Abstract
Background
It has been reported that contractility, as assessed using dobutamine infusion, is independently associated with reverse remodeling after CRT. Controversy, however, exists about the capacity of this approach to predict a long-term clinical response. This studys purpose was to assess whether long-term CRT clinical effects can be predicted according to acute inotropic response induced by biventricular stimulation (CRT on), as compared with AAI–VVI right stimulation pacing mode (CRT off), quantified at the time of implantation.
Methods
In 98 patients (ejection fraction 29 ± 10%), acute changes in left ventricular (LV) elastance (Ees), arterial elastance (Ea), and Ees/Ea, as assessed from slope changes of the force–frequency relation obtained when the heart rate increased, and also assessed while measuring triplane LV volumes and continuous noninvasive blood pressure, were related to death or rehospitalization during a 3-year follow-up. Other covariances tested were age, gender, disease etiology, QRS duration, amount of mitral regurgitation, LV diastolic volume, ejection fraction, and the degree of asynchrony and longitudinal strain at baseline.
Results
There was a marked increment in the Ees slope with CRT (interaction P = 0.004), no Ea change, and modest Ees/Ea increase (interaction P < 0.05). In Cox analysis, however, neither slope changes nor baseline values of Ees, Ea, and Ees/Ea were associated with long-term follow-up. Only ventricular diastolic volume (direct relation P = 0.002) and QRS duration (inverse relation P = 0.009) predicted death/rehospitalization.
Conclusions
Acute contractile recovery in CRT patients is not associated with 3 years prognosis. Instead, death or rehospitalization can be predicted from QRS duration and LV diastolic volume at baseline.
Abbreviations
CRT, biventricular stimulation;DYS, dyssynchrony;Ea, arterial elastance;EDV, end-diastolic volume;Ees, ventricular elastance;EF, ejection fraction;FFR, force–frequency relation;HR, hazard ratio;LV, left ventricle;MR, mitral regurgitation;r2, adjusted r squared;TUS, temporal uniformity of strain
Keywords
Dyssynchrony;Resynchronization;Force–frequency relation;Speckle-tracking echocardiography;Congestive heart failure
1. Introduction
Studies have reported that contractility, as assessed using dobutamine infusion, is independently associated with reverse remodeling after biventricular stimulation (CRT) [1] ; [2]. Controversy, however, exists about the capacity of the inotropic challenge to predict a long-term clinical response. A multicenter, prospective, observational study of left ventricular (LV) contractile reserve, as assessed using low-dose dobutamine infusion, was unable to demonstrate a significant difference in cardiac survival between patients who did or did not achieve an absolute increase in LV ejection fraction > 5 points during the drug infusion [3]. Only the combination of cardiac survival and/or heart failure hospitalization as a clinical end-point demonstrated a significant association with the inotropic challenge [3].
Increasing heart rates have long been recognized as a potential modulator of systolic function [4]. In the normal heart, the force of contractions is augmented by an increase in heart rate, while in the failing heart, alterations in the force–frequency relation (FFR) have been identified which potentially contribute to an impaired capacity for exercise [5]. FFR has been shown to be capable of recruiting cardiac contractility in the failing heart in excess of the CRT effect, with this positive FFR contributing significantly to the enhanced capacity for exercise that most of the patients exhibit after the device implantation [6].
Thus, the purpose of this study was to assess whether and to what extent the acute gain in contractility induced by CRT, as assessed during increasing heart rate, is affecting long-term follow-up in terms of rehospitalization or death of patients evaluated at the time of the device activation.
2. Materials and methods
Ninety-eight nonconsecutive patients (mean age 70.6 ± 8.3 years, 73 males) with congestive cardiomyopathy of nonischemic (n = 48) or ischemic etiology (n = 50), LV ejection fraction (EF) < 0.35 (0.30 ± 0.09), and QRS duration > 120 ms (170 ± 28 ms), with left bundle branch block morphology, except for 4 patients who exhibited a right bundle branch block with left axis deviation, were prospectively studied (Table 1). Ischemic cardiomyopathy was considered in the presence of a documented previous myocardial infarction or a significant coronary artery disease (luminal narrowing > 70%) shown through coronary angiography. Optimal revascularization had been previously performed in those patients. Nonischemic cardiomyopathy was considered only in the presence of angiographically “normal” coronary arteries. The patients, who fulfilled current criteria for CRT implantation, were selected to be in spontaneous sinus rhythm, except for 8 patients who were permanently bradyarrhythmic because of atrial fibrillation and 11 patients with pacing-induced rhythm for various degrees of atrioventricular block. In all these subjects a VVI pacemaker-induced regular rhythm could be guaranteed during the entire procedure (see below).
| N | 98 |
|---|---|
| Age, years | 71.2 ± 8.3 |
| Gender (M/F), n | 73/25 |
| BSA, m2 | 1.84 ± 0.18 |
| QRS width, ms | 170 ± 30 |
| Diabetes mellitus, % | 30.4 |
| NYHA functional class | 2.7 ± 0.7 |
| Device (ICD/PM), n | 70/28 |
| Etiology of heart disease, n | |
| Idiopathic | 31 |
| Ischemic | 43 |
| Valvular | 9 |
| Ischemic/valvular | 7 |
| Others (post-hypertensive, postpartum, tachicardiomyopathic, toxic) | 8 |
| Therapy, % | |
| ACE inhibitors/ARB blockers | 72 |
| Amiodarone | 33 |
| Antialdosterone | 20 |
| Anticoagulants | 24 |
| Antiplatelets | 64 |
| Beta-blockers | 76 |
| Ca++ channel blockers | 6 |
| Digitalis | 16 |
| Diuretics | 82 |
| Nitrates | 25 |
| ECG rhythm, n | |
| Spontaneous sinus rhythm | 79 |
| Permanent atrial fibrillation | 8 |
| Advanced atrioventricular block | 11 |
Four patients had been previously implanted with an aortic prosthesis (2 mechanical and 2 biological valves) while 7 had received a mechanical (n = 3) or a biological (n = 4) prosthesis in the mitral position. Three patients had received a mitral plus an aortic mechanical prosthesis, while 2 more patients had undergone mitral valve reconstructive surgery. In all subjects written informed consent was obtained in accordance with institutional human review studies committee guidelines and local institutional review board approval. The study complies with the Declaration of Helsinki.
