Patients in each group were matched with respect to age and sex. Patients were 89% male, with a mean age of 67.8 ± 8.2 years. While not statistically significant, there were more smokers in the high-risk group (40% vs 12.5%). No difference between BMI in the two study groups was detected, and the groups were otherwise well matched. Furthermore, there was no difference in echocardiographic data (right atrial volume, right atrial volume index, left atrial diameter, and left ventricular ejection fraction; p = 0.591, 0.180, 0.610, and 0.493 respectively) noted between the two study groups.
| Total | OSA low risk (N = 8) | OSA high risk (N = 10) | p | |
|---|---|---|---|---|
| Age (years) | 67.8 | 70.1 ± 6.9 | 66.0 ± 9.2 | 0.316 |
| Gender (% male) | 88.9 | 75 | 100 | 0.183 |
| BMI (kg/m2) | 27.6 | 26.5 ± 2.1 | 28.5 ± 5.4 | 0.337 |
| Smoker (%) | 27.8 | 12.5 | 40 | 0.314 |
| Hypertension (%) | 72.2 | 62.5 | 80 | 0.608 |
| Diabetes (%) | 16.7 | 12.5 | 20 | 1.000 |
| COPD (%) | 5.6 | 0 | 10 | 1.000 |
| Asthma (%) | 11.1 | 25 | 0 | 0.183 |
| History AF (%) | 0 | 0 | 0 | n/a |
| LVEF (%) | 55.4 | 56.8 ± 6.1 | 54.3 ± 8.2 | 0.493 |
| LAD (mm) | 38.8 | 38.1 ± 2.5 | 39.4 ± 4.8 | 0.610 |
| RAV (mL) | 50.4 | 52.3 ± 10.2 | 48.8 ± 15.3 | 0.591 |
| RAVI (mL/m2) | 27.4 | 29.0 ± 4.8 | 26.2 ± 4.0 | 0.180 |
| Beta-blocker use (%) | 61.1 | 75 | 50 | 0.367 |
| Presence of PCAF (%) | 50 | 50 | 50 | 1.000 |
| Length of stay (days) | 5.4 | 6.0 ± 2.1 | 4.9 ± 1.0 | 0.156 |
| CPAP use (%) | 0 | 0 | 0 | n/a |
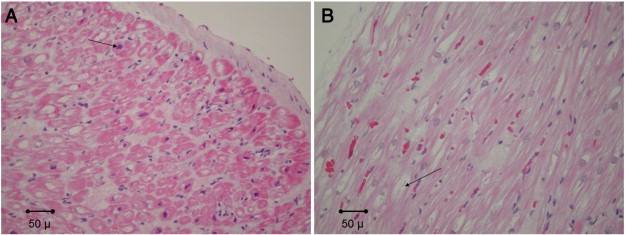
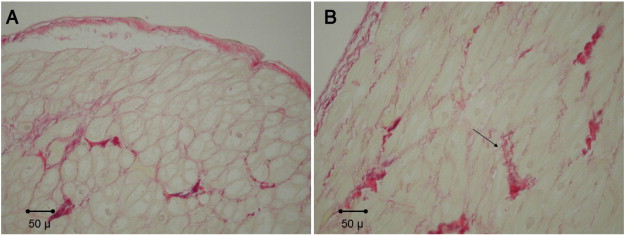
Table 2 demonstrates the results of our histological analysis. There was no statistical difference observed in atrial tissue between high- and low-risk OSA patients with respect to myocytolysis (p = 0.181), nuclear hypertrophy (p = 0.671), myocardial inflammation (p = n/a), amyloid deposition (p = n/a), or presence of thrombi (p = n/a), as quantified using routine H&E staining. Furthermore, statistical significance was not observed when using Sirius Red staining to assess collagen deposition. Both total tissue (p = 0.619) and myocardium (p = 0.776) were compared ( Fig. 1 ; Fig. 2). H&E stained sections showed variable degrees of myocytolysis and myocyte nuclear hypertrophy, which ranged from absent/mild to marked. No case showed more than mild inflammation, amyloid was not identified in a single case and no endocardial thrombus was present.
