Abstract
Spotted fever group (SFG) rickettsiosis is caused by obligatory intracellular Gram-negative bacteria that belong to the genus Rickettsia. Ticks belonging to the family Ixodidae can act as vectors, reservoirs or amplifiers of SFG rickettsiae. This study was conducted to estimate the seroprevalence of SFG rickettsioses in cattle, sheep and goats from Khartoum State, Sudan. Blood samples were collected from a total of 600 animals (sheep, goats and cattle) from 32 different farms distributed in three locations in Khartoum State during the period January to December 2012. Sera were tested for antibodies against SFG rickettsiae using IFAT. The prevalence of seropositivity was 59.3% in sheep, 60.1% in goats and 64.4% in cattle. Season was significantly (P < 0.05) associated with seroprevalence of SFG rickettsiae in cattle during winter. The SFG rickettsiae antibodies prevalence was significantly higher in female compared with male in sheep, but there were no significant differences between male and female in either cattle or goats. The prevalence was significantly higher in adult animals compared with young in both sheep and goats. With regard to management system, there was a significant difference in the prevalence in cattle raised in closed system compared with those raised in semi-intensive system. In contrast, there was significant difference in the seroprevalence of SFG in sheep where the prevalence was higher in the sheep raised in semi-intensive system compared with those raised in close system. There was no significant difference in the seroprevalence in goats with regard to management systems. The unexpected high prevalence of SFG rickettsia antibodies in domestic ruminants sera suggest that the veterinary and public health impact of these agents in Sudan need further evaluation especially in humans.
Introduction
Spotted fever group (SFG) rickettsiosis is caused by obligatory intracellular Gram-negative bacteria that belong to the genus Rickettsia (Parola et al. 2005). Ticks belonging to the family Ixodidae can act as vectors, reservoirs or amplifiers of SFG rickettsiae. These bacteria do not normally infect humans during their natural cycles between their arthropod and vertebrate hosts. Ecological characteristics of the tick vectors influence the epidemiology and clinical aspects of tick-borne diseases (Parola & Raoult 2001). Some rickettsiae, such as Rickettsia rickettsii, may be associated with several tick vectors from different genera (Parola et al. 2005). This contrasts with other rickettsiae, such as Rickettsia conorii, which appear to be associated with only one tick vector (Raoult & Roux 1997). Between these extremes, there are certain rickettsiae which are associated with several species within the same genus, such as association of Rickettsia africae and Rickettsia slovaca with Amblyomma spp. and Dermacentor spp., respectively (Parola & Raoult 2001). In sub-Saharan Africa, the main causative agents of SFG rickettsiae include R. conorii, R. africae, R. aeschlimannii, R. sibirica and R. massiliae (Parola 2006). The indirect immunofluorescence assay (IFA) is currently the test of choice for the serological diagnosis of rickettsial infection in humans and animals (Yu & DH 2003; Horta et al. 2004). However, the cross reactivity of IFA does not allow for identification of the infecting Rickettsia species. The geographic origin of the infection has been one of the best indicators of species identity (Horta et al. 2004). Testing a clinical serum sample against the possible Rickettsia species known to occur in a given area is ideal, because often homologous antibody titres are higher than heterologous antibody titres. In some cases, the differences in titres may be great enough to differentiate among the rickettsial species potentially stimulating the immune response (LaScola & Raoult 1997). A high proportion (80–100%) of cattle in endemic areas has serological evidence of exposure to SFG rickettsiae (Kelly et al. 1991). High seroprevalence (87%) has also been reported in goats (Parola et al. 1999), although in another study, seroprevalence rates in the three common domestic ruminants (cattle, goats and sheep) reflected the host preference of the vector Amblyomma variegatum, with the highest prevalence in cattle and much lower prevalence in goats and sheep (Kelly et al. 2010). A previous molecular detection of SFG rickettsia in Hyalomma spp. and Amblyomma spp. (A. lepidum, A. variegatum, H. dromedarii, H. truncatum, H. marginatum) collected in Sudan demonstrated that SFG rickettsia were highly prevalent in these ticks (Morita et al. 2004; Nakao et al. 2015). Entomological studies performed in Khartoum State confirmed that Ixodid ticks, the potential vectors of SFG rickettsia, are highly prevalent in domestic animals and that the close contact of humans with ticks is continual and permanent (Mohamed et al. 2004). Observations reported by Mohamed et al. (2004) indicated that seven species of Ixodidae ticks were infesting cattle in Khartoum State. Hyalomma anatolicum constituted 70.6% and 83.3% of the tick fauna in Soba and Kuku, while Rhipicephalus e. evertsi constituted 22.5% of the tick fauna in Soba and 6.2% in Kuku (Mohamed et al. 2004). Osman (1999) collected H. anatolicum, R. e. evertsi and ticks of the R. sanguineus group from sheep in the Khartoum University Farm. The aim of this study was to estimate the seroprevalence of SFG rickettsioses in cattle, sheep and goats from Khartoum State, Sudan.
