Summary
Background/Objective
The study aims to evaluate the alterations in the brain due to oxidative stress and lipid peroxidation resulting from obstructive jaundice.
Methods
Forty-one Wistar albino rats were used in this study. Simple laparotomy was performed in the sham group (n = 5). In the remaining 36 rats, the common bile duct (CBD) was found and ligated. They were divided into six groups. Group I, Group II, and Group III were sacrificed at the 3rd, 7th, and 14th day of ligation, respectively. In Group Id, Group IId, and Group IIId ligated bile ducts were decompressed at the 3rd, 7th, and 14th day, respectively. One week after decompression these rats were also sacrificed and samples were taken.
Results
After the CBD ligation, serum levels of bilirubin and malondialdehyde were found to be increased progressively in parallel to the ligation time of the CBD. After decompression these values decreased. In electron microscopy evaluation, the damage was found to be irreversible depending on the length of the obstruction period. In Group II, the damage was mostly reversible after the internal drainage period of 7 days. However in Group III, the tissue damage was found to be irreversible despite the decreased values of oxidative stress and bilirubin.
Conclusion
Ultrastructural changes in brain tissue including damage in the glial cells and neurons, were found to be irreversible if the CBD ligation period was >7 days and did not regress even after decompression. It is unreliable to trace these changes using blood levels of bilirubin and free radicals. Therefore, timing is extremely critical for medical therapies and drainage.
Keywords
astrocyte swelling;electron microscopy;malondialdehyde;obstructive jaundice;oxidative stress
1. Introduction
Obstructive jaundice (OJ) is the partial or total blockage of the extra- or intrahepatic biliary ducts that leads to a corruption of the normal biliary passage to the intestines. OJ may lead to high morbidity and mortality. In OJ, bile acids cause injury via free radicals and create oxidative stress in extrahepatic tissues.
Previous studies have suggested that altered gut barrier functions on the grounds of OJ, resulted in the migration of bacteria and endotoxins to the different organ systems.1 In animal models, by the means of oxidative stress, these toxins and molecules increase lipid peroxidation products. This affects extrahepatic tissues such as the kidneys, the lungs, and the brain. One of the well-known products is malondialdehyde (MDA), and is used as a marker of lipid peroxidation.2
In the brain, this oxidative stress may lead to neuropsychiatric abnormalities known as “hepatic encephalopathy” (HE) which at the end leads to symptoms varying from tremor and behavioral changes, to ataxia and coma which are eminently mortal.3
In 1991, Lockwood et al4 provided the first direct evidence in patients with ammonia and HE pathogenesis using positron emission tomography, which showed an increase in the cerebral metabolic rate of ammonia, and they suggested that ammonia was the main neurotoxin in the pathogenesis of HE. Astrocytes are responsible for the detoxification of ammonia during the conversion of glutamate to glutamine in the brain. Astrocytes regulate the extracellular matrix and influence neuronal excitability and neurotransmission.3
In clinical daily experience, if OJ patients are not operated on early and biliary drainage is not provided soon, the morbidity and mortality of the patients increase. These injuries in the end-organs are not reversible if the obstruction period gets longer. However, electron microscopic findings on functional changes regarding obstructive injury, pathophysiology of the reversibility, and the healing process are not fully understood. The unsolved dilemma is whether there is a period of reversibility, and what are the ultrastructural changes that lead to cell death.
The aim of the present study was to evaluate the alterations in the brain tissue resulting from OJ. After the release of the obstruction in different time periods, the hypothesis of whether the ultrastructural injury is reversible is also evaluated together with the correlations between OJ, oxidative stress, and injury.
2. Methods
2.1. Ethical approval
The study was approved, and the animals were treated according to the institutional guidelines of the Ethical Committee of Animal Research, Ankara University, Ankara, Turkey (protocol no: 37194).
2.2. Study design
Forty-one female Wistar albino rats, weighing 237 g (228–246 g) were used. Animals were housed in standard cages under appropriately dominated temperature and humidity conditions. They were fed with the standard rat diet (chow) and tap water. The night before the surgery feeding was stopped for preoperative fasting.
