Abstract
The present study was planned with an objective to test the pharmacokinetics of a new formulation of enrofloxacin (Flobac®SA) in buffalo calves. The drug was administered at the dose rate of 7.5 mg kg−1 body weight through the intravenous (i.v.) and intramuscular (i.m.) route followed by plasma collection and analysis at different time intervals. After analysis, using High Performance Liquid Chromatography – Ultraviolet, various pharmacokinetic parameters were calculated using visual fit for compartmental analysis, followed by integration with pharmacodynamic parameters against Escherichia coli and Pasteurella multocida. Although total area under plasma drug concentration time curve was higher through the i.v. route, mean residence time and metabolic conversion ratio was higher following administration by the i.m. route indicating longer persistence of the drug in body. Overall i.m. bioavailability of the parent compound with its metabolite was found to be 91%. Upon, Pharmacokinetic–Pharmacodynamic integration, all the parameters indicated significant antibacterial activity. It can be concluded that the dose of enrofloxacin used in the present study can be administered to contain infections caused by P. multocida and E. coli in buffalo calves.
Symbols and Abbreviations
HPLC
high-performance liquid chromatography
UV
ultraviolet
LOQ
limit of quantification
D
priming dosepriming/loading- a relatively large dose given at the beginning of therapy to get desired pharmacological/ antimicrobial effect at the earliest
D'
maintenance dose – a dose given during course of therapy to maintain desired pharmacological effect produced by the priming/loading dose
F
bioavailability defined as percent of drug available in the central compartment after drug administration
Introduction
Enrofloxacin [(1-cyclopropyl)-7-(ethyl-1-piperazinyl)- 6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid] is a fluoroquinolone antibacterial developed exclusively for veterinary use (Altreuther 1987). It is characterized by the presence of a fluorine atom at position 6 and the presence of a piperazinyl or pyrrolidinyl substituent in position 7 of the quinoline nucleus (Gips & Soback 1996). The mode of action of fluoroquinolones involves interactions with DNA gyrase, the originally recognized drug target and also topoisomerase IV, a related type II topoisomerase (Hooper 1999). It also possesses post-antibiotic effect (McKellar 1996). It is metabolized by de-ethylation to another fluoroquinolone ciprofloxacin (Kung et al. 1993), giving sustained activity at the same dose rate compared to other fluoroquinolones. It is indicated against various bacterial infections such as Escherichia coli, Staphylococcus aureus, Streptococcus pneumonia, Legionella pneumophila, Moraxella catarrhalis, Pseudomonas aeruginosa, Haemophilus influenza and Haemophilus ducreyi. Sanders (1988) resulting in septicaemia, respiratory tract, urinary tract, skin, soft tissue, bone and joint infections (McKellar et al. 1999; Sanjib et al. 2005). Recent reports indicate the use of enrofloxacin in bovine respiratory disease associated with Pasteurella multocida and Mannheimia (M.) haemolytica (Balaje et al. 2013).
The pharmacokinetic behaviour of enrofloxacin after intravascular administration has been determined in cattle (Kaartinen et al. 1995, 1997; Malbe et al. 1996), goats (Elmas et al. 2001; Elsheikh et al. 2002; Rao et al. 2002) and other ruminants (Gavrielli et al. 1995; Christensen et al. 1996) at the dose rate of 2.5–5 mg kg−1 body weight. Some studies on pharmacokinetics of enrofloxacin and ciprofloxacin have been performed in buffalo calves through intravenous (i.v.) and intramuscular (i.m.) routes (Saini & Srivastava 2001; Sharma et al. 2003; Balaje et al. 2013; Kumar & Jayachandran 2013). Enrofloxacin exhibited good absorption, large volume of distribution and half-life in the range of 2–6 h (Otero et al. 2009). Once kinetics of a drug is performed, its pharmacokinetic–pharmacodynamic (PK-PD) relationship has to be determined, which is the most significant predictor of the efficacy of fluoroquinolones (Balaje et al. 2013).