3. Echocardiographic measurements
Standard echocardiographic examinations were performed on all patients using a Vivid 7 or Vivid E9 digital ultrasound system (GE Medical Systems, Horten, Norway). Cardiac cycles were stored in digital, cineloop format for off-line analysis performed with a dedicated software package (EchoPac PC, BT11 version; GE Healthcare). Two-dimensional strain is a novel non-Doppler-based method to evaluate strain from standard two-dimensional acquisitions [7]. By tracing the endocardial contour on an end-systolic frame, the software will automatically track the contour on subsequent frames. Optimal tracking could be verified in real-time and corrected by adjusting the region of interest or manually correcting the contour. A minimum frame rate of 30 Hz was required for reliable operation of this software, and frame rates of 30 to 80 Hz were used for routine gray-scale imaging.
Two-dimensional longitudinal strains were assessed in 2 orthogonal apical views (4- and 2-chambers, 12 segments) starting from the septal and the inferior atrioventricular wall junction, respectively, and averaging strains among various segments. The two-dimensional strain software had to adequately track > 80% of the attempted segments in order to make the analysis acceptable.
Ventricular volumes were obtained using 3 real-time simultaneous longitudinal planes, as imaged from the apical approach, and then manually tracing the endocardial border with built-in software. The papillary muscles were excluded from the tracing. A triangular mesh was constructed by 3D interpolation between the traces, and end-diastolic volume and end-systolic volume were calculated by surface triangulation and summation of all triangles by the divergence theorem [8]. Bland and Altmans analysis of LV volumes and EF has previously demonstrated closer agreement between echocardiography and MRI results using triplane imaging, as compared with biplane imaging, both precontrast and with LV opacification [9].
Evaluation of mitral regurgitation (MR) was graded quantitatively according to the area of the regurgitant jet in a 4-chamber view using the color Doppler data [10] and expressed as a ratio relative to the atrial cavity area.
4. Asynchrony quantitation
Data for longitudinal strain curves were exported as digital matrixes to a proprietary open-source analysis software (StrATo version 2.0.3.0). Dyssynchrony (DYS) was quantified by TUS (temporal uniformity of strain), where a time plot of regional strains, arranged for ventricular location, was subjected to a Fourier analysis [11]. In cases of a perfectly synchronous ventricle, the plot appeared as a straight line, with power only in the 0-order Fourier term, whereas regionally clustered DYS generated an undulating plot with higher power in the 1-order term [12]. The TUS index, which reflects the 0-order relative to 1-order plus 0-order power, synthesized DYS motion data because this temporal variance index could distinguish between geographically clustered regions of shortening, which are out of phase compared to those dispersed throughout the wall [13]. Due to the large number of computations associated with dyssynchrony calculation, one single beat was used for each view. Intraobserver reproducibility for TUS measurements, as assessed in the longitudinal plane in 15 patients at baseline heart rate (CRT off/on) and computed as absolute mean difference ± the percentage coefficient of variation (SD/mean), averaged 0.11 ± 1.0%, a value similar to what our group previously reported [14].
5. Echocardiography protocol
Within 2–3 days after CRT implantation, patients underwent echocardiographic-guided optimization (n = 77) or using the Quick-Opt/Smart-Delay algorithm available with St. Jude/Boston Scientific devices (n = 21), respectively. Interventricular delay was set fixed at 0 ms. After optimization was obtained the device was programmed into one of 2 basic modes: either AAI (right atrial pacing for patients with spontaneous rhythm) or VVI (right ventricular pacing for patients with permanent atrial fibrillation or advanced atrioventricular block) pacing mode vs. DDD-CRT (biventricular) pacing mode, in a random sequence. In particular, during the AAI pacing mode, care was taken to confirm that the ventricular activation conformed to a left bundle branch pattern.
6. Force–frequency relation (FFR) testing
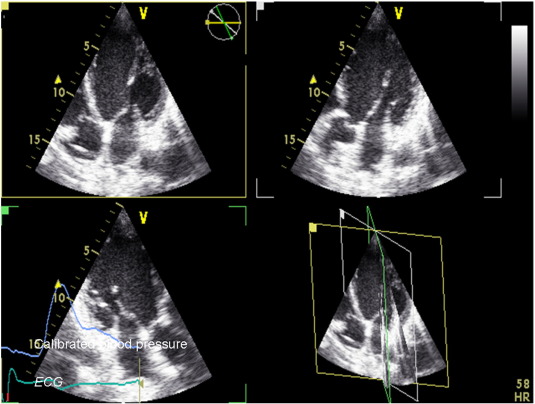
Pacing was initiated at spontaneous heart rate, or at fixed 70 beats/min in those patients with permanent atrial fibrillation or atrioventricular block, with 2 subsequent steps at 100 and 120 beats/min lasting for approximately 2 min. The other pacing mode was used to repeat the same sequence after a few minutes delay. During each step, continuous blood pressure measurements (taken with the right arm) were recorded using a digital photoplethysmography device capable of providing accurate beat-to-beat systolic and diastolic values (Finapress, Omeda 2300, Monitoring Systems) [15]. Continuous blood pressure signals were also visible on the screen of the echo machine (Fig. 1). After the protocol was completed, the device was reprogrammed in DDD-CRT mode with a fixed optimized AV and VV interval, with scheduled clinical follow-ups and device checks.