| Total | OSA low risk (N = 8) | OSA high risk (N = 10) | p | |
|---|---|---|---|---|
| Histological analysis | ||||
| Myocytolysis | ||||
| Absent/mild (%) | 5.6 | 0 | 10 | 0.181 |
| Moderate (%) | 83.3 | 75 | 90 | |
| Marked (%) | 11.1 | 25 | 0 | |
| Nuclear hypertrophy | ||||
| Absent/mild (%) | 44.4 | 50 | 40 | 0.671 |
| Moderate (%) | 55.6 | 50 | 60 | |
| Marked (%) | 0 | 0 | 0 | |
| Myocardial inflammation | ||||
| Absent/mild (%) | 100 | 100 | 100 | n/a |
| Moderate (%) | 0 | 0 | 0 | |
| Marked (%) | 0 | 0 | 0 | |
| Presence of amyloid (%) | 0 | 0 | 0 | n/a |
| Presence of thrombus (%) | 0 | 0 | 0 | n/a |
| Sirius Red staining (collagen) | ||||
| Total tissue (% pixels) | 29.8 ± 6.9 | 30.7 ± 5.6 | 29.0 ± 8.0 | 0.619 |
| Myocardium (% pixels) | 21.7 ± 6.6 | 21.2 ± 4.7 | 22.1 ± 8.1 | 0.776 |
|
|
|
Fig. 1. Representative routinely stained atrial tissue sections from patients with A. low-risk OSA, with focal myocyte nuclear hypertrophy (arrow), and B. high-risk OSA with focal myocytolysis (arrow). No significant differences were observed. No cases showed more than mild inflammation (hematoxylin and eosin stain, 200 ×). |
|
|
|
Fig. 2. Example of Sirius Red staining from atrial tissue from patients with A. low-risk OSA and B. high-risk OSA (positive staining at arrow). No significant differences were observed (Sirius red stain, 200 ×). |
4. Discussion
This study demonstrated no observable microscopic changes in the right atrial tissue in the high-risk OSA population as compared to the low-risk OSA population, as measured by H&E staining and Sirius Red staining. This is not in keeping with known structural findings as OSA is known to be associated with significant atrial remodeling. This has been characterized to include atrial enlargement, reduction in voltage, widespread conduction abnormalities, and longer sinus node recovery [9]. We postulated that these macrostructural changes would have associated changes on the microstructural level, which was not borne out in this study.
Much research has looked to investigate the relationship between OSA and cardiac arrhythmias. Among several pathogenic mechanisms arising from OSA that can lead to cellular remodeling in the atrium, intermittent hypoxia has been reported to be linked to an increased vulnerability to AF [37] ; [38]. Studies have shown that intermittent hypoxia can alter the expression and function of various cardiac ion channels thus promoting the generation of arrhythmias [39]. Given that microstructural changes associated with AF are well known [22] and that OSA predisposes to AF [3], we further postulated that similar changes would be seen in atrial tissue of patients with OSA as are seen in atrial tissue of patients with AF. We chose to assess primarily for myocytolysis, hypertrophy, inflammation, and fibrosis/collagen deposition, as changes in all of these microscopic findings have been linked to AF [22]. Unfortunately, our pilot study did not find any statistically significant changes.
It is worth noting that, while not reaching statistical significance in this population, smoking showed a trend towards the high-risk OSA group. Although there were no associated histological changes, because smoking is associated with ischemic heart disease this trend could, in larger studies, become significant enough to affect the histological analysis. Another factor worth mentioning in discussion is that all of the patients recruited in this study were inherently presenting with significant cardiovascular disease, requiring CABG. Cardiovascular disease is also known to promote cardiac structural changes [40]. The average BMI of our population was 27.6 kg/m2, and obesity, not only associated with heart disease, is in and of itself associated with cardiac structural changes and remodeling [41] ; [42]. These confounders likely make any microscopic changes caused by OSA difficult to tease out in such a small sample size; we would expect that in patients without ischemic disease, variation in histological changes caused by OSA would be more pronounced.
