Abstract
Background
Little is known concerning the effect of ezetimibe for secondary prevention in post-myocardial infarction (MI) patients. In this study, we investigated the secondary prevention effect of ezetimibe for post-MI patients.
Methods
This study is a retrospective analysis of Assessing Lipophilic vs. hydrophilic Statin therapy for Acute MI (ALPS-AMI study). The patients were divided into two groups: those administered a statin to control low density lipoprotein-cholesterol (LDL-C), the ezetimibe(−) group, and those administered ezetimibe in addition to a statin to control LDL-C, the ezetimibe(+) group. The endpoints were Major Adverse Cardiac and Cerebrovascular Event (MACCE), including all-cause death, recurrence of MI, stroke, and heart failure requiring hospitalization, and MACCE with revascularization.
Results
The ezetimibe(+) and ezetimibe(−) groups contained 113 and 337 patients, respectively. Incidences of MACCE and MACCE with revascularization were lower in the ezetimibe(+) group than in the ezetimibe(−) group (2.6% vs. 11.5%, p = 0.002; 23.0% vs. 36.7%, p = 0.014, respectively). Moreover, logistic regression analysis revealed ezetimibe(+) was a significant negative predictor of MACCE (OR 0.208, 95% CI 0.048 to 0.903, p = 0.047) and MACCE with revascularization (OR 0.463, 95% CI 0.258 to 0.831, p = 0.008). The preventive effect of ezetimibe against MACCE was observed in both moderate- and high-intensity lipid lowering treatment groups (0% vs. 17%; p = 0.077, 3.1% vs. 9.4%; p = 0.033).
Conclusions
In lipid-lowering therapy post-MI, ezetimibe and statin combination therapy improved MACCE with or without revascularization compared with statin monotherapy. These findings suggest that post-MI secondary prevention should be more intensive.
Keywords
Acute myocardial infarction;Ezetimibe;Secondary prevention;Statin
1. Introduction
Low-density lipoprotein cholesterol (LDL-C) is considered an important target for the prevention of atherosclerotic events [1]; [2] ; [3]. Insufficient control over LDL-C increases the atherosclerotic plaque burden and worsens plaque vulnerability [4]; [5] ; [6]. Previous ACC/AHA guidelines recommended an absolute decrease in LDL-C levels below 100 mg/dl, while the current guideline recommends a 30–50% decrease or more from baseline levels to prevent secondary atherosclerotic events. Moreover, the new ACC/AHA guideline recommends statins as the only agents to reduce LDL-C and improve lipid metabolism, and thus prognosis, for post-MI patients [7]. In many investigations, statin use was reported to improve plaque burden and vulnerability [4]; [5] ; [6] and the prognosis of atherosclerotic disease patients. However, although in the modern era statins are considered a necessary agent to improve atherosclerotic disease, statin monotherapy does not sufficiently prevent atherosclerotic disease [8]. Strict lipid-lowering therapy with another agent combined with a statin may be required to prevent secondary atherosclerotic events. Ezetimibe, a selective Niemann–Pick C1-like protein (NPC1L1) inhibitor, employs a different mechanism from statins to improve cholesterol metabolism by inhibiting intestinal cholesterol absorption [9]. Many investigations have reported favorable effects of ezetimibe for atherosclerosis [10]. Thus, we investigated the impact of combination therapy with ezetimibe and statin for secondary prevention of MI.
2. Materials and methods
This study is a retrospective analysis of prospectively collected data assessing lipophilic vs. hydrophilic statin therapy for acute MI (ALPS-AMI study) with a head-to-head comparison of the efficacy of lipophilic atorvastatin vs. hydrophilic pravastatin [11] ; [12]. This study was a prospective, randomized, open-labeled, blinded endpoint study that required patients at 20 participating sites in Nagano and Niigata prefectures of Japan. The inclusion criteria included: male or female, aged > 20 years, written informed consent, and percutaneous coronary intervention (PCI) to treat either ST-segment elevation or non-ST-segment elevation acute MI done within 96 h. Exclusion criteria included planned surgery for coronary artery bypass grafting, pregnancy, active liver or renal disease, malignant disease, withdrawal of informed consent, and serious arrhythmic events or the presence of hemodynamic instability (hypotension, congestive heart failure, or mechanical complication following acute MI). Patients were randomly allocated to receive 10 mg of either atorvastatin or pravastatin once daily, with the treatment goal to reduce the LDL-C level below 100 mg/dl. If necessary, the dose was increased to 20 mg in one month after admission of statin. If the treatment goal still was not achieved with statin monotherapy, then 10 mg ezetimibe was added in one month after increasing each statin dose up to 20 mg. Patients were enrolled from June 2008 to December 2010 and followed for at least 24 months. The study was performed in accordance with the Declaration of Helsinki ant the Good Clinical Practice Guidelines. The protocol was approved by each participating sites ethics committee, and was registered at the University Hospital Medical Information Network (UMIN000001521).