|
|
|
Fig. 1. Methodology adopted for the study. Ventricular volumes were obtained using real-time 3 apical simultaneous longitudinal planes and then by manually tracing the endocardial border with in-built software. Calibrated continuous blood pressure, together with ECG signal, was also available on the screen of the echo machine. |
7. Effective arterial elastance and ventricular–vascular coupling
Arterial load and stiffness were indexed by the effective arterial elastance (Ea), which is equal to the ratio of ventricular end-systolic pressure (estimated as systolic blood pressure times 0.9) divided by stroke volume, as assessed from the triplane volume [16]. Although Ea does not directly reflect stiffness per se, it combines various aspects of the total arterial input impedance into an effective stiffness, comprising resistance, compliance, and characteristic impedance of the arterial vascular bed [17]. Ventricular contractility (Ees) was indexed by the end-systolic pressure–volume ratio, neglecting the volume intercept of the end-systolic pressure–volume relationship [18]. Ventricular–vascular coupling was indexed by the Ees/Ea ratio.
8. Statistical analysis
Data are expressed as mean ± 1 SD (unless otherwise stated), with the Kolmogorov–Smirnov test used to assess normality of data. Differences between means were assessed using paired and unpaired t-tests as appropriate. A signed-rank test was used if data were not normally distributed.
A two-way repeated-measures analysis of variance was used to assess the effects of progressively increasing the heart rate over time on changes in ventricular volumes, Ees, Ea, and their ratio (Ees/Ea) in the overall population, with the effect of pacing mode (DDD-CRT vs. AAI–VVI) as a between-patient factor. The Holm–Sidak test was used for multiple pairwise comparisons. Furthermore, in each patient regression analysis (least-squares method) was used to assess the slope of the FFR relation obtained plotting Ees, Ea, and their ratio (dependent variables) against increasing heart rate (independent variable) during DDD-CRT vs. AAI–VVI pacing mode.
A best subsets regression model was then developed in order to identify which subset of baseline clinical or echocardiographic variables (slope of Ees, Ea, and Ees/Ea ratio obtained in AAI–VVI pacing mode and its significant CRT-induced acute change, along with age, QRS duration, amount of mitral regurgitation, LV diastolic volume, ejection fraction, TUS, and longitudinal strain at baseline) best contributed to predicting heart failure and rehospitalization or death. The adjusted r squared value (r2) was used to measure how well the selected set described the dependent variable, taking into account the number of independent variables. Then, a Cox stepwise regression model was developed in order to identify which of those selected variables, beside gender and etiology of cardiac disease (ischemic vs. nonischemic), predicted heart failure, rehospitalization, or death (whichever came first) during a 3-year follow-up period. Results of the Cox proportional hazards model are presented as hazard ratio (HR) [95% CI].
We reasoned that 98 patients would allow us to detect a 0.0053 mm Hg/ml/m2/beats/min Ees slope change (CRT off/on) during heart rate increments among those subjects that experienced rehospitalization due to heart failure, or death, over a 3-year follow-up period, compared to those who did not, with a power = 0.80 and α = 0.05, assuming a Ees slope SD equal to 0.013 mm Hg/ml/m2/beats/min.
Finally, a Kaplan–Meier survival analysis was performed using log-rank statistics and a post hoc Holm–Sidak test. A P value < 0.05 was considered to be significant. Statistical analyses were performed using SigmaPlot (version 12.5 for Windows, Jandel; San Rafael, CA) statistical software.
9. Results
Ventricular cavities at baseline (measured during AAI–VVI pacing mode) were markedly dilated (90.1 ± 27.1 ml/m2 for diastolic, 64.7 ± 23.8 ml/m2 for systolic volumes) and pump function was severely depressed (EF 0.29 ± 0.10). Longitudinal strain was also depressed (− 7.6 ± 3.5%), as reported in comparable populations [19].
9.1. Effects of FFR
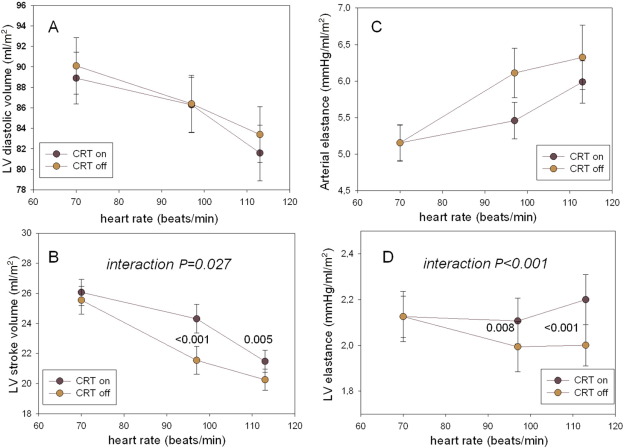
No patient complained of angina during the pacing protocol, and in all patients data were re-evaluated after CRT was activated. Fig. 2 illustrates assessment of FFR during different modes of stimulation, for ventricular volumes, Ea, and Ees.