Materials and methods
Study area
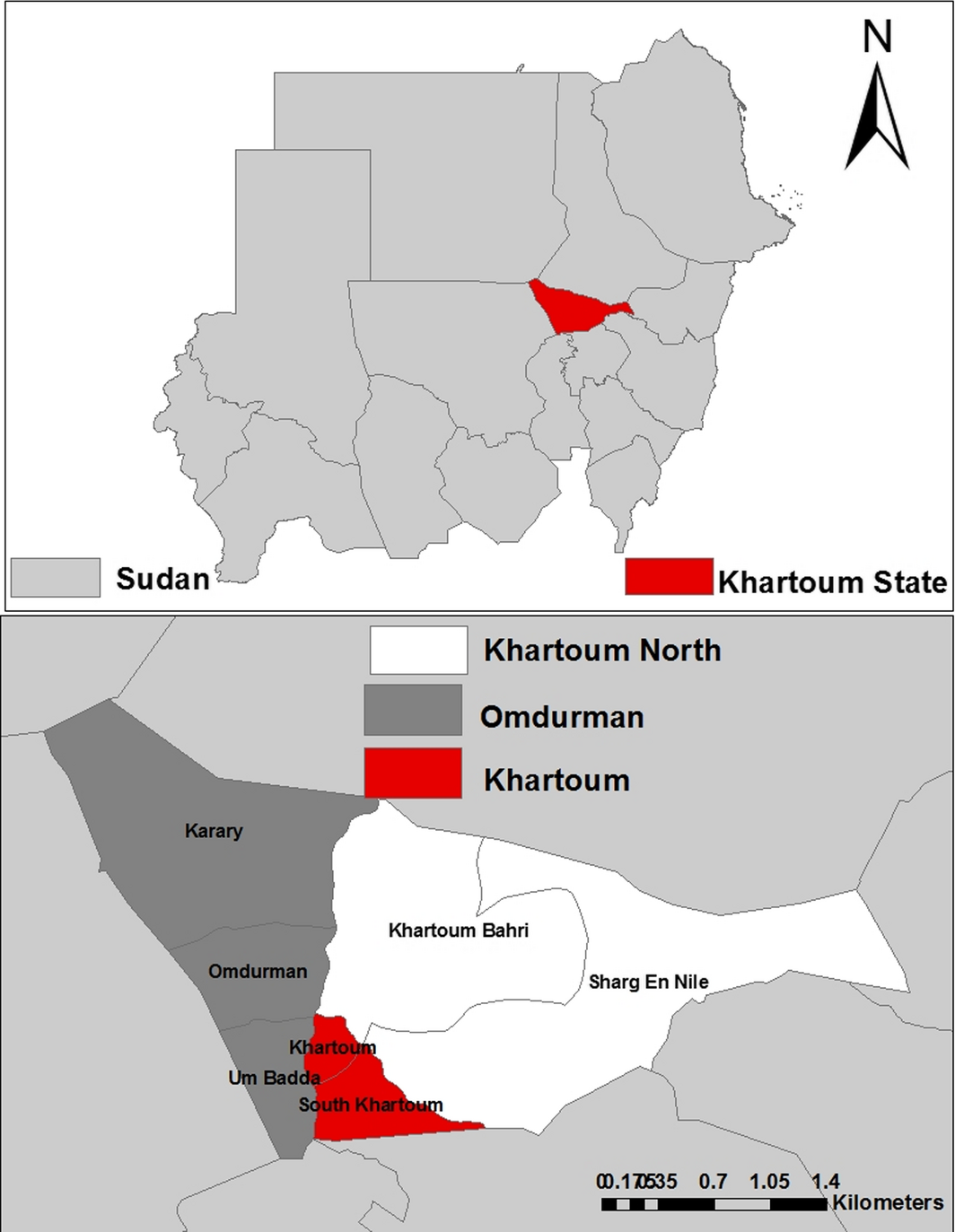
The survey was conducted during the period from January to December 2012 in three locations (Khartoum, Omdurman and Khartoum North) in Khartoum State in the poor savannah climatic zone, Sudan (15.16–16.65° North and 31.64–32.05° East) (Fig. 1).