2.1. Patient population
According to ALP-AMI study criteria, we screened 450 patients. The patients were divided into two groups: those administered only a statin to control LDL-C level, the ezetimibe(−) group, and those administered ezetimibe in addition to a statin to control LDL-C level, the ezetimibe(+) group. The endpoints were major adverse cardiac and cerebrovascular events (MACCE), including all-cause death, cardiovascular death, recurrence of myocardial infarction, stroke, and heart failure requiring hospitalization, and MACCE with revascularization.
2.2. Guideline of lipid lowering therapy for secondary prevention of post-myocardial infarction patient
In the 2013 ACC AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults, patients with a history of clinical atherosclerotic systemic cardiovascular disease including myocardial infarction were divided into 2 groups; patients ≤ 75 years old received high-intensity lipid lowering therapy, while those > 75 years old were assigned to moderate-intensity lipid lowering therapy. In the guideline, the target level of LDL-C was not set, and statin titration was not recommended. Furthermore, statin was only the recommended agent for lipid lowering therapy, and other agents including ezetimibe were not recommended. However, we consider that the effect of ezetimibe and statin combination therapy should be examined in each therapy intensity group.
2.3. Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are expressed as a number and percentage. Continuous variables were compared using the two-sided paired t-test, and categorical variables were compared using the chi-square test or Fishers exact test, as appropriate. All p values are two-sided, and results with p < 0.05 were considered statistically significant. A logistic regression model was subsequently used to analyze the incidence of MACCE and MACCE with revascularization. As the first step, potential predictors of MACCE and MACCE with revascularization incidence were separately assessed in logistic regression analyses. Then multiple logistic regression analysis was conducted for covariates that demonstrated an association with the incidence of MACCE and MACCE with revascularization (p ≤ 0.10). Results are expressed as odds ratios with 95% confidence intervals (95% CI). All analyses were performed using SPSS statistical software, version 13.0 (SPSS Inc., Chicago, Illinois).
3. Results
3.1. Baseline characteristics
Baseline clinical characteristics of the ezetimibe(+) and ezetimibe(−) groups are compared in Table 1. The ezetimibe(+) and ezetimibe(−) groups contained 113 and 337 patients, respectively. The ezetimibe(−) group was significantly older than the ezetimibe(+) group (62.1 ± 11.5 vs. 67.1 ± 10.9 years, p < 0.0001). There were no significant differences in history of hypertension or smoking. Diabetes mellitus was observed in 31 patients in the ezetimibe(+) group (27.4%) and 126 (37.3%) in the ezetimibe(−) group (p = 0.034). Total cholesterol and LDL-C were higher in the ezetimibe(+) group compared with those in the ezetimibe(−) group (228 ± 39.1 mg/dl vs. 194.7 ± 35.6 mg/dl, p < 0.0001; 153 ± 34.2 mg/dl vs. 123.6 ± 29.6 mg/dl, p < 0.0001). There were no significant differences in serum creatinine level (0.82 ± 0.24 mg/dl vs. 0.89 ± 0.64 mg/dl, p = 0.288), estimated glomerular filtration rate (73.88 ± 17.5 ml/min/1.73 m2 vs. 71.03 ± 20.1 ml/min/1.73 m2, p = 0.179), BNP (101.8 ± 160.1 pg/ml vs. 134.7 ± 179.9 pg/ml, p = 0.125), or left ventricular ejection fraction (55 ± 10.7% vs. 54.8 ± 12.2%, p = 0.87).