A subgroup analysis of our population looking at microscopic changes in the right atrial tissue in patients with known AF may have been helpful to clarify our above results. However this was not undertaken given the small sample size. Furthermore, AF was not highly prevalent in our study population (two confirmed cases in total), and none of the high-risk OSA population was known to have AF.
While we report no positive findings in this study, this marks, to our knowledge, the first effort to microscopically compare human atrial tissue in patients with and without OSA. Despite the lack of statistical significance, likely in part due to the small sample size, we feel that further, higher powered studies microscopically comparing atrial tissue between patients with and without OSA could possibly reveal changes that help explain the predisposition to AF. Expanding further analysis to the left atrium may also help in characterizing microscopic tissue changes associated with AF, as AF is predominantly a left atrial problem in a majority of patients.
5. Limitations
The lack of statistical significance in our findings is likely to have arisen from a small sample size (n = 18). Further limitation arises given the intrinsic differences that lie within the acquired tissue samples, and as a result of the premorbid medical conditions of these patients (as all were presenting for CABG). Furthermore, we were limited in that surgical constraints allowed us a collection of only the right atrial tissue and changes affecting the left atrium may be more pronounced than the right given that AF is largely associated with left atrial pathology. This study is also importantly limited by the use of the BQ as a surrogate for polysomnography; particularly limiting in a small study, using formal polysomnography to categorize patients definitively as having or not having sleep apnea would greatly strengthen any conclusions made.
6. Conclusions
In this pilot study there were no observable histological differences in human right atrial tissue from individuals at high- and low-risk for OSA. While these results suggest that OSA does not have an effect on the right atrial tissue at a microscopic level, further investigation with higher-powered studies would be required to more definitively come to a conclusion in this regard.
Disclosures
The authors have no disclosures or conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary material.
References
- [1] A. Baranchuk, C.S. Simpson, D.P. Redfearn, M. Fitzpatrick; Its time to wake up!: sleep apnea and cardiac arrhythmias; Europace, 10 (2008), pp. 666–667
- [2] J.M. Parish, V.K. Somers; Obstructive sleep apnea and cardiovascular disease; Mayo Clin Proc, 79 (2004), pp. 1036–1046
- [3] A. Baranchuk; Sleep apnea, cardiac arrhythmias, and conduction disorders; J Electrocardiol, 45 (2012), pp. 508–512
- [4] A.S. Gami, G. Pressman, S.M. Caples, R. Kanagala, J.J. Gard, D.E. Davison, et al.; Association of atrial fibrillation and obstructive sleep apnea; Circulation, 110 (2004), pp. 364–367
- [5] R. Kanagala, N.S. Murali, P.A. Friedman, N.M. Ammash, B.J. Gersh, K.V. Ballman, et al.; Obstructive sleep apnea and the recurrence of atrial fibrillation; Circulation, 107 (2003), pp. 2589–2594