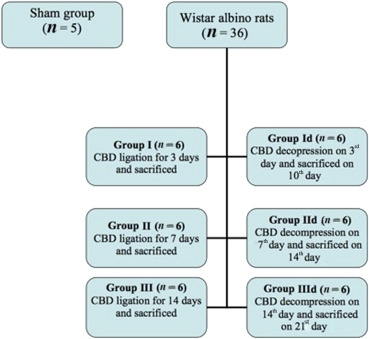
Rats were randomized into seven groups at the beginning of the study and kept in separate cages. Rats in the sham group (n = 5) underwent simple midline laparotomy. In the remaining 36 rats, which were randomized into three groups of 12 animals, after midline laparotomy, the common bile duct (CBD) was found and ligated. Half of the rats (n = 18, six from each group) in Group I, Group II, and Group III were sacrificed at the 3rd, 7th, and 14th day of the ligation, respectively, and samples were taken. In the remaining eighteen animals, ligated bile ducts were decompressed under anesthesia at Day 3, Day 7, and Day 14, respectively (groups designated as Group Id, Group IId, and Group IIId). One week after decompression, all these rats were sacrificed and samples were collected (Figure 1).
|
|
|
Figure 1. Study design. Forty one Wistar albino rats were used, all rats were randomized into seven groups with a sham group of five rats, remaining groups each with six rats. CBD = common bile duct. |
2.3. Surgical procedures
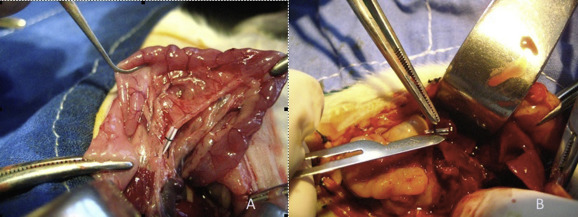
After topical povidone-iodine antisepsis, the animals were anesthetized with ketamine hydrochloride (50 mg/kg intraperitoneal) and xylazine hydrochloric acid (2 mg/kg intraperitoneal). All procedures were performed approximately at the same time of the day, between 11:30 AM and 01:30 PM, at the animal research laboratory of Ankara University Medical School. After sterile conditions were maintained, laparotomy was performed with a midline incision. In the sham operated group, after laparotomy was performed, the operation was terminated without manipulating the CBD (n = 5). In the remaining 36 rats, the CBD was isolated and ligated at the middle third by 5-0 polypropylene. Ligation was performed by placing a half transected catheter on the CBD and tying with a 4-0 silk suture ( Figure 2A). This ligation technique was described as a reliable modality for studies that require a reversal of OJ in animal models.5 The abdominal incision was then closed in two layers separately using 4-0 silk sutures.
|
|
|
Figure 2. Photograph of: (A) common bile duct ligation; (B) safe decompression of the dilated bile duct, over the catheter. |
Thirty-six OJ created rats were divided into six groups of six animals. Group I, Group II, and Group III were sacrificed on Day 3, Day 7, and Day 14, respectively, after the ligation. Group Id, Group IId, and Group IIId were taken to the laboratory again for decompression on Day 3, Day 7, and Day 14, respectively. The dilated CBD was found and decompressed by cutting the silk suture over the catheter. Care was taken to prevent any detrimental maneuver on the duct (Figure 2B). After the decompression, the abdomen was closed again and the rats were taken to their cages for recovery period. Seven days after the decompression, tissue samples and blood samples were taken and all rats were sacrificed with cardiac injection under ketamine/xylazine anesthesia.
2.4. Electron microscopy
After the brain tissues were removed, they were fixed in 2.5% glutaraldehyde, 0.1M phosphate buffer for 24 hours, and then postfixed with 1% osmium tetroxide. Ultrathin sections through the cerebral cortex were cut and mounted on copper grids. These were stained with 4% uranyl acetate-lead citrate, examined, and were photographed using transmission with an electron microscope (Carl-Zeiss EM 900; Carl Zeiss Inc., Thornwood, NY, USA).
2.5. Lipid peroxidation assay
MDA, the final product of lipid peroxidation, gives a reaction with thiobarbituric acid (TBA) and forms a colored complex that gives maximum adsorbance at 532 nm. For measurement of serum MDA, a fluorometric assay is used to measure this end product of the MDA-TBA reaction. By using the extraction index of MDA-TBA complex (532 nm = 1.56 × 105 mol/cm), MDA results are calculated in nmol/mL. Five hundred microliters of serum is mixed with 1 mL of TBA/trichloroacetic acid/hydrochloric acid mixture solution. After vortexing (REAX 2,000; Heidolph, Kelheim, Germany), the mixture was incubated at room temperature in microcentrifuge tubes for 5 minutes. Microcentrifuge tubes were centrifuged (Z233 MK; Hermle, Gosheim, Germany) at 10,000 rpm and supernatants were collected in glass tubes. Ten milliliters of butylated hydroxytoluene was added to each tube and vortexed. Tubes were closed and boiled for 15 minutes. If there was precipitation, it was again centrifuged at 10,000 rpm for 5 minutes. Then, samples were quantified under spectrophotometry (Milton- Roy Company Spectronic 3,000 Array, USA) at 532 nm.