| Ezetimibe(+) | Ezetimibe(−) | p value | |
|---|---|---|---|
| n = 113 | n = 337 | ||
| Female sex n = 450 (%) | 23 (20.3) | 61 (18.1) | 0.343 |
| Age (years) | 62.13 ± 11.5 | 67.1 ± 10.9 | < 0.0001 |
| BMI (kg/m2) | 24.17 ± 3.79 | 23.6 ± 3.7 | 0.303 |
| Hypertension n = 449 (%) | 55 (48.6) | 146 (43.3) | 0.205 |
| Diabetes mellitus n = 450 (%) | 31 (27.4) | 126 (37.3) | 0.034 |
| Smoking n = 450 (%) | 76 (67.2) | 212 (62.9) | 0.237 |
| Familial history n = 450 (%) | 31 (27.4) | 67 (19.8) | 0.062 |
| Hemoglobin (g/dl) | 14.56 ± 1.9 | 14.4 ± 2.4 | 0.69 |
| Creatinine (mg/dl) | 0.82 ± 0.24 | 0.89 ± 0.64 | 0.288 |
| eGFR (ml/min/1.73 m2) | 73.88 ± 17.5 | 71.03 ± 20.09 | 0.179 |
| T-chol (mg/dl) | 228 ± 39.1 | 194.7 ± 35.6 | < 0.0001 |
| HDL-C (mg/dl) | 47.1 ± 10.9 | 47.7 ± 11.8 | 0.666 |
| LDL-C (mg/dl) | 153 ± 34.2 | 123.6 ± 29.6 | < 0.0001 |
| TG (mg/dl) | 167.7 ± 142.3 | 126.3 ± 88.1 | < 0.0001 |
| Non-HDL (mg/dl) | 184 ± 40.2 | 148.7 ± 35.1 | < 0.0001 |
| HbA1c (%) | 5.79 ± 1.1 | 5.97 ± 1.17 | 0.164 |
| STEMI | 84 (74.3) | 246 (72.9) | 0.32 |
| pAf n = 433 (%) | 3 (2.6) | 12 (3.5) | 0.436 |
| NSVT n = 433 (%) | 31 (27.4) | 23 (6.8) | 0.003 |
| Killip class ≥ 2 | 9 (7.9) | 43 (12.7) | 0.107 |
| BNP (pg/ml) | 101.8 ± 160.1 | 134.7 ± 179.9 | 0.125 |
| LVEF (%) | 55 ± 10.7 | 54.8 ± 12.2 | 0.87 |
| Triple vessel disease (%) | 9 (7.9) | 21 (6.2) | 0.296 |
Abbreviations. BMI: body mass index, eGFR: estimated glomerular filtrated rate, T-chol: total cholesterol, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, TG: triglyceride, STEMI: ST-elevated myocardial infarction, pAf: paroxysmal atrial fibrillation, NSVT: non-sustained ventricular tachycardia, BNP: brain natriuretic peptide, LVEF: left ventricular ejection fraction.
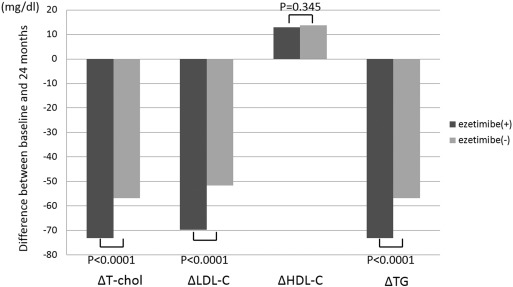
Table 2 revealed lipid parameters at twenty-four months and differences between values at baseline and 24 months. At 24 months, LDL-C and TG levels were still higher in the ezetimibe(+) group compared with those in the ezetimibe(−) group (94.6 ± 24.7 mg/dl vs. 83.7 ± 17.8 mg/dl, p < 0.0001; 163.8 ± 97.6 mg/dl vs. 132.9 ± 75.7 mg/dl, p = 0.004, respectively), while the difference of lipid parameters was higher in the ezetimibe(+) group compared with that in the ezetimibe(−) group (ΔLDL-C − 69.8 ± 32.1 mg/dl vs. − 51.7 ± 26.3 mg/dl, p < 0.0001; ΔTG − 73.1 ± 36.8 mg/dl vs. -56.9 ± 31.2 mg/dl, p < 0.0001) (Table 2, Fig. 1).
| Ezetimibe(+) | Ezetimibe(−) | p value | |
|---|---|---|---|
| n = 108 | n = 311 | ||
| 24 M T-chol (mg/dl) | 167.1 ± 29.5 | 155.2 ± 24.1 | 0.001 |
| 24 M HDL-C (mg/dl) | 49.2 ± 11.3 | 49.5 ± 11.8 | 0.865 |
| 24 M LDL-C (mg/dl) | 94.6 ± 24.7 | 83.7 ± 17.8 | < 0.0001 |
| 24 M TG (mg/dl) | 163.8 ± 97.6 | 132.9 ± 75.7 | 0.004 |
| ΔT-chol (mg/dl) | − 73.1 ± 36.8 | − 56.9 ± 31.2 | < 0.0001 |
| ΔLDL-C (mg/dl) | − 69.8 ± 32.1 | − 51.7 ± 26.3 | < 0.0001 |
| ΔHDL-C (mg/dl) | 12.9 ± 7.1 | 13.8 ± 9.2 | 0.345 |
| ΔTG (mg/dl) | − 73.1 ± 36.8 | − 56.9 ± 31.2 | < 0.0001 |
Abbreviations. Same as in Table 1. 24 M: 24 months.
Δ indicates the difference between values at baseline and 24 months.