|
|
|
Figure 1. Map of Khartoum State showing locations where samples were collected. |
Study design
This is a cross-sectional survey that was carried out in three locations in Khartoum State. Sample size estimation of the animals was calculated using the following formula (Thrusfield 2007):
|
|
where n is the required number of individuals to be examined; z is a constant = 1.96; P is a known or estimated prevalence; Q = (1 − P) and L are the allowable error. As the prevalence of SFG rickettsiae in different regions of the Sudan has never been substantially determined, this study assumed an expected prevalence of SFG rickettsiae in domestic ruminants to be 50% (Thrusfield 2007). The number of animals estimated using this formula was 384. The sample size was increased to 600, in order to allow for more reliable statistical analysis.
Blood collection
The investigation was carried out in compliance with the animal welfare code of Sudan. Six hundred blood samples from sheep, goats and cattle (200 from each species) from 32 farms distributed in the three locations in Khartoum State were collected. Five ml jugular venous blood was collected from each animal in a plain container. Sera were separated by centrifugation at 1500 rpm for 10 min and then stored at −20°C until tested.
Immunofluorescent antibody test for detection of SFG rickettsial infection
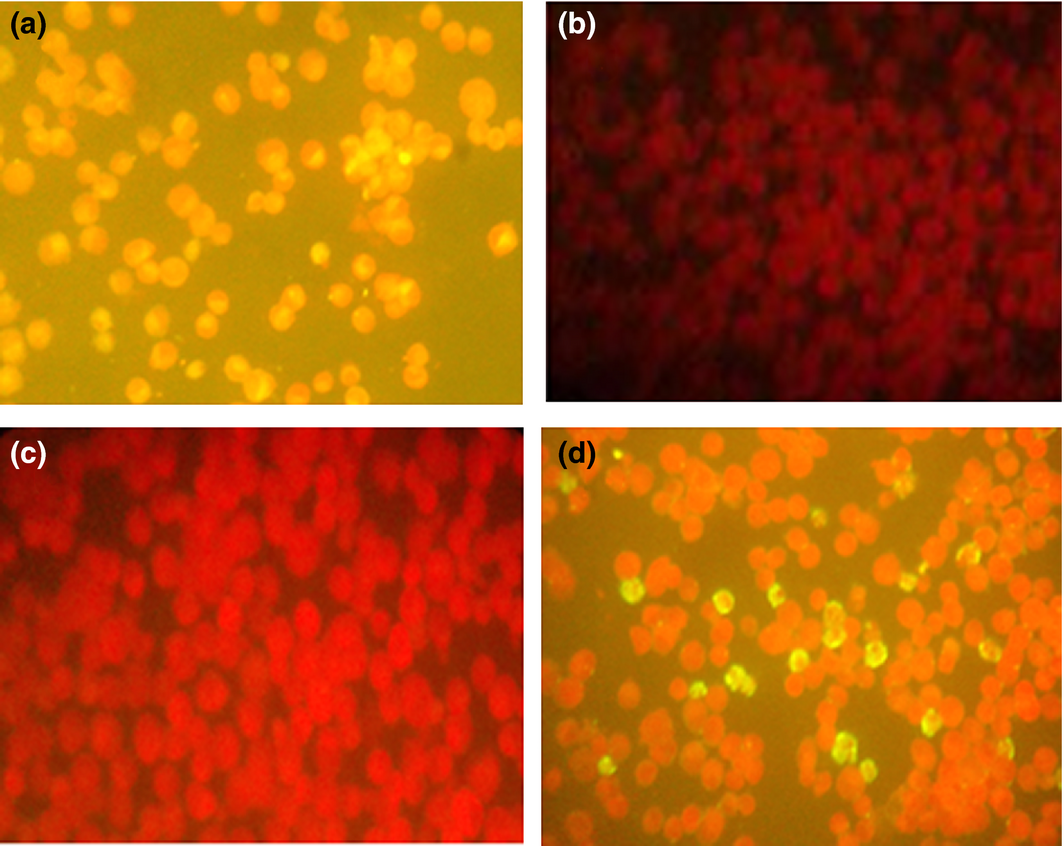
Rickettsia SFG antibodies were detected by indirect fluorescent antibody test (IFAT) using different species IgG conjugates (Fuller Laboratories, Fullerton, California, USA). Twelve-well masked slides containing acetone-fixed Vero cells, some of which were infected with the bitterroot strain of R. rickettsii (chemically killed) were used. The procedure was performed according to the manufacturers instructions (Raoult & Dasch 1989) (Fig. 2). Serum was considered to contain antibodies against the rickettsiae if it displayed a reaction at the 1:64 dilution.