2.6. Statistical analysis
Statistical analyses were performed using SPSS software version 12.0 (SPSS Inc. Chicago, IL, USA). Descriptive analyses were presented using medians and interquartile range. Nonparametric tests were used to compare the parameters between groups. The Mann-Whitney U test was performed to test the significance of pairwise differences using Bonferroni correction to adjust for multiple comparisons. The Wilcoxon test was used to compare the change in total, direct bilirubin, and MDA levels between ligation and decompression groups. An overall 5% type-I error level was used to infer statistical significance.
3. Results
All samples procured from the 41 Wistar albino rats were analyzed successfully.
3.1. Serum bilirubin and MDA levels
Total bilirubin and direct bilirubin levels in Group I, Group II, and Group III were significantly higher when compared with the sham group (p = 0.004, p = 0.008, and p = 0.004, respectively). The serum total bilirubin levels were further increased with the delayed obstruction period. With the decompression of the CBD, in all groups with decompression (Group Id, Group IId, and Group IIId), the total and direct bilirubin values were decreased. All of the value declines were also statistically significant ( Table 1).
| Total Bilirubin (mg/dL) | p value | Direct Bilirubin (mg/dL) | p value | |
|---|---|---|---|---|
| Sham | 0.02 (0.02–0.03) | 0.004υ (S vs. I) | 0.01 (0.001–0.01) | 0.004υ (S vs. I) |
| 0.008υ (S vs. II) | 0.008υ (S vs. II) | |||
| 0.004υ (S vs. III) | 0.004υ (S vs. III) | |||
| Group I | 5.46 (4.53–5.87) | 0.043ψ | 5.02 (3.99–5.4) | 0.043ψ |
| Group Id | 1.9 (1.08–3.71) | 1.65 (0.83–2.95) | ||
| Group II | 8.62 (6.9–10.76) | 0.043ψ | 7.94 (6.12–9.29) | 0.043ψ |
| Group IId | 0.42 (0.36–1.98) | 0.29 (0.22–1.63) | ||
| Group III | 7.74 (5.14–9.80) | 0.028ψ | 6.82 (4.43–8.75) | 0.028ψ |
| Group IIId | 0.46 (0.4–0.58) | 0.28 (0.24–0.47) |
All values are shown as medians (IQR 25–75), ψ Wilcoxon test, υ Mann–Whitney U test.
Abbreviations; S: Sham group, I: Group I, II: Group II, III: Group III, Id: I decompression, IId: II decompression, IIId: III decompression.
MDA levels were found to be increased in all ligation groups when compared with the control group. After decompression, serum MDA levels decreased in all groups (Table 2).
| MDA (nmol/ml) | p value | |
|---|---|---|
| Control | 1.57 (1.34–1.67) | 0.004υ (S vs. I) |
| 0.004υ (S vs. II) | ||
| 0.004υ (S vs. III) | ||
| Group I | 3.98 (3.82–4.04) | 0.028ψ |
| Group Id | 1.55 (1.48–1.64) | |
| Group II | 4.98 (4.78–5.24) | 0.028ψ |
| Group IId | 3.48 (3.38–3.59) | |
| Group III | 5.12 (4.89–5.48) | 0.028ψ |
| Group IIId | 1.51 (1.39–1.62) |
All values are shown as medians (IQR 25–75), ψ Wilcoxon test, υ Mann–Whitney U test.
Abbreviations; IQR: Interquartile range, MDA: Malondialdehyde acid, S: Sham group, I: Group I, II: Group II, III: Group III.