|
|
|
Fig. 1. The difference in the lipid parameters between values of baseline and 24 months after, comparison between the ezetimibe(+) and ezetimibe(−) groups. T-chol, LDL-C and TG were decreased significantly in the ezetimibe group compared with those in the ezetimibe(−) group between values at baseline and 24 months. HDL-C was increased in both ezetimibe(+) and ezetimibe(−) groups, however there were no significant differences in ΔHDL-C between the ezetimibe(+) and ezetimibe(−) groups. |
3.2. MACCE and MACCE with revascularization
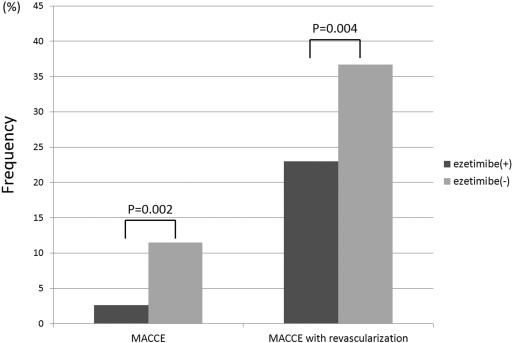
The composite endpoints, incidence of MACCE and MACCE with revascularization, were lower in the ezetimibe(+) group than the ezetimibe(−) group (2.6% vs. 11.5%, p = 0.002; 23.0% vs. 36.7%, p = 0.004) (Fig. 2) All-cause death and cardiovascular death were not observed in the ezetimibe(+) group, but occurred in 24 (7.1%) and 15 (4.4%) patients (p = 0.001 and 0.012), respectively, in the ezetimibe(−) group. There were no significant differences in heart failure requiring hospitalization, stroke, or any revascularization between the two groups.
|
|
|
Fig. 2. Comparison of MACCE and MACCE with revascularization between the ezetimibe(+) and ezetimibe(−) groups. Among all patients, the incidence of MACCE and MACCE with revascularization was lower in the ezetimibe(+) group compared with that in the ezetimibe(−) group. |
3.3. Analysis according to lipid lowering therapy intensity
According to the 2013 ACC/AHA guideline patients were divided into 2 groups; patients ≤ 75 years old received high-intensity lipid lowering therapy, while those > 75 years old were assigned to moderate-intensity lipid lowering therapy.
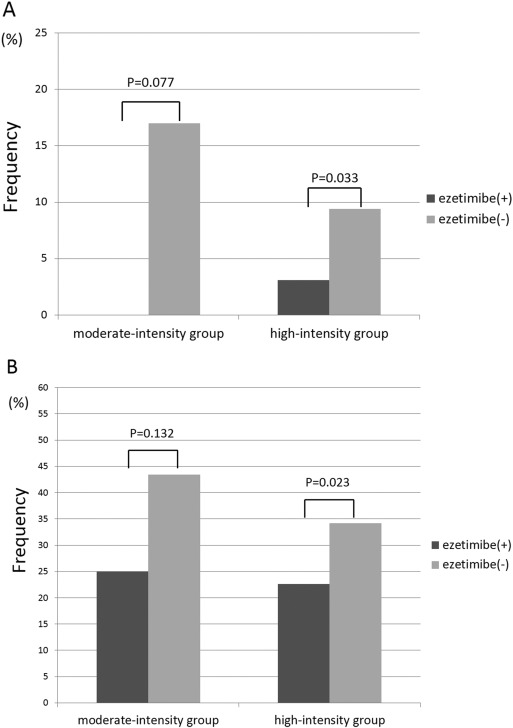
High-intensity lipid lowering therapy group included 347 patients and moderate-intensity lipid lowering therapy group included 113 patients, respectively. The incidence of MACCE was lower in the ezetimibe(+) group compared with that in the ezetimibe(−) group in both the high-intensity and moderate-intensity groups (0% vs. 17%, p = 0.077, 3.1% vs. 9.4%, p = 0.033, respectively) (Fig. 3A). The incidence of MACCE with revascularization was also lower in the ezetimibe(+) group compared with that in the ezetimibe(−) group in patients receiving both high-intensity and moderate-intensity statin therapies (25% vs. 43.4%, p = 0.132; 22.6% vs. 34.2%, p = 0.023, respectively) (Fig. 3B).
|
|
|
Fig. 3. Comparison of MACCE and MACCE with revascularization between the ezetimibe(+) and ezetimibe(−) groups according to statin therapy intensity. (A) In both the moderate- and high-intensity groups, the incidence of MACCE decreased in patients administered ezetimibe (ezetimibe(+)) compared with that in statin monotherapy (ezetimibe (−)). (B) In both the moderate-intensity group and high-intensity group, the incidence of MACCE with revascularization was also decreased in the ezetimibe(+) group compared with that in the ezetimibe(−) group. |
3.4. Multivariate analysis
In univariate analysis, age, serum creatinine level, and BNP were significantly associated with the incidence of MACCE (71.1 ± 10.2 vs. 65.4 ± 11.3, p = 0.002; 1.16 ± 1.44 vs. 0.84 ± 0.36, p = 0.0001; 206.1 ± 218.9 vs. 116.8 ± 167, p = 0.004) (Table 3). Serum creatinine level and BNP were also significantly associated with the incidence of MACCE with revascularization (0.96 ± 0.92 vs. 0.82 ± 0.22, p = 0.018; 152.4 ± 181.1 vs. 111.9 ± 169.5, p = 0.038) (Table 4), and female sex was a negative predictor for MACCE with revascularization (13.1% vs. 21.6%; p = 0.017) (Table 5). Table 5 demonstrated multivariate analysis, included p < 0.05 variables in univariate analysis, and revealed incidence of MACCE and MACCE with revascularization. BNP (OR 1.002, 95% CI 1.000 to 1.003, p = 0.036) and ezetimibe(+) therapy (OR 0.204, 95% CI 0.047 to 0.885, p = 0.034) were associated with the incidence of MACCE (Table 5). Moreover, multivariate analysis for MACCE with revascularization revealed that BNP (OR 1.001, 95% CI 1.000 to 1.003, p = 0.038) and ezetimibe(+) therapy (OR 0.445, 95% CI 0.249 to 0.795, p = 0.006) were independent risk factors. (Table 5) In the multivariate analysis, ezetimibe(+) and BNP were significant predictors for both MACCE and MACCE with revascularization in this study population.