|
|
|
Figure 2. IFAT results for SFG rickettsiae: (a) positive control, (b) negative control, (c) negative sample and (d) positive sample. |
Statistical analysis
Data were compared using Pearsons chi-squared test. A P ≤ 0.05 was considered indicative of a statistically significant difference. All the data analyses were performed using the SPSS 20.0 for Windows software package (SPSS, Chicago, IL, USA).
Results
The prevalence of SFG antibodies was found to be 64.4%, 60.1% and 59.3% in cattle, goats and sheep, respectively (Table 1). There was no significant difference in prevalence of SFG rickettsiae antibodies among different animal groups. The seasonally related prevalence of SFG rickettsiae antibodies was significantly (P = 0.027) higher in cattle during winter season. The SFG rickettsiae antibodies rate was significantly (P = 0.02) higher in female compared with male in sheep, and in older animals compared with young ones in both sheep (P = 0.04) and goats (P = 0.000). According to location, the infection rate was significantly (P = 0.01) higher in Khartoum North in goats. According to the management systems there was significant (P = 0.02) difference in the prevalence in cattle raised in closed system compared to those raised in semi-intensive management system. In contrast, there was significant (P = 0.000) difference in the prevalence of SFG antibodies in sheep raised in semi-intensive management system compared with those raised in close system (Tables 2–4).