|
|
|
Fig. 2. Assessment of FFR during different modes of stimulation, as far as diastolic and ventricular filling volumes, Ees, and Ea are concerned. There is no difference in diastolic volume between AAI/VVI (CRT off) vs. biventricular pacing mode (CRT on) during FFR, although cavity declines significantly with heart rate increments (P < 0.001, A). Such ventricular volumetric decrement during FFR was true for stroke volumes too (P < 0.001 for trend), but with a significant interaction between time-changes in ejected blood during DDD-CRT (− 10 ± 44%) compared to AAI–VVI pacing mode (− 12 ± .45%, P = 0.027 for interaction) (B). This relative smaller reduction in stroke volume with DDD-CRT developed with no difference in Ea between the 2 pacing modes (NS for interaction), although overall Ea increased progressively with increasing heart rates (P < 0.001 for trend, C). As far as inotropic challenge was concerned, Ees increased significantly during heart rate increments in DDD-CRT, whereas it decreased in AAI–VVI pacing mode (D, interaction P < 0.001). For Ea and Ees, data are displayed as absolute changes, normalized to the index (CRT off) baseline value. |
There were no differences in diastolic volumes between DDD-CRT vs. AAI–VVI pacing modes during FFR, with volumes declining significantly with heart rate increments (P < 0.001 for trend, Fig. 2A). Such ventricular volumetric decrement during FFR was also true for stroke volumes (P < 0.001 for trend), but with a significant interaction between time-changes in ejected blood during DDD-CRT pacing mode (− 10 ± 44%) as compared with AAI–VVI pacing mode (− 12 ± 45%, P = 0.027 for interaction) (Fig. 2B). A relatively smaller reduction in stroke volume with DDD-CRT pacing mode developed, with no difference in Ea between the 2 pacing modes (NS for interaction), although overall Ea increased progressively with increasing heart rates (P < 0.001 for trend, Fig. 2C).
As far as inotropic challenge was concerned, Ees increased significantly during heart rate increments in DDD-CRT pacing mode, whereas it decreased in AAI–VVI pacing mode ( Fig. 2D, interaction P < 0.001). This caused a modest improvement in the Ees/Ea ratio during DDD-CRT pacing mode (P = 0.03 for interaction), although the ratio overall decreased significantly during FFR testing (P < 0.001 for trend [data not shown]).
9.2. Assessment of discoordination and pacing mode
Effects of CRT vs. AAI–VVI pacing modes, as assessed at baseline, demonstrated a significant improvement in ventricular discoordination (TUS from 0.56 ± 0.16 to 0.61 ± 0.14, P < 0.03), albeit not mirrored by any significant change in longitudinal strain generation (from − 7.6 ± 3.5% to − 7.7 ± 3.1%, P = 0.31). There was also a borderline reduction in the amount of mitral regurgitation with CRT on (jet area/atrial area from 0.11 ± 0.11 to 0.09 ± 0.10, P = 0.067), compared to with AAI–VVI pacing mode, as computed at baseline.
9.3. Clinical outcomes
Follow-up data (mean duration 849 ± 443 days), obtained from hospital records and/or telephone interviews with patients or their relatives, were available for all subjects. A total of 24 patients reached the end-point (8 deaths, 16 patients with rehospitalization due to heart failure).
According to paired testing only the acute change in the Ees slope (from − 0.003 ± 0.013 to + 0.002 ± 0.013 mm Hg/ml/m2/beats/min, P = 0.004), taken as a cumulative descriptor of the inotropic ventricular response to CRT, was included in the multivariate analysis, besides the other analysis already indicated covariates. Changes in the Ea and in Ees/Ea slopes were not significant and thus they were not considered further.
Three variables (diastolic ventricular volume, QRS duration, and acute change in Ees slope) were finally identified (best adjusted r2 0.082) and used, with gender and etiology of cardiac disease (ischemic vs. nonischemic), in the Cox regression analysis. The Cox model showed that only diastolic ventricular volume (HR 1.03 [95% CI 1.01–1.04], P = 0.001) and QRS duration (HR 0.98 [95% CI 0.96–1.00], P = 0.019) at baseline, during AAI–VVI pacing mode, predicted heart failure exacerbations, requiring hospitalization or leading to death, at follow-up. No prognostically significant contribution could be obtained from the other covariates included in the model.
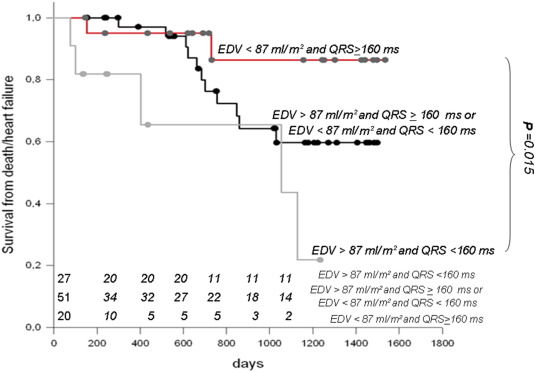
In order to confirm that greater cardiac remodeling at baseline and shorter QRS duration affected survival after CRT, we performed a Kaplan–Meier analysis classifying the CRT population based on the median end-diastolic volume (EDV) (87 ml/m2) and QRS duration (164 ms) for the entire population. Thus, 3 groups were created according to the values of EDV and QRS compared to the related medians (Group 1, n = 27: EDV < 87 ml/m2 and QRS ≥ 160 ms; Group 2, n = 51: EDV > 87 ml/m2 and QRS ≥ 160 ms or EDV < 87 ml/m2 and QRS < 160 ms; Group 3, n = 20: EDV > 87 ml/m2 and QRS < 160 ms).