| MACCE(+) | MACCE(−) | OR | 95% CI | p value | |
|---|---|---|---|---|---|
| n | 42 | 416 | |||
| Female sex (%) | 7 (16.6) | 79 (18.9) | 0.853 | 0.366–1.991 | 0.837 |
| Hypertension (%) | 17 (40.4) | 186 (44.7) | 0.837 | 0.439–1.597 | 0.628 |
| Diabetes mellitus (%) | 13 (30.9) | 146 (35.0) | 0.829 | 0.418–1.644 | 0.361 |
| Familial history (%) | 6 (14.2) | 92 (22.1) | 0.587 | 0.240–1.436 | 0.734 |
| pAf (%) | 3 (7.1) | 12 (2.8) | 2.622 | 0.708–9.710 | 0.147 |
| STEMI (%) | 33 (78.5) | 301 (72.3) | 3.728 | 0.873–15.912 | 0.062 |
| Age (years) | 71.1 ± 10.2 | 65.4 ± 11.3 | 1.049 | 1.017–1.082 | 0.002 |
| BMI (kg/m2) | 23.5 ± 3.3 | 23.7 ± 3.8 | 0.982 | 0.900–1.072 | 0.69 |
| Hemoglobin (g/dl) | 13.8 ± 2.1 | 14.5 ± 2.3 | 0.824 | 0.694–0.980 | 0.056 |
| Creatinine | 1.16 ± 1.44 | 0.84 ± 0.36 | 1.655 | 1.026–2.668 | 0.0001 |
| BNP | 206.1 ± 218.9 | 116.8 ± 167 | 1.002 | 1.001–1.004 | 0.004 |
| HbA1c | 5.73 ± 0.65 | 5.94 ± 1.19 | 0.820 | 0.564–1.191 | 0.295 |
| Ezetimibe(+) (%) | 3 (7.1) | 39 (9.4) | 0.208 | 0.063–0.688 | 0.004 |
Abbreviations. pAf: paroxysmal atrial fibrillation, STEMI: ST elevated myocardial infarction, BMI: body mass index, BNP: brain natriuretic peptide, HbA1c: hemoglobin A1c.
| MACCE + revascularization(+) | MACCE + revascularization(−) | OR | 95% CI | p value | |
|---|---|---|---|---|---|
| n | 153 | 305 | |||
| Female sex (%) | 20 (13.1) | 66 (21.6) | 0.545 | 0.316–0.938 | 0.031 |
| Hypertension (%) | 61 (39.8) | 142 (46.5) | 0.756 | 0.510–1.122 | 0.195 |
| Diabetes mellitus (%) | 56 (36.6) | 103 (33.7) | 1.132 | 0.755–1.699 | 0.603 |
| Familial history (%) | 25 (16.3) | 73 (23.9) | 0.621 | 0.375–1.026 | 0.070 |
| pAF (%) | 6 (3.9) | 9 (2.9) | 1.343 | 0.469–3.847 | 0.586 |
| STEMI (%) | 115 (75.1) | 219 (71.8) | 1.517 | 0.848–2.714 | 0.165 |
| Age (years) | 67.5 ± 11.4 | 65.1 ± 11.2 | 1.019 | 1.001–1.037 | 0.33 |
| BMI (kg/m2) | 23.5 ± 3.1 | 23.8 ± 4.01 | 0.978 | 0.927–1.032 | 0.415 |
| Hemoglobin (g/dl) | 14.3 ± 1.9 | 14.5 ± 2.4 | 0.945 | 0.856–1.043 | 0.259 |
| Creatinine (mg/dl) | 0.96 ± 0.92 | 0.82 ± 0.22 | 1.984 | 0.922–4.267 | 0.018 |
| BNP (pg/dl) | 152.4 ± 181.1 | 111.9 ± 169.5 | 1.001 | 1.000–1.002 | 0.038 |
| HbA1c (%) | 5.87 ± 0.94 | 5.95 ± 1.25 | 0.944 | 0.787–1.133 | 0.537 |
| Ezetimibe(+) (%) | 26 (16.9) | 87 (28.5) | 0.513 | 0.314–0.839 | 0.008 |
Abbreviations as in Table 3.