3.2. Electron microscopic evaluation of the brain tissue
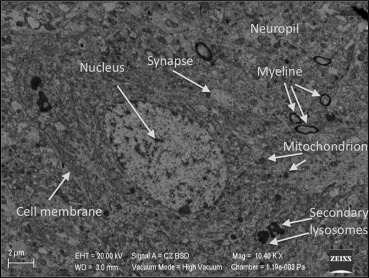
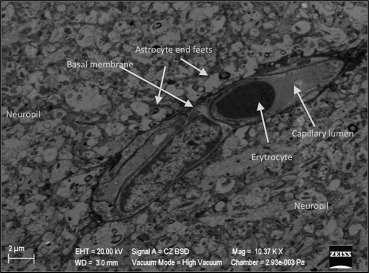
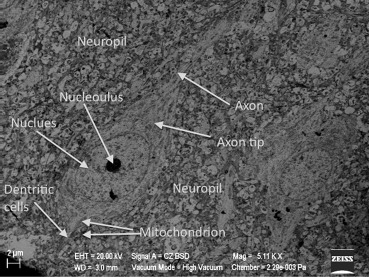
Electron microscopic evaluation of the sham operated group revealed that all organelles were in their normal configurations. Figure 3 shows a normal neuron cross-section with organelle-like mitochondria, endoplasmic reticulum, lysosome, nucleus, and more. The small figure on the left upper side of Figure 3 shows a normal neuropil-capillary relation. The end-feet of the astrocytes surrounding the capillary basal lamina and completely intact basal membrane can be seen, both needed for the healthy blood–brain barrier (Figure 4).
|
|
|
Figure 3. Electron microscopic view of the brain tissue in the sham group. All organelles are in their normal configurations. |
|
|
|
Figure 4. Normal neuropil-capillary relation in the sham group. |
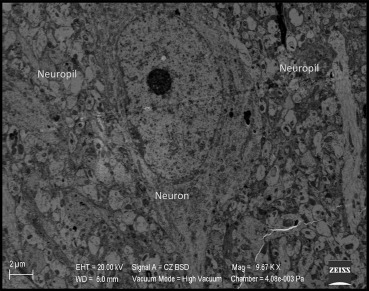
In Group I, neurons were still in their normal configurations with their organelles. The neuropil-capillary relation seems to be almost unchanged when compared with the sham group.
Seven days after decompression, the neuron cross-section was normal and no change was found in the organelles. When comparing the sham group with Group I and Group Id, the neuropil and precapillary astrocyte connections also remained unchanged (Figure 5 ; Figure 6).
|
|
|
Figure 5. Normal neuron and neuropil in Group I. |
|
|
|
Figure 6. Normal neuron and neuropil in Group Id. |
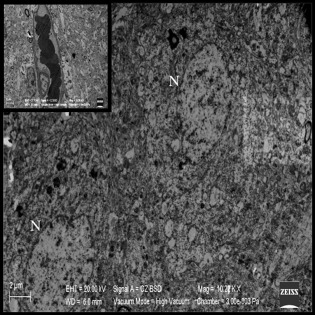
When examining Group II, we found precapillary astrocytic edema in only some of the capillaries. Also, a small amount of electron dense neurons were seen. These two changes represent the apoptosis and blood–brain barrier deterioration (Figure 7).
|
|
|
Figure 7. Precapillary astrocytic edema (smaller figure on the bottom right) and electron dense neurons (EDN) in Group II. |
After decompression (Group IId), the quantity of electron dense neurons and precapillary astrocytic edema were significantly decreased. This finding represents the recovery of the damage after decompression (Figure 8).
|
|
|
Figure 8. Reversibility of precapillary astrocytic edema and neuronal injury after decompression in Group IId. The small figure on the top left; normal neuropil capillary interaction, the BBB is intact. BBB = Blood Brain Barrier; N = neuron. |
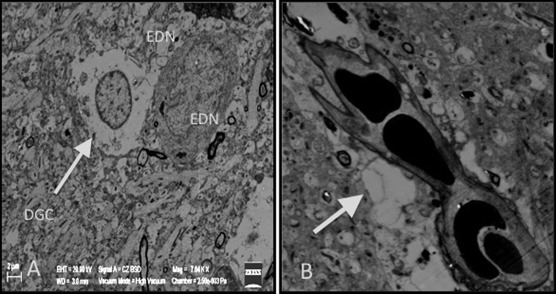
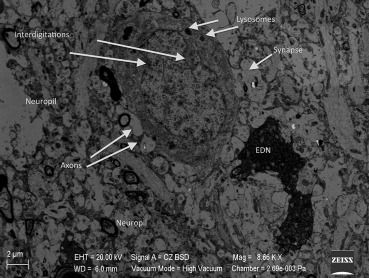
In Group III, swollen and degenerated glial cells as well as deformation of the neurons were extremely noticeable. These findings were present in nearly all cross-sections. In Figure 9, degenerated glial cells and apoptotic electron dense neurons are seen together. These apoptotic neurons were increased in quantity when compared with the other groups. In nearly all capillaries, edema was observed in the astrocyte end-feet and at the basal membrane of the blood–brain barrier. In this group, we found that there was an increased number of secondary lysosomes in neurons and an expansion of synapses neighboring the neuronal membrane. Flattening and separation of the myelin formation were also detected in the neuropil (Figure 9 ; Figure 10).