- [6] C.Y. Ng, T. Liu, M. Shehata, S. Stevens, S.S. Chugh, X. Wang; Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation; Am J Cardiol, 108 (2011), pp. 47–51
- [7] A. Baranchuk, H. Pang, G.E.J. Seaborn, P. Yazdan-Ashoori, D.P. Redfearn, C.S. Simpson, et al.; Reverse atrial electrical remodelling induced by continuous positive airway pressure in patients with severe obstructive sleep apnoea; J Interv Card Electrophysiol, 36 (2013), pp. 247–253
- [8] A. Baranchuk, B. Parfrey, L. Lim, F. Morriello, C.S. Simpson, W.M. Hopman, et al.; Interatrial block in patients with obstructive sleep apnea; Cardiology, 18 (2011), pp. 171–175
- [9] H. Dimitri, M. Ng, A.G. Brooks, P. Kuklik, M.K. Stiles, D.H. Lau, et al.; Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation; Heart Rhythm, 9 (2012), pp. 321–327
- [10] A. Baranchuk, C.S. Simpson, D.P. Redfearn, K.A. Michael, M. Fitzpatrick; Understanding the association between sleep apnea & cardiac arrhythmias; Electrofisiología Arritmias, 1 (2008), pp. 5–6
- [11] T.D. Bradley, J.S. Floras; Obstructive sleep apnoea and its cardiovascular consequences; Lancet, 373 (2009), pp. 82–93
- [12] G.C. Digby, A. Baranchuk; Sleep apnea and atrial fibrillation; 2012 update; Curr Cardiol Rev, 8 (2012), pp. 265–272
- [13] A.S. Gami, D.O. Hodge, R.M. Herges, E.J. Olson, J. Nykodym, T. Kara, et al.; Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation; J Am Coll Cardiol, 49 (2007), pp. 565–571
- [14] A.S. Hersi; Obstructive sleep apnea and cardiac arrhythmias; Ann Thorac Med, 6 (2010), pp. 10–17
- [15] W.T. McNicholas, M.R. Bonsignore; Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms, and research priorities; Eur Respir J, 29 (2007), pp. 156–178
- [16] K. Todd, W.F. McIntyre, A. Baranchuk; Obstructive sleep apnea and atrial fibrillation; J Nat Sci Sleep, 2 (2010), pp. 39–45
- [17] S. Nattel; Atrial electrophysiology and mechanisms of atrial fibrillation; J Cardiovasc Pharmacol Ther, 8 (2003), pp. S5–S11
- [18] J. Ausma, M. Wijffels, F. Thoné, L. Wouters, M. Allessie, M. Borgers; Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat; Circulation, 96 (1997), pp. 3157–3163
- [19] A. Boldt, U. Wetzel, J. Weigl, J. Garbade, J. Lauschke, G. Hindricks, et al.; Expression of angiotensin II receptors in human left and right atrial tissue in atrial fibrillation with and without underlying mitral valve disease; J Am Coll Cardiol, 42 (2003), pp. 1785–1792
- [20] A. Frustaci, C. Chimenti, F. Bellocci, E. Morgante, M.A. Russo, A. Maseri; Histological substrate of atrial biopsies in patients with lone atrial fibrillation; Circulation, 96 (1997), pp. 1180–1184
- [21] S. Kostin, G. Klein, Z. Szalay, S. Hein, E.P. PBauer, J. Schaper; Structural correlate of atrial fibrillation in human patients; Cardiovasc Res, 54 (2002), pp. 361–379
- [22] A. Kourliouros, I. Savelieva, A. Kiotsekoglou, M. Jahangiri, J. Camm; Current concepts in the pathogenesis of atrial fibrillation; Am Heart J, 157 (2009), pp. 243–252
- [23] D. Li, S. Fareh, T.K. Leung, S. Nattel; Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort; Circulation, 100 (1999), pp. 87–95
- [24] G. Mariscalco, K.G. Engström, S. Ferrarese, G. Cozzi, V.D. Bruno, F. Sessa, et al.; Relationship between atrial histopathology and atrial fibrillation after coronary bypass surgery; J Thorac Cardiovasc Surg, 131 (2006), pp. 1364–1372