| 95% CI | OR | p value | |
|---|---|---|---|
| MACCE | |||
| Age | 0.999–1.072 | 1.035 | 0.054 |
| BNP | 1.000–1.003 | 1.002 | 0.036 |
| Creatinine | 0.939–2.145 | 1.419 | 0.097 |
| Ezetimibe(+) | 0.047–0.885 | 0.204 | 0.034 |
| MACCE with revascularization | |||
| Female sex | 0.337–1.245 | 0.648 | 0.193 |
| BNP | 1.000–1.003 | 1.001 | 0.038 |
| Creatinine | 0.829–2.550 | 1.454 | 0.191 |
| Ezetimibe(+) | 0.249–0.795 | 0.445 | 0.006 |
Abbreviations. BNP: brain natriuretic peptide.
4. Discussion
In the present study, ezetimibe and statin combination therapy decreased the incidence of all-cause death and cardiovascular death as well as the incidence of MACCE and MACCE with revascularization in post-MI patients.
Statins are only agent recommended for secondary prevention of atherosclerotic events in new guidelines [7] based on their favorable effects of lowering LDL-C, increasing high-density lipoprotein cholesterol (HDL-C) levels, and stabilizing coronary artery atherosclerotic plaques. In recent years, it was recommended that LDL-C level should be decreased as much as possible by lipid lowering therapy. However, the theory has changed, and the most recent guideline does not set target levels for LDL-C control. It is recommended only that a sufficient dose of statin should be admitted according to the desired treatment intensity. However, the recurrence rate of MI remains high [13], and given the high mortality of patients with recurrent MI, often due to lost cardiac function and complicated with renal dysfunction, secondary prevention should be more intensive [14]. Therefore, another approach for lipid lowering therapy could be valuable for the secondary prevention of atherosclerotic disease.
Ezetimibe is a comparatively new cholesterol-lowering drug with a different mechanism from statins [15], and thus could provide another quality lipid control treatment. It reduces cholesterol by inhibiting the absorption of LDL-C from the intestinal mucous membrane. Buchwald et al. reported that partial ideal bypass improved the blood lipid levels of acute MI patients and their morbidity due to coronary heart disease [16]. Ezetimibe and statin combination therapy has also been reported to reduce LDL-C level more than statin monotherapy [17]; [18] ; [19]. The balance between cholesterol absorption and synthesis was revealed to correlate with atherosclerosis progression [20] and may be improved by ezetimibe. In the present study, we measured campesterol-to-lathoserol ratio, reflecting the balance between absorption and synthesis of a cholesterol marker, in 72 patients: 17 ezetimibe(+) and 55 ezetimibe(−) and found that it decreased significantly in the ezetimibe(+) group (2.36 ± 1.01 vs. 5.58 ± 2.85; p < 0.001). Ezetimibe has also demonstrated favorable effects on atherosclerotic plaques, acting to improve plaque vulnerability by reducing plaque burden and improving thinning of the fibrous cap of plaques similar to statin therapy [21].
In the present study, ezetimibe and statin combination therapy improved all-cause death and cardiovascular death compared with statin monotherapy. Lin et al. reported that post-acute coronary syndrome patients treated with ezetimibe combined with statins had a lower risk of re-hospitalization due to acute coronary syndrome recurrence and revascularization than those administered statin monotherapy [22]. In addition, the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), the only prospective randomized trial to compare ezetimibe with statin combination therapy and statin monotherapy [23], revealed a clinical mortality benefit of ezetimibe and simvastatin combination therapy in patients presenting with acute coronary syndrome. The clinical benefit of ezetimibe combined with high-potency statin therapy in patients with renal dysfunction has also been established: a sufficient dose of simvastatin plus ezetimibe reduced the incidence of major atherosclerotic events in chronic kidney disease patients compared with statin monotherapy [24].
By contrast, Pauriah et al. reported no observed mortality benefit of ezetimibe and statin combination therapy compared with high-potency statin therapy in post-MI patients [25]. Several points differ between this previous study and our study. In the previous study, criteria for ezetimibe administration depended on the judgment of clinicians, and the intensity of statin therapy varied across individuals in the ezetimibe and statin combination therapy group. In the present study, ezetimibe was added after statin administration reached a pre-established maximum dose. Statin therapy is currently the main strategy for lipid lowering in the secondary prevention of atherosclerotic disease. It appears that an appropriate strategy for ezetimibe administration is to use combination therapy only after statins have been extended to a sufficient dose.
We observed a clinical benefit of ezetimibe in both moderate-intensity (≤ 75 years old) and high-intensity (> 75 years old) therapy groups. To the best of our knowledge, no prior investigations have assessed the effects of ezetimibe according to lipid lowering therapy intensity. Our results indicated that ezetimibe was effective in post-MI patients in both intensity groups.