Event-free survival curves for the 3 groups, shown in Fig. 3, were significantly different (log-rank test P = 0.012). Group 3 event-free survival was less than 1/3 than that of Group 1 at the end of follow-up. Furthermore, Group 3 event-free survival rapidly decreased in the first 1000 days after CRT. In contrast, Group 1 event-free survival stayed around 90% until the end of the observation period. The difference, based on a post hoc Holm–Sidak test, was statistically significant (P = 0.015). Group 2 exhibited an intermediate trend, with improved event-free survival compared to Group 3, but worse than that of Group 1, although not at a significant level (Fig. 3).
|
|
|
Fig. 3. Survival curves were obtained by dividing patients into 3 groups according to values of ventricular diastolic volume (EDV) and QRS duration compared to the related medians. Three groups were thus created: Group 1, n = 27: EDV < 87 ml/m2 and QRS ≥ 160 ms; Group 2, n = 51: EDV > 87 ml/m2 and QRS ≥ 160 ms or EDV < 87 ml/m2 and QRS < 160 ms; Group 3, n = 20: EDV > 87 ml/m2 and QRS < 160 ms. Event-free survival curves were significantly different among the 3 groups (log-rank test P = 0.012). Group 3 event-free survival rate was less than 1/3 of the rate of Group 1 at the end of follow-up, and rapidly decreased in the first 1000 days after CRT. In contrast, Group 1 event-free survival was maintained around 90% until the end of the observation period. The difference, based on a post hoc Holm–Sidak test was statistically significant (P = 0.015). Group 2 exhibited an intermediate trend, with improved event-free survival as compared with Group 3, but worse relative to Group 1, although not at a significant level. EDV = end-diastolic volume. |
Results could not be explained by differences in the drug distribution among the 3 groups. The percentages of patients treated with beta-blockers (78%, 82%, 75%), diuretics (85%, 84%, 90%), and ACE inhibitors/AT1 antagonists (85%, 71%, 70%) were not dissimilar among groups.
9.4. Variability of analysis
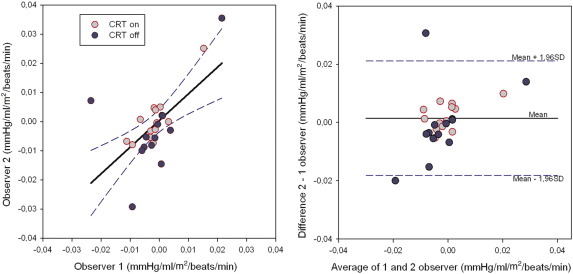
Interobserver reproducibility of Ees slopes with CRT on/off was determined by the blind operator 12 months apart for 13 randomly selected patients. The correlation coefficient for the Ees slopes between the 2 measurements was 0.62 (P < 0.001) (Fig. 4, left). A plot of the average between the repeated Ees slope measurements against their difference demonstrated no over- or underestimation, but the dispersion of the data was slightly larger for CRT off compared to CRT on ( Fig. 4, right).
|
|
|
Fig. 4. Plot of regression between 2 Ees slope measurements performed 12 months apart by a different reader (left). There is a significant correlation between the 2 measurements (r = 0.62, P < 0.001). Also, a plot of the average of the 2 measurements against their difference showed good agreement (right), but the dispersion of the data was slightly larger for CRT off as compared with CRT on. |
10. Discussion
Our study confirmed that in patients with advanced heart failure and intraventricular conduction abnormalities, the correction of baseline asynchrony with CRT activation resulted in an acute increase in contractility, which mediated some improvement in ventricular–vascular coupling. The acute increment in the FFR steepness with CRT, however, was unable to predict rehospitalization due to heart failure, or death, during a 3-year follow-up. Rather, QRS duration (direct relation) and ventricular diastolic volume (inverse relation), as assessed at the time of device implantation, did stratify patients long-term.
10.1. Correction of asynchrony and acute recruitment of contractility
In our study, we provided further evidence that asynchrony correction with CRT improved contractility in heart failure patients. This same effect was shown by Vollmann et al., who demonstrated that biventricular pacing improved the blunted force–frequency relation that was present in 22 subjects with heart failure and ventricular conduction delay [6]. In their study, progressive increases in positive dP/dtmax were demonstrated with increasing heart rate with CRT on, without major changes in preload [6].
It was not clear why the FFR was steeper with CRT. Our data suggested that different pacing modes affected asynchrony, which led to disparities in the FFR response, previously reported as unexplainable on the basis of acute changes in intrinsic myocyte calcium handling or the state of various related proteins [20]. The same beneficial effect from CRT should be anticipated for the LV diastolic properties, given that acute increases in heart rate accelerate early relaxation in patients with heart failure [21]. Using speckle-tracking echocardiography we confirmed that asynchrony modulation, as assessed longitudinally, was associated with a positive inotropic response in CRT pacing mode that favored, only a modest improvement in ventricular–vascular coupling [22] ; [23].
10.2. Prediction of long-term clinical outcome according to acute recruitment of contractility
Many papers have been published on the use of an inotropic challenge in order to predict prognosis in CRT patients. Most of these papers have focused on the short-term anti-remodeling effect of CRT. Ypenburg et al. [1] showed that myocardial contractile reserve (defined as > 7.5% points increment in ejection fraction during low-dose dobutamine infusion) predicted, with a 76% sensitivity and a 86% specificity, reverse remodeling 6 months after CRT in 31 consecutive heart failure patients. Similarly, Tucillio et al. [2] demonstrated in 42 CRT patients that a 25% relative increment in ejection fraction during a dobutamine stress-echo test was a strong predictive factor 6 months after CRT (cut-off for CRT response was an LV end-systolic volume reduction of 15%).
Other papers have examined survival and/or re-hospitalization due to heart failure for long-term periods [3] ; [24]. While the different studies on the short-term anti-remodeling effect are relatively concordant, this is not true for long-term clinical effects. In the only available randomized study on this topic, conducted in 221 CRT patients, it was clear that the end-point was reached only when the 5% point increment in ejection fraction induced by low-dose dobutamine infusion (5–20 μg/kg/min) was contrasted against the combined findings of death and re-hospitalizations due to heart failure that were documented during the 15-month follow-up [3]. No difference was detected using mortality alone.