- [25] P. Ramos, I. Almendros, C. Rubies, M. Torres, M. Batlle, R. Farre, et al.; Obstructive sleep apnea induces atrial fibrosis; A Chronic Murine Model. Heart Rhythm 2012 — 33rd Annual Scientific Sessions (2012), pp. PO1–PO113
- [26] C. Rücker-Martin, F. Pecker, D. Godreau, S.N. Hatem; Dedifferentiation of atrial myocytes during atrial fibrillation: role of fibroblast proliferation in vitro; Cardiovasc Res, 55 (2002), pp. 38–52
- [27] K.-U. Thiedemann, V.J. Ferrans; Left atrial ultrastructure in mitral valvular disease; Am J Pathol, 89 (1977), pp. 575–604
- [28] J. Xu, G. Cui, F. Esmailian, M. Plunkett, D. Marelli, A. Ardehali, et al.; Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation; Circulation, 109 (2004), pp. 363–368
- [29] N.C. Netzer, R.A. Stoohs; Using the Berlin questionnaire to identify patients at risk for sleep apnea syndrome; Ann Intern Med, 131 (1999), pp. 485–491
- [30] E.M. van Oosten, A. Hamilton, D. Petsikas, D. Payne, D.P. Redfearn, S. Zhang, et al.; Effect of pre-operative obstructive sleep apnea on the frequency of atrial fibrillation after coronary artery bypass grafting; Am J Cardiol, 113 (2013), pp. 919–923
- [31] D. Martinez, R. Silva, C. Klein, C. Fiori, D. Massierer, C. Cassol, et al.; High risk for sleep apnea in the Berlin questionnaire and coronary artery disease; Sleep Breath, 16 (2012), pp. 89–94
- [32] Histology special stain methods, techniques, protocols. IHC WORLD, LLC; Available from: http://www.ihcworld.com/special_stains.htm (2003–2011)
- [33] M.C. Castonguay, Y. Wang, J.L. Gerhart, D.V. Miller, J.M. Stulak, W.D. Edwards, et al.; Surgical pathology of atrial appendages removed during the Cox-maze procedure; Am J Surg Pathol, 37 (2013), pp. 890–897
- [34] J.B. Grammer, J. Bohm, A. Dufour, M. Benz, R. Lange, R. Bauernschmitt; Atrial fibrosis in heart surgery patients; Basic Res Cardiol, 100 (2005), pp. 288–294
- [35] M. Borgers, F. Thone, L. Wouters, J. Ausma, B. Shivalkar, W. Flameng; Structural correlates of regional myocardial dysfunction in patients with critical coronary artery stenosis: chronic hibernation?; Cardiovasc Pathol, 2 (1993), pp. 237–245
- [36] B. Maisch, B. Bultman, S. Factor, H.J. Grone, K.K. Hufnagel, U. Kuhl, et al.; World Heart Federation consensus conferences definition of inflammatory cardiomyopathy (myocarditis): report from two expert committees on histology and viral cardiomyopathy; Heartbeat, 4 (1999), pp. 3–4
- [37] F. Gramley, J. Lorenzen, B. Jedamzik, K. Gatter, E. Koellensperger, T. Munzel, et al.; Atrial fibrillation is associated with cardiac hypoxia; Cardiovasc Pathol, 19 (2010), pp. 102–111
- [38] Z. Lu, L. Nie, B. He, L. Yu, M. Salim, B. Hunag, et al.; Increase in vulnerability of atrial fibrillation in an acute intermittent hypoxia model: importance of autonomic imbalance; Auton Neurosci, 177 (2013), pp. 148–153
- [39] L.A. Shimoda, J. Polak; Hypoxia and ion channel function; Am J Physiol Cell Physiol, 300 (2011), pp. C951–C967
- [40] J.N. Cohn; Structural changes in cardiovascular disease; Am J Cardiol, 76 (1995), pp. 34E–37E
- [41] K.C. Zalesin, B.A. Franklin, W.M. Miller, E.D. Peterson, P.A. McCullough; Impact of obesity on cardiovascular disease; Med Clin N Am, 95 (2011), pp. 919–937
- [42] E.D. Abel, S.E. Litwin, G. Sweeney; Cardiac remodeling in obesity; Physiol Rev, 88 (2008), pp. 389–419
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?