In the present study, the criteria for ezetimibe administration depended on LDL-C level. Because LDL-C level tended to be higher in younger patients, patients in the ezetimibe(+) group were younger, and mean BNP level was lower than in the ezetimibe(−) group. However, we also observed that ezetimibe was a negative predictor for the incidence of MACCE and MACCE with revascularization after adjusting for diabetes mellitus, age, BNP, female sex, and serum creatinine level (MACCE: 95% CI 0.072 to 0.803, OR 0.241, p = 0.021; MACCE with revascularization: 95% CI, 0.334 to 0.910, OR 0.552, p = 0.02).
5. Study limitations
This study has several limitations. The original ALPS-AMI study focused on only 500 MI patients. This sample size limited the assessment of these patients' prognosis. Levels of serum creatinine kinase, indicating myocardial necrosis, were not recorded; thus the scale of myocardial infarction was difficult to estimate. Furthermore, administration of ezetimibe was dependent on LDL-C level, creating an inherent bias between the ezetimibe(+) and ezetimibe(−) groups. In addition, it should be emphasized that the ezetimibe(+) group was small in comparison with the ezetimibe(−) group, which could impact our results concerning the mortality benefit of ezetimibe. Further prospective randomized trial focused on ezetimibe is needed to establish the benefits of ezetimibe in this and other patient populations.
6. Conclusion
Ezetimibe and statin combination therapy improved the prognosis of post-MI patients compared with statin monotherapy. This favorable effect extended to both moderate- and high-intensity stain therapy groups. Ezetimibe and statin combination therapy may improve the mortality of post-MI patients.
Conflict of interest
We have no conflicts of interest.
Acknowledgments
The authors would like to thank Minako Aono for her kind help.
References
- [1] L. Bowman, K. Wallendszus, R. Bulbulia, K. Rahimi, R. Haynes, S. Parish, R. Peto, R. Collins, Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group AJ; Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial; Lancet, 376 (2010), p. 10
- [2] A.J.K.S. Taylor, P.J. Flaherty, L.C. Coyle, T.T. Markwood, M.N. Vernalis; ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness; Circulation, 106 (2002), p. 6
- [3] S.J. Nicholls, TE, I. Sipahi, A.W. Grasso, P. Schoenhagen, T. Hu, K. Wolski, T. Crowe, M.Y. Desai, S.L. Hazen, S.R. Kapadia, S.E. Nissen; Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis; J. Am. Med. Assoc., 297 (2007), p. 10
- [4] M. Kawasaki, K. Sano, M. Okubo, H. Yokoyama, Y. Ito, I. Murata, et al.; Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three-dimensional integrated backscatter intravascular ultrasound; J. Am. Coll. Cardiol., 45 (2005), pp. 1946–1953
- [5] C.W. Lee, S.J. Kang, J.M. Ahn, H.G. Song, J.Y. Lee, W.J. Kim, et al.; Comparison of effects of atorvastatin (20 mg) versus rosuvastatin (10 mg) therapy on mild coronary atherosclerotic plaques (from the ARTMAP trial); Am. J. Cardiol., 109 (2012), pp. 1700–1704
- [6] T. Hiro, T. Kimura, T. Morimoto, K. Miyauchi, Y. Nakagawa, M. Yamagishi, et al.; Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study); J. Am. Coll. Cardiol., 54 (2009), pp. 293–302
- [7] N.J.R.J. Stone, A.H. Lichtenstein, C.N. Merz, C.B. Blum, R.H. Eckel, A.C. Goldberg, D. Gordon, D. Levy, D.M. Lloyd-Jones, P. McBride, J.S. Schwartz, S.T. Shero, S.C. Smith Jr., K. Watson, P.W. Wilson; 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Circulation (November 12 2013)
- [8] P. Libby; The forgotten majority: unfinished business in cardiovascular risk reduction; J. Am. Coll. Cardiol., 46 (2005), pp. 1225–1228
- [9] T. Sudhop; Inhibition of intestinal cholesterol absorption by ezetimibe in humans; Circulation, 106 (2002), pp. 1943–1948
- [10] I. Staprans, X.M. Pan, J.H. Rapp, A.H. Moser, K.R. Feingold; Ezetimibe inhibits the incorporation of dietary oxidized cholesterol into lipoproteins; J. Lipid Res., 47 (2006), pp. 2575–2580
- [11] Y. Kashima, A. Izawa, K. Aizawa, M. Koshikawa, H. Kasai, T. Tomita, et al.; Rationale and design of assessment of lipophilic vs. hydrophilic statin therapy in acute myocardial infarction (the ALPS-AMI) study; J. Cardiol., 54 (2009), pp. 76–79