The present study aims at exploring the pharmacokinetics of a new preparation of enrofloxacin, thereby arriving at PK-PD integration as well as calculation of dosage regimen, using pharmacodynamic data previously established in the laboratory.
Materials and methods
The experiment was conducted on six male buffalo calves of 6–12 months age (weight 90–120 kg). The experiment protocol followed the guideline on proper care and handling of animals and was approved by the Institutional Animal Ethics Committee. Calves were given both i.v. and i.m. administrations in a complete crossover design with a washout period of 3 weeks minimum between two treatments. Enrofloxacin 10% (Flobac SA®; Intas Pharmaceuticals Pvt. Ltd., Ahmedabad, India) was administered through jugular vein and deep gluteal muscles of hindquarters at the dose rate of 7.5 mg kg−1 body weight by the i.v. and i.m. route, respectively. Blood samples (4–5 mL) were collected by jugular venipuncture (using contra-lateral vein from that to which the i.v. dose was administered) in tubes containing 8–10 units heparin per mL of blood at 0, 2.5, 5, 10, 15, 30 min, 1 h, 2, 4, 8, 12, 24, 36, 48, 60 and 72 h following enrofloxacin administration. Subsequently, plasma was separated and analysed. Enrofloxacin and Ciprofloxacin hydrochloride standards were purchased from Sigma Aldrich, St. Louis, Missouri and M.P. Biomedicals, Santa Ana, California USA. All the reagents used for the analysis were of high-performance liquid chromatography (HPLC) grade. Plasma concentrations of enrofloxacin and ciprofloxacin hydrochloride were determined using HPLC-Ultraviolet (HPLC-UV) (Perkin Elmer series 200, Waltham, MA, USA) as per the method previously established in the laboratory (Balaje et al. 2013).