The results of our analysis were uncertain because we could not use results of acute improvement in contractility with CRT to stratify patients long-term. It is possible that the FFR-induced increment in inotropy in our study (+ 3 ± 0.9% points absolute increment in ejection fraction between CRT vs. AAI–VVI pacing modes at maximum heart rate achieved [data not shown]), which was less than what we obtain with dobutamine infusion (> 5 points absolute increment in the LODO-CRT trial [3]), was inadequate for evoking any obvious beneficial effect during the follow-up. In any case, it remains unclear whether a response to early surrogate testing would identify the chronic responders to CRT with respect to hard clinical end points [25]. This reasoning is in line with another report that described how the acute improvement in dP/dtmax was not correlated to the clinical outcome, although dP/dtmax, measured at baseline and during CRT, was a predictor of 1-year survival free from mortality, heart transplantation, or LV assist device implantation [26]. Thus, it might be that the acute response in contractility, in our study as well as in other studies, might have been a measure of response to CRT than an indicator of prognosis per se.
10.3. Ventricular elastance (Ees) and long-term prognosis in heart failure patients
It must be stressed that the Ees slope change with CRT in our study was highly significant ( Fig. 2B), almost double its baseline value, although we could not use it to stratify patients long-term. In a very recent study another group demonstrated comparable results. In a large population of 446 chronic heart failure participants in the Penn Heart Failure Study, with a reasonable proportion (24%) of CRT-implanted subjects, Ky et al. [27] showed that noninvasive Ees was unable to exert prognostic stratification (combined endpoint of death, cardiac transplantation, or ventricular assist device placement and cardiac hospitalization) over a 6-year follow-up, in either unadjusted or adjusted models. Unlike that study, however, in our study we could not stratify patients long-term based on ventricular elastance, arterial elastance, or their ratio. These findings were unexpected given that the same Penn Heart Failure Study group demonstrated that Ea and ventricular–arterial coupling were able to exert long-term prognostic stratification [27].
We have no clear-cut explanation for these discrepancies apart, from the values of Ea being much higher in our study compared to Kys study [27]. This difference is the consequence of the lower values of systolic LV pressures (median, 25th and 75th percentiles: 109 [96–124] mm Hg vs. 120 [102–140] mm Hg) and larger stroke volumes (59 [45–76] ml vs. 45 [35–57] ml) generated in their study population compared to our study population. These differences, along with their prognostic implications, underline potential margins of therapeutic improvement in our cohort, while reinforcing the importance of an aggressive afterloading therapy in subjects with depressed pump performance. They also attenuate the pathophysiological “weight” of LV chamber elastance alone, compared to the arterial properties and the ventricular–vascular coupling, in the long-term prognosis of such patients [27] ; [28].
10.4. QRS duration and degree of adverse remodeling pre-CRT as predictors of long-term clinical outcome
In our study ventricular diastolic volume (direct relation P = 0.001) and QRS duration (inverse relation P = 0.019), as measured at baseline, predicted death or rehospitalization long term. These findings confirm previous reports that have shown that the degree of LV adverse remodeling [29] and baseline QRS duration [29] ; [30], alone or in combination, predicted CRT response.
Sipahi et al. [30] conducted a meta-analysis of 5813 patients and found that there was a significant relationship between baseline QRS duration and risk ratio, with a benefit of CRT in patients with severely prolonged QRS intervals. Also Park et al. [29] showed in 100 patients that QRS durations, along with LV end-diastolic volume, predicted CRT response (defined as a decrease in LV end-systolic volume of ≥ 15% and/or an absolute increase of 5% in ejection fraction) at 6-months follow-up.
These studies, however, contrast with others which suggest that QRS duration is not predictive of clinical and echocardiographic responses during short-term follow-up [30]; [31] ; [32]. For example Mollema et al. [32] found that QRS duration in 242 subjects was not predictive for clinical (improvement of ≥ 1 grade in New York Heart Association class) and echocardiographic (decrease > 10% in LV end-systolic volume) responses to CRT 6 months after implantation. Similarly, Molhoek et al. [33] showed that QRS duration at baseline is not predictive for clinical response to CRT (improvement of NYHA class, 6-minute walking distance and quality of life score) in 61 patients over 6 months follow-up. They demonstrated that responders exhibit a shortening QRS duration only after CRT, but individual response varied highly.
A direct relationship exists between increased QRS duration and advanced LV dilatation in patients with ischemic cardiomyopathy, a large amount of nonviable muscle in the posterolateral wall, and poor LV function [34]. In our study, where no significant differences could be found in terms of QRS duration or baseline LV remodeling between ischemic vs. nonischemic patients, the combination of longer QRS duration and smaller end-diastolic volume at baseline imposed better survival rates, based on the composite clinical endpoint of worsening heart failure and cardiac death (Fig. 3). Also in the large population of 446 participants in the Penn Heart Failure Study a clear negative prognostic impact of LV end-diastolic volume and risk of cardiac hospitalization was evident [27], reflecting the tight relationship between chamber remodeling and the degree of contractile dysfunction.
11. Limitations
Some limitations in our work need to be considered. DYS could not be assessed during the entire FFR protocol, and thus potential deviations from what we have described during baseline AAI/VVI vs. CRT pacing modes could not be assessed. This limitation in the study design could also explain discrepancies between our study results and our previous publications [11] ; [12] and other groups' studies [13]. It is likely that new 3D speckle tracking echocardiography will represent a more robust method for reconciling quantification of intraventricular mechanical dyssynchrony during CRT therapy along the various axes [35].
Ventricular elastance was not estimated using the single-beat algorithm proposed by Chen et al. [36], due to the difficulty in obtaining timing landmarks during the acquisition protocol, which prevented us from computing the intercept (V0), beside the slope (Ees), of the end-systolic pressure–volume relationship. We acknowledge this limitation but considering the findings of the Penn Heart Failure Study, which used single-beat LV systolic elastance [27], we are confident that the use of a more sophisticated approach to assess cardiac contractility would not have substantially modified our final conclusions.