| Animal type | Positive | Negative | P |
|---|---|---|---|
| Cattle | 125 (64.4%) | 69 (35.6) | 0.383a |
| Sheep | 118 (59.3%) | 81 (40.7%) | |
| Goats | 119 (60.1%) | 79 (39.9%) | |
|
a No significant differences in seroprevalence rate were found among the three kinds of animals tested. | |||
| Risk factor | Group | No. positive | No. negative | Percentage positive | P |
|---|---|---|---|---|---|
| Season | Summer† | 16 | 9 | 64.0 | 0.027* |
| Winter‡ | 109 | 60 | 64.5 | ||
| Age (years) | <3 | 26 | 11 | 70.3 | 0.121 |
| 3–6 | 38 | 27 | 58.5 | ||
| 6.1–9 | 41 | 27 | 60.3 | ||
| >9 | 20 | 4 | 83.3 | ||
| Location | Khartoum | 38 | 23 | 62.3 | 0.13 |
| Omdurman | 44 | 15 | 74.6 | ||
| Khartoum North | 43 | 31 | 58.1 | ||
| Sex | Male | 16 | 7 | 69.6 | 0.58 |
| Female | 109 | 62 | 63.7 | ||
| Management | Intensive | 105 | 48 | 68.6 | 0.02* |
| Semi-intensive | 20 | 21 | 48.8 | ||
*P < 0.05. †March–June. ‡November–February. | |||||
| Risk factor | Group | No. positive | No. negative | Percentage positive | P |
|---|---|---|---|---|---|
| Season | Summer† | 36 | 19 | 65.5 | 0.36 |
| Winter‡ | 44 | 38 | 53.7 | ||
| Autumn§ | 38 | 24 | 61.3 | ||
| Age (years) | <1 | 25 | 30 | 45.5 | 0.04* |
| 1–4 | 58 | 35 | 62.4 | ||
| >4 | 35 | 16 | 68.6 | ||
| Location | Khartoum | 37 | 26 | 58.7 | 0.2 |
| Omdurman | 26 | 26 | 50.0 | ||
| Khartoum North | 55 | 29 | 65.5 | ||
| Sex | Male | 46 | 45 | 50.5 | 0.02* |
| Female | 72 | 36 | 66.7 | ||
| Management | Intensive | 86 | 76 | 53.1 | 0.000* |
| Semi-intensive | 32 | 5 | 86.5 | ||
*P < 0.05. †March–June. ‡November–February. §July–September. | |||||
| Risk factor | Group | No. positive | No. negative | Percentage positive | P |
|---|---|---|---|---|---|
| Season | Summer† | 41 | 16 | 71.9 | 0.09 |
| Winter‡ | 49 | 37 | 57.0 | ||
| Autumn§ | 29 | 26 | 52.7 | ||
| Age (years) | <1 | 24 | 35 | 40.7 | 0.000* |
| 1–3 | 38 | 30 | 55.9 | ||
| >3 | 57 | 14 | 80.3 | ||
| Location | Khartoum | 27 | 30 | 47.4 | 0.01* |
| Omdurman | 31 | 26 | 54.4 | ||
| Khartoum North | 61 | 23 | 72.6 | ||
| Sex | Male | 24 | 14 | 63.2 | 0.67 |
| Female | 95 | 65 | 59.4 | ||
| Management | Intensive | 115 | 79 | 59.3 | 0.1 |
| Semi-intensive | 4 | 0 | 100 | ||
*P < 0.05. †March–June. ‡November–February. §July–September. | |||||
Discussion
In the present study, the seroprevalence of SFG rickettsiae using IFAT was found to be 59.3%, 60.1% and 64.4% in sheep, goats and cattle, respectively, comparable with that reported in Guadeloupe in the French, West Indies, in which the seroprevalence of R. africae was 55.3% in cattle at a titre ≥ 1:100 and 63.3% in goats at a titre ≥ 1:100 (Parola et al. 1999). However, our results were in contrast with 15.7% and 65% found in sheep and goats, respectively, in Catalonia (Ortuno et al. 2012). Also, the seroprevalence of SFG rickettsiae reported in domestic ruminants in this study is higher than that reported for goats (20%) and cattle (30%) in Sri Lanka and for sheep (7.5%) in Tanzania using the same technique (Kovacova et al. 1996). In addition, the seroprevalence of SFG rickettsiae reported herein in cattle (64.4%) is higher than that reported for cattle (9.6%) in Japan and for cattle (30%) from the north Zimbabwe, but lower than that reported in cattle from southern Zimbabwe in which almost 100% of 52 cattle tested were found to have antibodies reactive with R. conorii (Jilintai et al. 2008) (Kelly et al. 1991). Furthermore, our results were higher than those reported in Kenya in which the seroprevalence was found to be 43% in goats, 23% in sheep and 1% in cattle (Maina et al. 2014). These differences in seroprevalence may reflect geographic, ecologic and climate differences that affect the diversity, abundance and distribution of ticks found in a particular area or region.