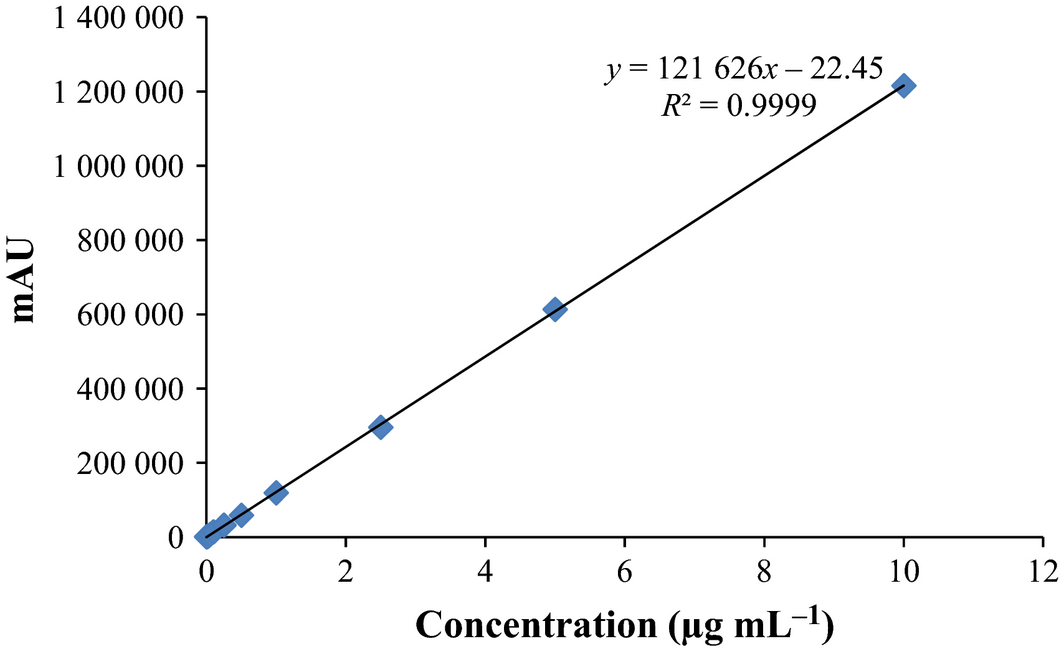
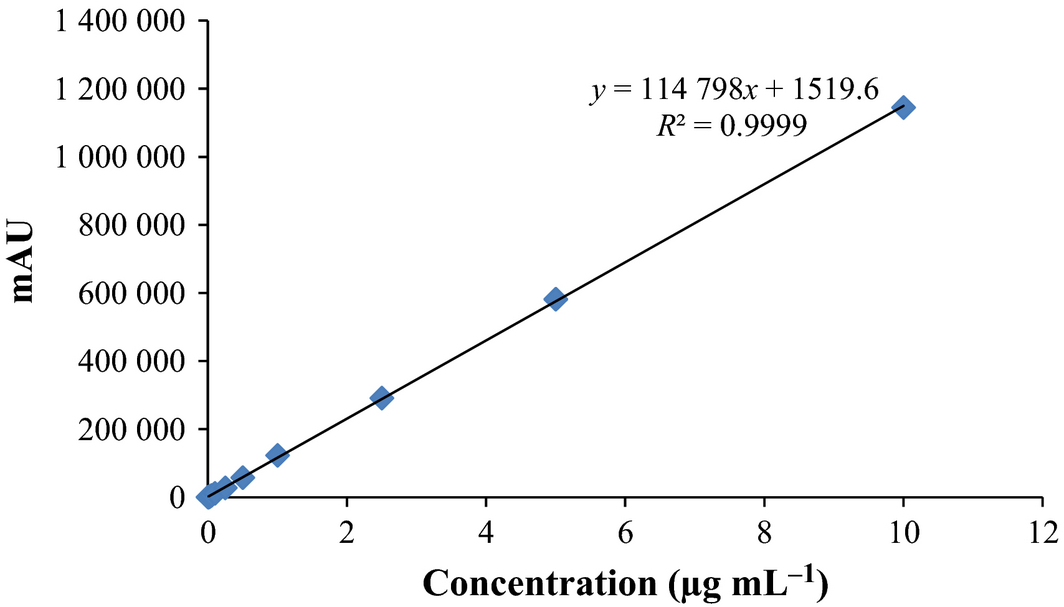
The retention time of ciprofloxacin and enrofloxacin was about 6 ± 0.5, 7 ± 0.5 min, respectively, with a peak separation of 1 min. The calibration curves for ciprofloxacin and enrofloxacin are presented in Figs 1 and 2, respectively, where y is peak area and x is the concentration (μg mL−1). The linearity (R2 = 0.9999) of assay method was evaluated by constructing a calibration curve in the range of 0.01–10 μg mL−1. Limit of quantification (LOQ) of enrofloxacin and ciprofloxacin was 0.02 and 0.01 μg mL−1. The recoveries validated via repetitive analysis (n = 3) of the plasma samples spiked with enrofloxacin and ciprofloxacin were in the range of 90–105%. The precision (interday and intraday) of the assay was less than 5% with an accuracy of more than 95%. The best suited model for pharmacokinetic analysis was determined by visual fit. Various pharmacokinetic parameters were calculated from the log plasma concentration-time profile of enrofloxacin and ciprofloxacin, using Microsoft Excel for each animal according to standard equations given by Gibaldi & Perrier (1982). The metabolite conversion ratio (MCR), priming and maintenance dose were estimated by the following formulae:
|
|
|
|
|
|
|
|
|
Figure 1. Standard curve for ciprofloxacin. |
|
|
|
Figure 2. Standard curve for enrofloxacin. |
Statistical analysis was performed on various pharmacokinetic and pharmacodynamic parameters using Students t test (P > 0.05).