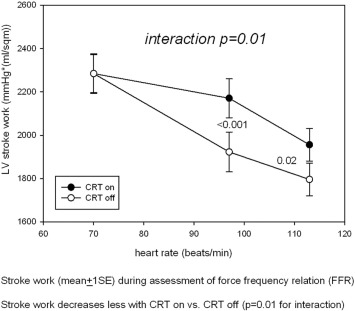
Invasively assessed stroke work (SW) has been reported as a sensitive predictor of long-term CRT response in a 41-patient study [37], in which responders (n = 29) exhibited an acute increase in SW with CRT activation significantly higher (+ 57 ± 33% vs. + 10 ± 30%, P = 0.001) compared to nonresponders (n = 12). Furthermore, the acute inotropic recruitment did stratify long-term, whereas the acute increase in dP/dtmax did not, showing no difference between the 2 groups (+ 15 ± 18% vs. + 6 ± 15%, NS) [37]. In our study, computation of SW slope (derived as mean arterial blood pressure multiplied by stroke volume) against heart rate increments with CRT (on/off) demonstrated a significant interaction (P = 0.01), with CRT on exhibiting higher SW compared to CRT off (Appendix). However, substitution of Ees with SW in the Cox analysis did not change our final conclusion. Differences in the follow-up duration and end-points (death and/or rehospitalization due to heart failure within 3 years vs. reverse remodeling > 15% within 1 year) could in part explain the discrepancy.
12. Conclusion
Correction of DYS is associated with acute contractile recovery in heart failure patients during CRT pacing mode vs. AAI/VVI pacing mode. Acute contractile recovery at time of device implantation, however, is not associated with 3 years prognosis. Rather, death or rehospitalization can be predicted from QRS duration and LV diastolic volume at baseline. Whether a contractility-guided approach to CRT implementation will help in better selection of heart failure patients and long-term prognosis could not be confirmed in this study.
Disclosures
No conflicts to disclose.
Appendix A. Supplementary data
The following are the supplementary data related to this article.
|
|
|
Supplementary figure. Stroke work (mean ± 1 SE) during assessment of force frequency relation (FFR). Stroke work decreases less with CRT on vs. CRT off (P = 0.01 for interaction). |
References
- [1] C. Ypenburg, A. Sieders, G.B. Bleeker, et al.; Myocardial contractile reserve predicts improvement in left ventricular function after cardiac resynchronization therapy; Am. Heart J., 154 (2007), pp. 1160–1165
- [2] B. Tuccillo, C. Muto, R. Iengo, et al.; Presence of left ventricular contractile reserve, evaluated by means of dobutamine stress-echo test, is able to predict response to cardiac resynchronization therapy; J. Interv. Card. Electrophysiol., 23 (2008), pp. 121–126
- [3] M. Gasparini, C. Muto, S. Iacopino, et al.; Low-dose dobutamine test associated with interventricular dyssynchrony: a useful tool to identify cardiac resynchronization therapy responders: data from the low dose dobutamine stress-echo test in cardiac resynchronization therapy (LODO-CRT) phase 2 study; Am. Heart J., 163 (2012), pp. 422–429
- [4] C. Leclercq, J.M. Hare; Ventricular resynchronization: current state of the art; Circulation, 109 (2004), pp. 296–299
- [5] D. Kass; Left ventricular versus biventricular pacing in cardiac resynchronization therapy: the plot in this tale of two modes; J. Cardiovasc. Electrophysiol., 15 (2004), pp. 1348–1349
- [6] D. Vollmann, L. Luthje, P. Schott, G. Hasenfuss, C. Unterberg-Buchwald; Biventricular pacing improves the blunted force–frequency relation present during univentricular pacing in patients with heart failure and conduction delay; Circulation, 113 (2006), pp. 953–959
- [7] K. Serri, P. Reant, M. Lafitte, et al.; Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy; J. Am. Coll. Cardiol., 47 (2006), pp. 1175–1181
- [8] R.N. Goldman; Area of planar polygons and volume of polyhedra; J. Arvo (Ed.), Graphics Gems II, Academic Press, San Diego (2004)
- [9] S. Malm, S. Frigstad, E. Sagberg, P.A. Steen, T. Skjarpe; Real-time simultaneous triplane contrast echocardiography gives rapid, accurate, and reproducible assessment of left ventricular volumes and ejection fraction: a comparison with magnetic resonance imaging; J. Am. Soc. Echocardiogr., 19 (2006), pp. 1494–1501
- [10] F. Helmcke, N.C. Nanda, M.C. Hsiung, et al.; Color Doppler assessment of mitral regurgitation with orthogonal planes; Circulation, 75 (1987), pp. 175–183
- [11] B. Bertola, E. Rondano, M. Sulis, et al.; Cardiac dyssynchrony quantitated by time-to-peak or temporal uniformity of strain at longitudinal, circumferential and radial level: implications for resynchronization therapy; J. Am. Soc. Echocardiogr., 22 (2009), pp. 665–671
- [12] C. Cavallino, E. Rondano, A. Magnani, et al.; Baseline asynchrony, assessed circumferentially using temporal uniformity of strain, besides coincidence between site of latest mechanical activation and presumed left ventricular lead position, predicts favourable prognosis after resynchronization therapy; Int. J. Cardiovasc. Imaging, 28 (2012), pp. 1011–1021
- [13] R.H. Helm, C. Leclercq, O.P. Faris, et al.; Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: implications for assessing cardiac resynchronization; Circulation, 111 (2005), pp. 2760–2767
- [14] E. Facchini, M. Varalda, C. Sartori, D. Burkhoff, P.N. Marino; Systolic heart failure and cardiac resynchronization therapy: a focus on diastole; Int. J. Cardiovasc. Imaging, 30 (2014), pp. 897–905