|
|
|
Figure 9. (A) Increase in the apoptotic, degenerated glial cells (DGC) and electron dense neurons (EDN) in Group III. (B) Precapillary astrocytic edema (thick arrow). |
|
|
|
Figure 10. Interdigitations in the neuron nucleus and a noticeable increase in the amount of lysosomes in Group III, enlargement of the synapses, and an electron dense neuron (EDN). |
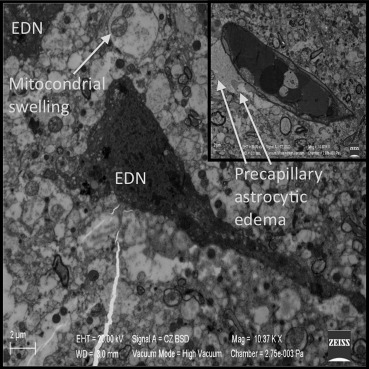
In Group IIId, damage in the glial cells, neurons, and precapillary astrocytes was present and despite decompression, the damage did not decrease when compared with Group III. This finding suggests that the damage is irreversible in this group. The quantity of electron dense neurons were increased. Precapillary astrocytic edema remained, and moreover, swelling and cristalysis of the mitochondria were found in the axons. This change provides more proof of the irreversible damage (Figure 11).
|
|
|
Figure 11. Irreversible damage in the glial cells, neurons, and precapillary astrocytes, despite the decompression in Group IIId. EDN = electron dense neuron. |
4. Discussion
Despite new medical and surgical therapies available and used today, OJ still has high morbidity and mortality rates. The present study reveals some important changes and highlights some unknown parts of brain injury pathogenesis in OJ. There are many studies about traumatic brain injury, HE, stroke, and other neurological disorders.6 ; 7 Although the mechanisms and details are not fully understood about these disorders, several studies that were published by experienced scientists discovered some important details about the disease progression and these findings gave enthusiasm to investigators for new research.
Hyperammonemia has a critical role in the pathogenesis of HE, however, a direct correlation between the severity of HE and ammonia levels has not been found. HE was found to be present even in some patients with normal ammonia levels. Therefore, some other factors are charged in the progression of HE. Interleukin-1β, which is formed by astrocytes in peripheral inflammation, induces nitric oxide, superoxide, and prostaglandins. These increase the detrimental effects of hyperammonia in the brain.8 ; 9
In acute and chronic liver failure, different abnormal alterations of the brain functions occur by means of HE. These brain disturbances include abnormal transmission, energy impairment, brain edema, and neuroinflammation.7 Previous studies suggested the role of oxidative stress which plays an important role in the pathogenesis of acute central nervous system injury.10 In the formation of HE, many studies have reported the role of activating microglial cells and chronic glial edema with subsequent alterations of glioneuronal communication in the brain.11; 12 ; 13
In OJ, reactive oxygen species (ROS) formation leads to lipid peroxidation in tissues, and this finally results in cell injury. The increase in MDA level is an indicator for injury formation. The decreased levels of MDA after decompression, reveals both a decrease in the oxidative injury and an increase in the activity of the antioxidant systems. In our study groups, after CBD ligation, serum levels of the total bilirubin and direct bilirubin were found to be increased progressively in parallel to the ligation time of the CBD. When bilirubin levels for Group II and Group III were compared, one would expect higher levels as the obstruction proceeds. However, our results show that there was not a significant difference in bilirubin levels between Group II and Group III. Furthermore MDA levels were not increased in Group IIId when compared with Group IId. Prolonged obstruction did not cause higher bilirubin levels and more severe oxidative injury to the brain tissue. We believe that the limited numbers of animals in each study group might cause this controversy. It should be noted that, regarding the laboratory findings, one defective result might affect the overall group result more than expected. Moreover, when the obstruction period prolongs antioxidant systems are also taking affect, and there is a point in time where oxidants and antioxidants are nearly on balance. This could be another explanation, however, this hypothesis could only be shown and proven by new research designs that strongly emphasize this topic.