Results and discussion
Pharmacokinetics
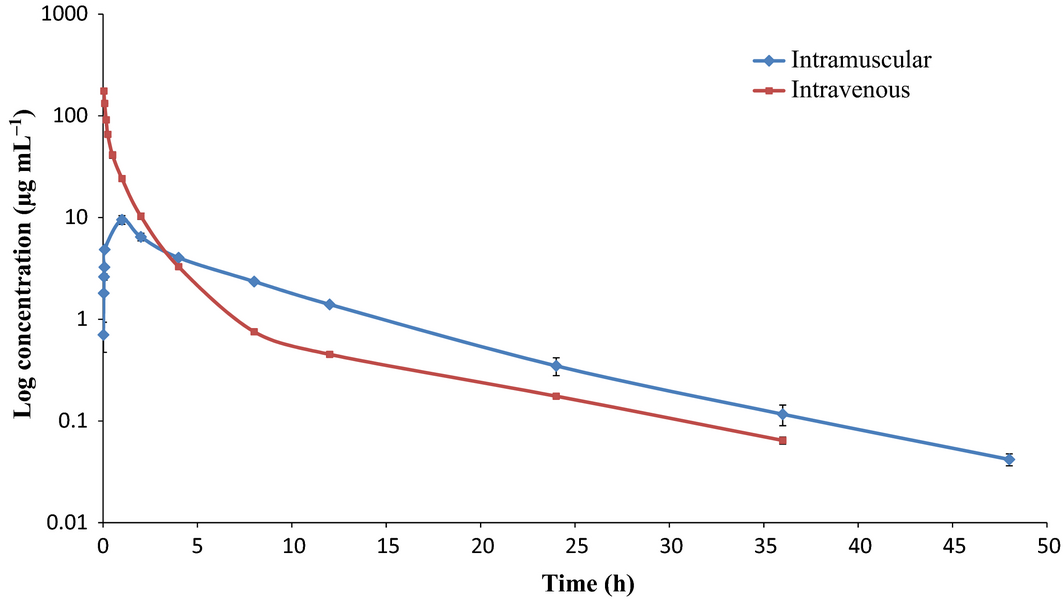
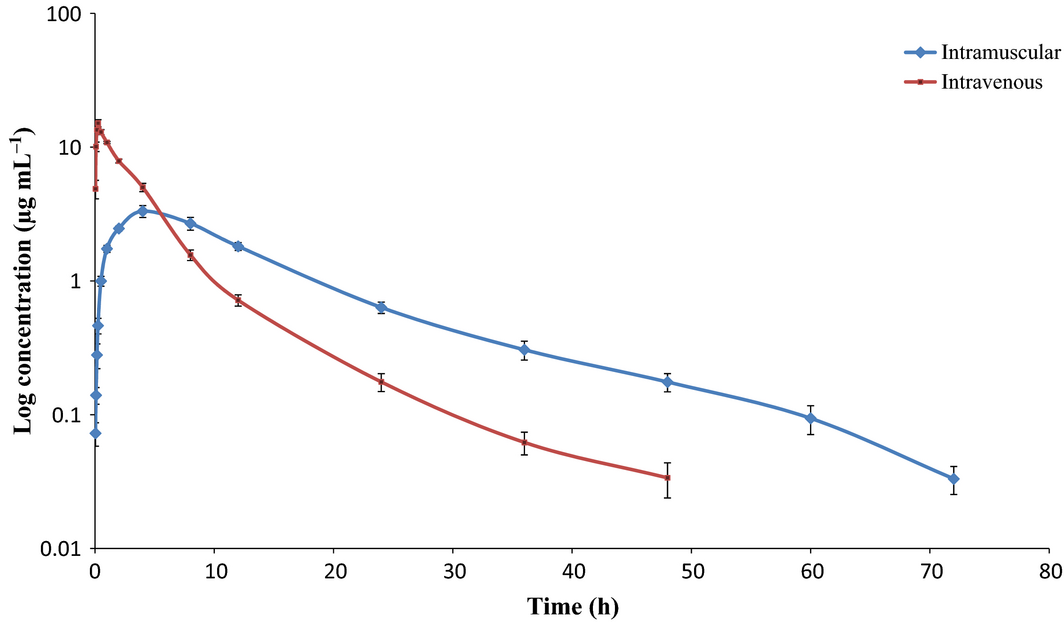
Upon single administration of enrofloxacin (7.5 mg kg−1 body weight), concentration – the time curve was plotted and compartments were decided by visual fit. After i.v. administration of enrofloxacin, both parent drug and ciprofloxacin followed the two-compartment open model whereas enrofloxacin followed the two-compartment open model and ciprofloxacin the one-compartment open model after i.m. administration of enrofloxacin (Figs 3,4). In previous studies, a mono-compartment open model was used for pharmacokinetic analysis (Balaje et al. 2013; Kumar & Jayachandran 2013). The two-compartment open model followed in the present study was in confirmation with previous study where kinetics in several ruminant species was conducted after administration of ciprofloxacin intramuscularly (Javed et al. 2009). Ahangar & Srivastava (2000) also reported the two-compartment model for enrofloxacin following i.v. administration at dose rate of 5 mg kg−1 body weight. All the pharmacokinetic parameters calculated are presented in Table 1. Since, enrofloxacin gets metabolized to ciprofloxacin, a pharmacologically active metabolite, it is important to consider the role of the metabolite along with the parent drug for analysing the pharmacokinetics and dynamics of drug preparation.