- [12] A. Izawa, T. Miura, S. Ebisawa, H. Kitabayashi, H. Yamamoto, S. Sakurai, M. Kagoshima, T. Tomita, Y. Miyashita, J. Koyama, U. Ikeda, on behalf of the ALPS-AMI Investigators; Assessment of lipophilic vs. hydrophilic statin therapy in acute myocardial Infarction– ALPS-AMI Study –; Circ. J., 79 (2014), pp. 161–168
- [13] L. Shen, B.R. Shah, A. Nam, D. Holmes, K.P. Alexander, D.L. Bhatt, et al.; Implications of prior myocardial infarction for patients presenting with an acute myocardial infarction; Am. Heart J., 167 (2014), pp. 840–845
- [14] A.A.T.U. Motivala, V.S. Ramanath, F. Saab, D.G. Montgomery, J. Fang, E. Kline-Rogers, N. May, G. Ng, J. Froehlich, H. Gurm, K.A. Eagle; A prior myocardial infarction: how does it affect management and outcomes in recurrent acute coronary syndromes?; Clin. Cardiol., 31 (2008), p. 7
- [15] A.E. Pesaro, C.V. Serrano Jr., J.L. Fernandes, A.B. Cavalcanti, A.H. Campos, H.S. Martins, et al.; Pleiotropic effects of ezetimibe/simvastatin vs. high dose simvastatin; Int. J. Cardiol., 158 (2012), pp. 400–404
- [16] H.V.R. Buchwald, J.P. Matts, J.M. Long, L.L. Fitch, G.S. Campbell, M.B. Pearce, A.E. Yellin, W.A. Edmiston, R.D. Smink Jr., et al.; Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH); N. Engl. J. Med., 323 (1990), p. 10
- [17] K. Okada, K. Kimura, N. Iwahashi, T. Endo, H. Himeno, K. Fukui, et al.; Clinical usefulness of additional treatment with ezetimibe in patients with coronary artery disease on statin therapy; Circ. J., 75 (2011), pp. 2496–2504
- [18] E. Moutzouri, E.N. Liberopoulos, C.C. Tellis, H.J. Milionis, A.D. Tselepis, M.S. Elisaf; Comparison of the effect of simvastatin versus simvastatin/ezetimibe versus rosuvastatin on markers of inflammation and oxidative stress in subjects with hypercholesterolemia; Atherosclerosis, 231 (2013), pp. 8–14
- [19] M. Averna, A. Zaninelli, C. Le Grazie, G.F. Gensini; Ezetimibe/simvastatin 10/20 mg versus simvastatin 40 mg in coronary heart disease patients; J. Clin. Lipidol., 4 (2010), pp. 272–278
- [20] K. Nasu, M. Terashima, M. Habara, E. Ko, T. Ito, D. Yokota, et al.; Impact of cholesterol metabolism on coronary plaque vulnerability of target vessels: a combined analysis of virtual histology intravascular ultrasound and optical coherence tomography; JACC Cardiovasc. Interv., 6 (2013), pp. 746–755
- [21] M. Habara, K. Nasu, M. Terashima, E. Ko, D. Yokota, T. Ito, et al.; Impact on optical coherence tomographic coronary findings of fluvastatin alone versus fluvastatin + ezetimibe; Am. J. Cardiol., 113 (2014), pp. 580–587
- [22] C.F. Lin, C.S. Gau, F.L. Wu, F.Y. Hsiao, C.H. Bai, L.J. Shen; Impact of ezetimibe coadministered with statins on cardiovascular events following acute coronary syndrome: a 3-year population-based retrospective cohort study in Taiwan; Clin. Ther., 33 (2011), pp. 1120–1131
- [23] C.P. Cannon, for the IMPROVE-IT Investigators; IMPROVE-IT trial: a comparison of ezetimibe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes after acute coronary syndromes; Paper Presented at: American Heart Association Scientific Sessions (November 17, 2014) (Chicago, IL)
- [24] C.L.M. Baigent, C. Reith, J. Emberson, D.C. Wheeler, C. Tomson, C. Wanner, V. Krane, A. Cass, J. Craig, B. Neal, L. Jiang, L.S. Hooi, A. Levin, L. Agodoa, M. Gaziano, B. Kasiske, R. Walker, Z.A. Massy, B. Feldt-Rasmussen, U. Krairittichai, V. Ophascharoensuk, B. Fellström, H. Holdaas, V. Tesar, A. Wiecek, D. Grobbee, D. de Zeeuw, C. Grönhagen-Riska, T. Dasgupta, D. Lewis, W. Herrington, M. Mafham, W. Majoni, K. Wallendszus, R. Grimm, T. Pedersen, J. Tobert, J. Armitage, A. Baxter, C. Bray, Y. Chen, Z. Chen, M. Hill, C. Knott, S. Parish, D. Simpson, P. Sleight, A. Young, R. Collins, SHARP Investigators; The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection) a randomised placebo-controlled trial..pdf; Lancet, 377 (2011), p. 12
- [25] M.E.D. Pauriah, S. Ogston, A.Y. Noman, A. Majeed, J.C. Wyatt, A.M. Choy, T.M. Macdonald, A.D. Struthers, C.C. Lang; High-potency statin and ezetimibe use and mortality in survivors of an acute myocardial infraction; Heart, 100 (2014), p. 6
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?