After decompression these values were found to be decreased as predicted. MDA levels were found to be increased in all ligation groups and decreased after decompression. These results are all statistically significant and show that the model of CBD ligation used is rather effective.
In OJ, bile salt concentration increases in liver cells. Inflammatory cells like leukocytes and macrophages secrete ROS which cause cell injury in hepatocytes.14 ; 15 Bile salts effect the mitochondria electron transport chain negatively, and augment free oxygen radical production that causes oxidative stress.16 Additionally, bile salts decrease the antioxidant enzymes like superoxide dismutase and catalase. ROS cause injury by protein denaturation, mutations in DNA, and lipid peroxidation. With all of these changes, the cell membrane stabilization is destroyed and cell death occurs. In OJ, augmented ROS in the tissue may lead to peroxidation in the unsaturated fatty acids in the cell membrane. This peroxidation eventually results in the formation of MDA. Therefore, MDA formation is the indicator for lipid peroxidation. Different studies showing an increase of MDA levels in experimental models of OJ confirms our results.17 ; 18
Free oxygen radicals are formed from oxygen, which is used in mitochondria, in order to provide energy for brain tissues. It has been shown that oxidative stress plays a major role in the pathogenesis of acute central nervous system injury. Free radicals damage lipids, proteins, and nucleic acids in the brain and this damage leads to necrosis and apoptosis in the brain cells. The damage exacerbates as the cell antioxidant mechanisms also weaken.19 ; 20 Furthermore, acute brain injury induces excitotoxic amino acids especially glutamate. This leads to an increase in ROS and results in parenchymatous destruction.21 ; 22 Electron microscopic evaluation in our study revealed some important ultrastructural changes in the brain tissue due to oxidative stress at different time periods. In the early phase of oxidative stress, necrosis and apoptosis was not significant in our model. In Group I rats, the bilirubin and MDA levels were increased, however, the electron microscopic neuropil-capillary relation remained steady. When obstruction proceeds with time (Group II rats), precapillary astrocytic edema with destruction in tight junctions and electron dense neurons were found to be increased.
The role of free radicals in the pathogenesis of ischemia was described in several studies.23 ; 24 It was shown that, especially superoxide, which formed in the reperfusion phase, combines with nitric oxide and forms peroxynitrite.25 Love19 suggested that in transient brain ischemia, free radicals and related reactive chemical species are responsible from the majority of the damage. In other related studies, authors suggested that activation of nuclear factor-κB in astrocytes and microglias results in increased expression of nitric oxide synthase and increased nitric oxide production. These cells form proinflammatory cytokines, neurotoxic free oxygen radicals, and excitotoxins that are responsible for ischemic neuronal death.26 ; 27 Chan28 showed that the increase in free oxygen radicals causes damage in the blood–brain barrier, which leads to apoptosis, necrosis, or both. In our study, by the means of blood–brain barrier deterioration, we found that in Group II the damage regressed after the internal drainage period of 7 days. However, in Group III, in some brain parts the tissue damage was increased despite the decreased values of oxidative stress and bilirubin. These findings of programmed cell death (apoptosis) are presumably because of the injured neuropil-capillary relation that provokes a destructed blood–brain barrier and this leads to oxidative injury affecting the neuronal cells.
Neurological symptoms like mental status, intellectual functioning impairments, alertness and/or motor function deficits are frequently seen in the patients with OJ. These symptoms and the correlation of these symptoms with the disease are not fully understood. Oxidative injury resulting from jaundice and several other reasons were charged for these neurological complications. Although there are multiple studies based on CBD ligation related encephalopathy and impaired brain functions in rats, more studies are needed to combine these with ultrastructural findings to investigate clinical neurological manifestations of the disease.29 ; 30 In our study, the animals with bile duct obstruction were morbidly weak starting from nearly the first days of the obstruction. There were some noticeable motor disturbances especially in the late stages of the obstruction.
There are some limitations of this study. In daily practice, patients with hepatic coma have shown improvement months down the line after supportive measures are instituted. Thereby, in our study, the findings could resolve if the decompression time extended >7 days. However, it seems to be controversial and still an open question as to whether there is an actual stage for reversibility in neuronal death or not.31 Moreover, it should be kept in mind that these findings are based on an animal model and might not be directly converted to human models.
Another important limitation is that the ultrastructural change of the brain during OJ might be caused by different mechanisms. In addition to oxidative stress, there are numerous mechanisms like metabolic dysregulations, extrahepatic cholestasis, bacterial transformation, and other organ dysfunctions that have been reported to contribute to psychoneurological changes during OJ.32; 33 ; 34 The experimental design does not rule out other factors that may lead to ultrastructural changes in the brain tissue.