|
|
|
Figure 3. Semi logarithmic plot of enrofloxacin after administration at 7.5 mg kg−1 body weight through intravenous & intramuscular route. |
|
|
|
Figure 4. Semi logarithmic plot of ciprofloxacin after administration of enrofloxacin at 7.5 mg kg−1 body weight through intravenous and intramuscular route. |
| Parameter | Ciprofloxacin i.m. | Ciprofloxacin i.v. | Enrofloxacin i.m. | Enrofloxacin i.v |
|---|---|---|---|---|
| A' (μg mL−1) | 3.59 ± 0.42 | 9.17 ± 3.53 | 4.29 ± 1.65 | – |
| Ka (h−1) | 0.85 ± 0.12 | 9.77 ± 2.68* | 3.53 ± 0.99 | – |
| t1/2Ka (h) | 0.89 ± 0.12 | 0.09 ± 0.02* | 0.28 ± 0.06 | – |
| A (μg mL−1) | – | 14.0 ± 0.51 | 4.86 ± 1.30 | 167.7 ± 4.24* |
| ∞ (h−1) | – | 0.37 ± 0.03 | 0.17 ± 0.03 | 3.10 ± 0.17* |
| t1/2∞ (h) | – | 1.90 ± 0.17 | 3.62 ± 0.26 | 0.23 ± 0.01* |
| B (μg mL−1) | 3.50 ± 0.45 | 1.73 ± 0.14* | 3.04 ± 0.87 | 1.39 ± 0.08 |
| β (h−1) | 0.07 ± 0.003 | 0.09 ± 0.007* | 0.09 ± 0.005 | 0.09 ± 0.003 |
| t1/2β (h) | 10.9 ± 0.63 | 7.95 ± 0.56* | 8.20 ± 0.53 | 8.12 ± 0.31 |
| Cmax (μg mL−1) | 3.32 ± 0.34 | 15.1 ± 0.92 | 9.52 ± 0.93 | – |
| Tmax (h) | 4.00 ± 0 | 0.25 ± 0 | 1 ± 0 | – |
| AUC (μg h mL−1) | 49.1 ± 4.54 | 18.6 ± 1.02* | 32.0 ± 9.09 | 70.5 ± 2.33* |
| AUMC (μg h2 mL−1) | 836.2 ± 91.2 | 223.4 ± 16.9* | 375.4 ± 82.2 | 207.2 ± 7.58 |
| Vdarea (L kg−1) | – | – | 1.46 ± 1.02 | 1.25 ± 0.08 |
| Kel (h−1) | 0.14 ± 0.007 | 0.59 ± 0.18 | 0.37 ± 0.13 | 2.41 ± 0.11* |
| MRT (h) | 17.0 ± 0.98 | 12.1 ± 1.11* | 13.4 ± 1.50 | 2.95 ± 0.14* |
| td (h) | – | – | 42.9 ± 2.75 | 42.4 ± 1.62 |
| K12/K21 | 0.69 ± 0.05 | 4.82 ± 1.84 | 1.64 ± 0.70 | 6.14 ± 0.68* |
| MCR | i.m. – 1.54 ± 0.27* | i.v. – 0.27 ± 0.021 | ||
i.v., intravenous; i.m., intramuscular; A', zero time intercept of regression line of absorption phase; Ka, absorption rate constant; t1/2Ka, absorption half life; A, zero time intercept of regression line of distribution phase; ∞, distribution rate constant; t1/2∞, distribution half life; B, zero time intercept of regression line of elimination phase; β, elimination rate constant; t1/2β, elimination half life, Cmax, maximum concentration achieved; Tmax, time taken to achieve maximum concentration; AUC, total area under plasma drug concentration time curve calculated by trapezoidal method; AUMC, total area under the first moment of plasma drug concentration time curve; Vdarea, volume of distribution; Kel, elimination rate constant from central compartment; MRT, mean residence time; td, dosing interval; K12/K21, ratio of rate constants for transfer of drug between central and peripheral compartments; MCR, metabolite conversion ratio. *Significant difference at P > 0.05 level of significance when compared within enrofloxacin and ciprofloxacin between different routes of administration. | ||||