In this study, gender as expected, seemed to be irrelevant to the prevalence of SFG rickettsia antibodies in both cattle and goats. On the contrary, it was significantly higher (P < 0.05) in female compared with male in sheep. This may reflect a higher degree of attractiveness of females to tick vectors (Elhassan et al. 2014), but could be due to the continued replacement of males in the farms compared with females so that females were probably exposed to several tick seasons (Palmer et al. 1998). In cattle, unlike sheep and goats, seasons seemed to be relevant to seroprevalence of SFG rickettsiae antibodies. The prevalence was significantly (P < 0.05) higher in winter season. This could be attributed to the crowding of cattle together for warmth which will increase the possibility of contact to ticks and infection transmission. In this study, breeds seemed to be irrelevant to the prevalence of SFG rickettsia antibodies in cattle, sheep and goats (data not shown). With respect to geographic location only, the prevalence in goats was significantly (P < 0.05) higher in Khartoum North compared to the other two locations. The reasons of this situation could be due to the small sample size, but most of the goat samples from Khartoum North location were collected from intensively managed farms with poor tick control programmes. The prevalence of seropositivity was significantly (P < 0.05) higher in older animals compared with young ones in both sheep and goats. This may be due to maternal immunity transfer protecting young animals. Protection will decrease with animals’ ages and thus adult animals will have more exposure to infection and a higher prevalence (Jegede et al. 2015). However, there was no significant difference (P > 0.05) between different age groups in cattle.
The results obtained indicated that high prevalence of SFG rickettsiae antibodies in Khartoum State may pose a serious health problem. The results to the best of our knowledge represent the first serological detection of SFG rickettsiae in animals in Khartoum State. The seroprevalence of SFG in the three animal species reported herein might have been underestimated due to small numbers tested for each animal species. Ideally more than 300 animals for each species have been tested. In addition, due to limitations of study design, the question of domestic ruminants acting as a reservoir could not be answered definitively. Therefore, further investigations are urgently needed to screen domestic ruminants sera over a longer period for possible rickettsaemia. These investigations are also needed in other parts of the country, in order to obtain more reliable data with regard to ruminants’ role in maintaining rickettsial natural life cycles. On the other hand, further investigations including human and ticks to assess the actual risk of rickettsial diseases in the country is required to implement necessary therapeutics and control.
Acknowledgements
This work was supported by Central Laboratory, Ministry of Higher Education and Scientific Research and Bank of Animal Resources, Khartoum, Sudan.
Source of funding
This study was funded by Bank of Animal Resources, Khartoum, Sudan.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributions
Nagwa M. Eisawi drafted the manuscript; Nagwa M. Eisawi and Mohammed O. Hussien carried out the IFA assays; Azza B. Musa done the statistical analysis; Abdel Rahim M. El Hussein and Dina A. Hassan contributed to the concept, design and supervision of the study as well as revision of the manuscript.
References
- Elhassan A.M., Mansour M.E., Shamon A.A. & El Hussein A.M. (2014) A serological survey of Akabane virus infection in cattle in Sudan. ISRN Veterinary Science2014, 123904.
- Horta M.C., Labruna M.B., Sangioni L.A., Vianna M.C., Gennari S.M., Galvao M.A.et al. (2004) Prevalence of antibodies to spotted fever group rickettsiae in humans and domestic animals in a Brazilian spotted fever-endemic area in the state of Sao Paulo, Brazil: serologic evidence for infection by Rickettsia rickettsii and another spotted fever group Rickettsia. American Journal of Tropical Medicine and Hygiene71, 93–97.
- Jegede O.C., Onoja E., Obeta S.S., Opara M., Olayem O.D. (2015) Haemoparasitism in small ruminants in Gwagwalada Metropolis, Federal Capital Territory, Abuja, North Central Nigeria. International Journal for Agro Veterinary and Medical Sciences9, 186–191.
- Jilintai S.N., Matsumoto K., Hayakawa D., Suzuki M., Hata H., Kondo S.et al. (2008) Serological and molecular survey of Rickettsial infection in cattle and sika deer in a pastureland in Hidaka District, Hokkaido, Japan. Japanese journal of Infectious Diseases61, 315–317.