There are many studies on acute and chronic liver failure and their effects on brain functions.35 ; 36 However, this is one of the first studies that shows important ultrastructural changes in the brain tissue related to OJ. Furthermore, as far as we know, this is the first study that emphasizes the reversibility of brain injury before neuronal death occurs in OJ.
To summarize, the strengths of the present study are, firstly, based on electron microscopic findings and oxidative stress it is suggested that 7–14 days is a critical time for the reversibility of brain injury. Secondly, it shows some important details of the ultrastructural damage developed in the brain tissue. We think that these finding are extremely important, and if the critical time is missed major ultrastructural damage starts especially in the neurons, blood–brain barrier, and mitochondria. In daily clinical knowledge, as the signs of alterations in the brain functions and many other neurological findings start, most clinicians are aware of the irreversibility of the disease. It is also unreliable to trace neurological changes with serum levels of bilirubin and free radicals. Therefore, it is critical to plan the medical therapies and timing of drainage.
For patients with OJ, further studies are needed to focus on the timing of the drainage procedures, in relation to encephalopathy progression. Because there are so many controversies to be solved, this study may provide a base for future research to revise the specific method for gathering data.
References
- 1 S.F. Assimakopoulos, C.E. Vagianos, N. Patsoukis, C. Georgiou, V. Nikolopoulou, C.D. Scopa; Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats; Acta Physiol Scand, 180 (2004), pp. 177–185
- 2 P. Ljubuncic, Z. Tanne, A. Bomzon; Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease; Gut, 47 (2000), pp. 710–716
- 3 R. Jalan, D. Shawcross, N. Davies; The molecular pathogenesis of hepatic encephalopathy; Int J Biochem Cell Biol, 35 (2003), pp. 1175–1181
- 4 A.H. Lockwood, E.W. Yap, W.H. Wong; Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy; J Cereb Blood Flow Metab, 11 (1991), pp. 337–341
- 5 M.T. Oruç, M.M. Ozmen, U. Han; A new technique for inducing and releasing obstructive jaundice in rats; Eur Surg Res, 43 (2009), pp. 354–359
- 6 M. Moriyama, A.R. Jayakumar, X.Y. Tong, M.D. Norenberg; Role of mitogen-activated protein kinases in the mechanism of oxidant-induced cell swelling in cultured astrocytes; J Neurosci Res, 88 (2010), pp. 2450–2458
- 7 R. Garcia-Martinez, J. Cordoba; Acute-on-chronic liver failure: the brain; Curr Opin Crit Care, 17 (2011), pp. 177–183
- 8 V. Lachmann, B. Görg, H.J. Bidmon, V. Keitel, D. Häussinger; Precipitants of hepatic encephalopathy induce rapid astrocyte swelling in an oxidative stress dependent manner; Arch Biochem Biophys, 536 (2013), pp. 143–151
- 9 J. Licinio, M.L. Wong; Pathways and mechanisms for cytokine signaling of the central nervous system; J Clin Invest, 100 (1997), pp. 2941–2947
- 10 D. Konstantinou, A. Mavrakis, K. Grintzalis; Quantification of superoxide radical in the brain of rats with experimentally induced obstructive jaundice; Neurochem Res, 33 (2007), pp. 1101–1105
- 11 R.F. Butterworth; Altered glial-neuronal crosstalk: cornerstone in the pathogenesis of hepatic encephalopathy; Neurochem Int, 57 (2010), pp. 383–388
- 12 I. Zemtsova, B. Görg, V. Keitel, H.J. Bidmon, K. Schrör, D. Häussinger; Microglia activation in hepatic encephalopathy in rats and humans; Hepatology, 54 (2011), pp. 204–215
- 13 R. Rodrigo, O. Cauli, U. Gomez-Pinedo, A. Agusti, V. Hernandez-Rabaza; Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy; Gastroenterology, 139 (2010), pp. 675–684