Drug absorption was rapid after i.m. administration based on the low absorption half-life (0.28 h) followed by prolonged distribution (t1/2∞ = 3.6 h) but the drug elimination followed similar pattern even though drug was administered through different routes. Ciprofloxacin on the other hand showed prolonged residence in the body of buffalo calves after i.m. dosing of enrofloxacin compared to intravenous administration, which might be due to absorption of parent drug from the site of administration and its slower metabolism forming ciprofloxacin in liver resulting in longer elimination half-life (10.9 h) as well as mean residence time (MRT) (17 h). Both the maximal concentration and time taken to achieve the maximum concentration for ciprofloxacin was higher and earlier after i.v. route than i.m. route.
Since enrofloxacin has an active metabolite (ciprofloxacin), while calculating bioavailability, the total area under plasma drug concentration time curve (AUC) of both parent and metabolite have been taken into consideration. Bioavailability (F) of the drug after drug administration through the i.m. route was 91%. This indicated that the parent drug was well absorbed through the i.m. route. Enrofloxacin metabolically converted to ciprofloxacin and also persisted for longer duration in the animal body, resulting in greater AUC of ciprofloxacin after injection of enrofloxacin by the i.m. route than i.v.
Volume of distribution, an indicator of the drug distribution was not significantly altered irrespective of the route of administration as it depends on the type of compound rather than the dose or route of administration.
MRT of enrofloxacin administered intravenously was many fold lesser compared to that after the i.m. route indicating rapid elimination via the first route. On comparing MRT of enrofloxacin to ciprofloxacin following any route of administration, it was found to be higher for later indicating that the parent drug gets metabolized and the metabolite persists in the system for a longer time than the parent compound itself. This further supports the longer persistence of ciprofloxacin following either route.
MCR indicates the conversion of the parent drug (enrofloxacin) to active metabolite (ciprofloxacin). When enrofloxacin was administered intravenously, there was lower conversion of the parent compound to metabolite, indicated by significantly lower MCR, as seen by lower AUC of ciprofloxacin obtained.
PK-PD integration
The maximum plasma concentration achieved after drug administration (Cmax)/minimum inhibitory concentration (MIC) and/or AUC/MIC ratios are the main PK–PD parameters correlating with efficacy of concentration-dependent antimicrobials (McKellar et al. 2004). Using the pharmacodynamic parameters recently established in our laboratory (Balaje et al. 2013; Beri et al. 2015), PK-PD indices were calculated to analyse the apparent effectiveness of the drug preparation in vivo. The results of PK-PD integration are presented in Table 2.