- [15] Z.I. Whinnett, J.E. Davies, K. Willson, et al.; Haemodynamic effects of changes in atrioventricular and interventricular delay in cardiac resynchronisation therapy show a consistent pattern: analysis of shape, magnitude and relative importance of atrioventricular and interventricular delay; Heart, 92 (2006), pp. 1628–1634
- [16] K. Sunagawa, W.L. Maughan, D. Burkoff, K. Sagawa; Left ventricular interaction with arterial load studied in isolated canine ventricle; Am. J. Phys., 245 (1983), pp. H773–H780
- [17] D.A. Kass; Age-related changes in ventricular–arterial coupling: pathophysiologic implications; Heart Fail. Rev., 7 (2002), pp. 51–62
- [18] H. Suga, K. Sagawa; Instantaneous pressure–volume relationships and their ratio in the exercised, supported canine left ventricle; Circ. Res., 35 (1974), pp. 117–126
- [19] F. Knebel, S. Schattke, H. Bondke, et al.; Circumferential 2D-strain imaging for the prediction of long term response to cardiac resynchronization therapy; Cardiovasc. Ultrasound, 6 (2008), pp. 28–34
- [20] D.A. Kass; Cardiac resynchronization therapy and cardiac reserve. How you climb a staircase may alter its steepness; Circulation, 113 (2006), pp. 923–925
- [21] S. Esfandiari, F. Fuchs, R.V. Wainstein, et al.; Heart rate-dependent left ventricular diastolic function in patients with and without heart failure; J. Card. Fail., 21 (2015), pp. 68–75
- [22] G.S. Nelson, R.D. Berger, B.J. Fetics, et al.; Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block; Circulation, 102 (2000), pp. 3053–3059
- [23] P. Steendijk, S.A. Tulner, J.J. Bax, et al.; Hemodynamic effects of long-term cardiac resynchronization therapy: analysis by pressure–volume loops; Circulation, 113 (2006), pp. 1295–1304
- [24] R.K. Altman, D. McCarty, A.A. Chen-Tournoux, et al.; Usefulness of low-dose dobutamine echocardiography to predict response and outcome in patients undergoing cardiac resynchronization therapy; Am. J. Cardiol., 108 (2011), pp. 252–257
- [25] B. Bozkurt, K. Ramasubbu; Guiding left ventricular lead positioning and refining ability to predict response and nonresponse to cardiac resynchronization therapy using dP/dtmax; J. Am. Coll. Cardiol., 58 (2011), pp. 1137–1139
- [26] M.D. Bogaard, P. Houthuizen, F.A. Bracke, et al.; Baseline left ventricular dP/dtmax rather than the acute improvement in dP/dtmax predicts clinical outcome in patients with cardiac resynchronization therapy; Eur. J. Heart Fail., 13 (2011), pp. 1126–1132
- [27] B. Ky, B. French, A. May Khan, et al.; Ventricular–arterial coupling, remodeling, and prognosis in chronic heart failure; J. Am. Coll. Cardiol., 62 (2013), pp. 1165–1172
- [28] M. St John Sutton, J. Cerkvenik, Borlaug BA, et al.; Effects of cardiac resynchronization therapy on cardiac remodeling and contractile function: results from Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE); J. Am. Heart Assoc., 4 (9) (Sep 11 2015), p. e002054 doi: 10.1161/JAHA.115.002054 (assessed December 19, 2015)
- [29] M.Y. Park, R.K. Altman, M. Orencole, et al.; Characteristics of responders to cardiac resynchronization therapy: the impact of echocardiographic left ventricular volume; Clin. Cardiol., 35 (2012), pp. 777–780
- [30] I. Sipahi, T.P. Carrigan, D.Y. Rowland, B.S. Stambler, J.C. Fang; Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials; Arch. Intern. Med., 171 (2011), pp. 1454–1462
- [31] S.G. Duckett, M. Ginks, A.K. Shetty, et al.; Invasive acute hemodynamic response to guide left ventricular lead implantation predicts chronic remodeling in patients undergoing cardiac resynchronization therapy; J. Am. Coll. Cardiol., 58 (2011), pp. 1128–1136
- [32] S.A. Mollema, G.B. Bleeker, E.E. van der Wall, M.J. Schalij, J.J. Bax; Usefulness of QRS duration to predict response to cardiac resynchronization therapy in patients with end-stage heart failure; Am. J. Cardiol., 100 (2007), pp. 1665–1670
- [33] S.G. Molhoek, L. Van Erven, M. Bootsma, P. Steendijk, E.E. Van Der Wall, M.J. Schalij; QRS duration and shortening to predict clinical response to cardiac resynchronization therapy in patients with end-stage heart failure; Pacing Clin. Electrophysiol., 27 (2004), pp. 308–313
- [34] O. De Winter, N. Van de Veire, F. Van Heuverswijn, G. Van Pottelberge, T.C. Gillebert, J. De Sutter; Relationship between QRS duration, left ventricular volumes and prevalence of nonviability in patients with coronary artery disease and severe left ventricular dysfunction; Eur. J. Heart Fail., 8 (2006), pp. 275–277
- [35] C. Thebault, E. Donal, A. Bernard, et al.; Real-time three-dimensional speckle tracking echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony; Eur. J. Echocardiogr., 12 (2011), pp. 26–32
- [36] C.H. Chen, B. Fetics, E. Nevo, et al.; Noninvasive single-beat determination of left ventricular end-systolic elastance in humans; J. Am. Coll. Cardiol., 38 (2001), pp. 2028–2034
- [37] G.J. De Roest, C.P. Allaart, S.A. Kleijn, et al.; Prediction of long-term outcome of cardiac resynchronization therapy by acute pressure–volume loop measurements; Eur. J. Heart Fail., 15 (2013), pp. 299–307
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?