- 14 F. Bingol, S. Aydin, S. Acikgoz; Free radicals; Medic J Ankara Hosp, 28 (1993), pp. 1–23
- 15 B. Halliwell; Oxygen radicals as key mediators in neurological disease: fact or fiction?; Ann Neurol, 32 (1992), pp. 10–15
- 16 S. Singh, G. Shackleton, E. Ah-Sing, J. Chakraborty, M.E. Bailey; Antioxidant defenses in the bile duct-ligated rat; Gastroenterology, 103 (1992), pp. 1625–1629
- 17 N. Alptekin, G. Mehmetçik, M. Uysal, G. Aykaç-Toker; Evidence for oxidative stress in the hepatic mitochondria of bile duct ligated rats; Pharmacol Res, 36 (1997), pp. 243–247
- 18 M. Moran, M.T. Oruc, M.M. Ozmen, et al.; Effect of erythropoietin on oxidative stress and liver injury in experimental obstructive jaundice; Eur Surg Res, 43 (2009), pp. 228–234
- 19 S. Love; Oxidative stress in brain ischemia; Brain Pathol, 9 (1999), pp. 119–131
- 20 Y. Gilgun-Sherki, Z. Rosenbaum, E. Melamed, D. Offen; Antioxidant therapy in acute central nervous system injury: current state; Pharmacol Rev, 54 (2002), pp. 271–284
- 21 J.T. Coyle, P. Puttfarcken; Oxidative stress, glutamate, and neurodegenerative disorders; Science, 262 (1993), pp. 689–695
- 22 K. Ohara, M. Aoyama, M. Fujita, K. Sobue, K. Asai; Prolonged exposure to ammonia increases extracellular glutamate in cultured rat astrocytes; Neurosci Lett, 462 (2009), pp. 109–112
- 23 I.M. Cojocaru, M. Cojocaru, V. Sapira, A. Ionescu; Evaluation of oxidative stress in patients with acute ischemic stroke; Rom J Intern Med, 51 (2013), pp. 97–106
- 24 D. Radak, I. Resanovic, E.R. Isenovic; Link between oxidative stress and acute brain ıschemia; Angiology, 65 (2014), pp. 667–676
- 25 C. Cazevieille, A. Muller, F. Meynier, C. Bonne; Superoxide and nitric oxide cooperation in hypoxia/reoxygenation-induced neuron injury; Free Radic Biol Med, 14 (1993), pp. 389–395
- 26 A. Schneider, A. Martin-Villalba, F. Weih, J. Vogel, T. Wirth, M. Schwaninger; NF-kappaB is activated and promotes cell death in focal cerebral ischemia; Nat Med, 5 (1999), pp. 554–559
- 27 M.P. Mattson, S. Camandola; NF-kappaB in neuronal plasticity and neurodegenerative disorders; J Clin Invest, 107 (2001), pp. 247–254
- 28 P.H. Chan; Reactive oxygen radicals in signaling and damage in the ischemic brain; J Cereb Blood Flow Metab, 21 (2001), pp. 2–14
- 29 C. Giménez-Garzó, D. Salhi, A. Urios, et al.; Rats with mild bile duct ligation show hepatic encephalopathy with cognitive and motor impairment in the absence of cirrhosis: effects of alcohol ingestion; Neurochem Res, 40 (2015), pp. 230–240
- 30 R. Leke, D.L. Oliveira, B.H. Mussulini, et al.; Impairment of the organization of locomotor and exploratory behaviors in bile duct-ligated rats; PLoS One, 7 (2012), p. e36322
- 31 G.C. Higgins, P.M. Beart, Y.S. Shin, M.J. Chen, N.S. Cheung, P. Nagley; Oxidative stress: Emerging mitochondrial and cellular themes and variations in neuronal injury; J Alzheimers Dis, 20 (2010), pp. 453–473
- 32 P.W. Kaplan, R. RSutter; Seeing more clearly through the fog of encephalopathy; J Clin Neurophysiol, 30 (2013), pp. 431–434
- 33 D.N. Ruskin, S.A. Masino; The nervous system and metabolic dysregulation: emerging evidence converges on ketogenic diet therapy; Front Neurosci, 6 (2012), p. 33
- 34 A.M. Dell'anna, S. Scolletta, K. Donadello, F.S. Taccone; Early neuroprotection after cardiac arrest; Curr Opin Crit Care, 20 (2014), pp. 250–258
- 35 V. Felipo; Hepatic encephalopathy: effects of liver failure on brain function; Nat Rev Neurosci, 14 (2013), pp. 851–858
- 36 O. Cauli, M. Llansola, A. Agustí, R. Rodrigo, V. Hernández-Rabaza; Cerebral edema is not responsible for motor or cognitive deficits in rats with hepatic encephalopathy; Liver Int, 34 (2014), pp. 379–387
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?