- Kelly P.J., Mason P.R., Manning T. & Slater S. (1991) Role of cattle in the epidemiology of tick-bite fever in Zimbabwe. Journal of Clinical Microbiology29, 256–259.
- Kelly P., Lucas H., Beati L., Yowell C., Mahan S. & Dame J. (2010) Rickettsia africae in Amblyomma variegatum and domestic ruminants on eight Caribbean islands. Journal of Parasitology96, 1086–1088.
- Kovacova E., Sixl W., Stunzner D., Urvolgyi J. & Kazar J. (1996) Serological examination of human and animal sera from six countries of three continents for the presence of rickettsial antibodies. European Journal of Epidemiology12, 85–89.
- LaScola B. & Raoult D. (1997) Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. Journal of Clinical Microbiology35, 2715–2727.
- Maina A.N., Jiang J., Omulo S.A., Cutler S.J., Ade F., Ogola E.et al. (2014) High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural western Kenya: implications for human health. Vector-Borne and Zoonotic Diseases14, 693–702.
- Mohamed A.S., Osman M.O., Elrabaa F.M. (2004). The occurrence of ixodid tick species of cattle in two localities in Khartoum District. The Sudan Journal of Veterinary Science and Animal Husbandry43, 1–2.
- Morita C., El Hussein A.R., Matsuda E., Abdel Gabbar K.M., Muramatsu Y., Abdel Rahman M.B.et al. (2004) Spotted fever group rickettsiae from ticks captured in Sudan. Japanese Journal of Infectious Diseases57, 107–109.
- Nakao R., Qiu Y., Salim B., Hassan S.M. & Sugimoto C. (2015) Molecular Detection of Rickettsia africae in Amblyomma variegatum Collected from Sudan. Vector Borne Zoonotic Dis.15, 323–325.
- Ortuno A., Pons I., Quesada M., Lario S., Anton E., Gil A.et al. (2012) Evaluation of the presence of Rickettsia slovaca infection in domestic ruminants in Catalonia, Northeastern Spain. Vector Borne Zoonotic Dis12, 1019–1022.
- Osman I.A., (1999) Some studies on malignant ovine theileriosis in Northern Sudan. MVSc thesis, Department of Parasitology, University of Khartoum.
- Palmer G.H., Abbott J.R., French D.M. & McElwain T.F. (1998) Persistence of Anaplasma ovis infection and conservation of the msp-2 and msp-3 multigene families within the genus Anaplasma. Infection and Immunity66, 6035–6039.
- Parola P. (2006) Rickettsioses in sub-Saharan Africa. Annals of the New York Academy of Sciences1078, 42–47.
- Parola P. & Raoult D. (2001) Ticks and tick-borne bacterial diseases in humans: an emerging infectious threat. Clinical Infectious Diseases32, 897–928.
- Parola P., Vestris G., Martinez D., Brochier B., Roux V. & Raoult D. (1999) Tick-borne rickettiosis in Guadeloupe, the French West Indies: isolation of Rickettsia africae from Amblyomma variegatum ticks and serosurvey in humans, cattle, and goats. American Journal of Tropical Medicine and Hygiene60, 888–893.
- Parola P., Paddock C.D. & Raoult D. (2005) Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clinical Microbiology Reviews18, 719–756.
- Raoult D. & Dasch G.A. (1989) Line blot and western blot immunoassays for diagnosis of Mediterranean spotted fever. Journal of Clinical Microbiology27, 2073–2079.
- Raoult D. & Roux V. (1997) Rickettsioses as paradigms of new or emerging infectious diseases. Clinical Microbiology Reviews10, 694–719.
- Thrusfield M. (2007) Veterinary Epidemiology. 3rd Edn. Blackwell Science Ltd: UK pp: 274–282.
- Yu X.J., Walker D.H. (2003) The Order Rickettsiales. In: The Prokaryotes: An Evolving Electronic Resource for the Microbiology Community. Third Edition. (eds M. Dworkin), Springer-Verlag: New York. (http://link.springer-ny.com/link/service/books/10125).
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?