| Parameter | Ciprofloxacin i.m. | Ciprofloxacin i.v. | Enrofloxacin i.m. | Enrofloxacin i.v. | Enrofloxacin i.m. | Enrofloxacin i.v. |
|---|---|---|---|---|---|---|
| Escherichia coli | Pasteurella multocida | |||||
| Cmax/MIC | 369.0 ± 37.7 | 1680.9 ± 102.7* | 432.5 ± 42.2 | – | 190.3 ± 18.6 | – |
| AUC/MIC | 5457.0 ± 504.8 | 2062.0 ± 113.6* | 1453.3 ± 413.2 | 3204.8 ± 105.7* | 639.5 ± 181.8 | 1410.1 ± 46.5* |
| Cmax/MBC | 207.6 ± 21.2 | 945.5 ± 57.8* | 264.3 ± 25.8 | – | 158.6 ± 15.5 | – |
| AUC/MBC | 3069.6 ± 37.7 | 1159.9 ± 63.9* | 888.1 ± 252.5 | 1958.5 ± 64.6* | 532.9 ± 151.5 | 1175.1 ± 38.8* |
| Cmax/MPC | 87.4 ± 8.92 | 398.1 ± 24.3* | 56.6 ± 5.52 | – | 6.26 ± 0.61 | – |
| AUC/MPC | 1292.5 ± 119.6 | 488.4 ± 26.9* | 190.3 ± 54.10 | 419.7 ± 13.8* | 21.0 ± 5.98 | 46.4 ± 1.53* |
PK-PD, pharmacokinetic–pharmacodynamic; i.v., intravenous; i.m., intramuscular; Cmax, maximum concentration achieved; MIC, minimum inhibitory concentration; AUC, total area under plasma drug concentration time curve calculated by trapezoidal method; MBC, minimum bactericidal concentration; MPC, mutant prevention concentration. *Significant difference at P > 0.05 level of significance when compared within enrofloxacin and ciprofloxacin between different routes of administration. | ||||||
Balaje et al. (2013) reported MIC, minimum bactericidal concentration (MBC) and mutant prevention concentration (MPC) values of enrofloxacin against P. multocida isolates as 0.05, 0.06 and 1.52 μg mL−1, respectively. Whereas, Beri et al. (2015) reported MIC, MBC and MPC values of 0.009, 0.016 and 0.038 & 0.022, 0.036 and 0.168 μg mL−1 for ciprofloxacin and enrofloxacin, respectively, against E. coli and its clinical isolates. Pharmacodynamic parameters of ciprofloxacin against Pasteurella spp. is yet to be established in our laboratory.
As the fluoroquinolones are concentration-dependent drugs with post-antibiotic effect, PK-PD indices such as Cmax/MIC or Cmax/MBC or Cmax/MPC and AUC/MIC or AUC/MBC or AUC/MPC are used for interpreting the effectiveness of preparation. These PK-PD parameters were also calculated for danofloxacin in camels (Aliabadi et al. 2003).
It was proposed that Cmax/MIC and/or AUC24 h/MIC for ciprofloxacin should be more than 10 and 125, respectively for successful therapy and prevention of emergence of resistance (Forrest et al. 1993). Nightingale et al. (2000) suggested that for effective eradication of Gram positive and negative bacteria, an AUC/MIC ratio should be more than 30 and 100, respectively. AUC/MPC ratio of 9–12 was effective in controlling mutations when the inoculum size was between 105 and 107 cfu mL−1 (Ferran et al. 2007).
The PK/PD indices, such as the Cmax/MPC and AUC24 h/MPC were selected in order to predict the in vivo applicability of the in vitro MPC concept in agreement with other studies (Toutain et al. 2002; Olofsson et al. 2006). When the bacterial load is very high (in case of acute infection) or in chronic cases there might be chances that some of microorganisms are less sensitive (mutant) to that particular antimicrobial this led to the concept of MPC.
In the present study, values of Cmax/MIC and AUC/MIC for ciprofloxacin were found to be more than that recommended by Forrest et al. (1993). The results from the present study indicate that enrofloxacin and ciprofloxacin can be used effectively for Gram positive and Gram negative bacteria because of their high AUC/MIC ratio (>>>100). With respect to the ability to control mutants, it was found that AUC/MPC ratio was very high in case of E. coli (>>>9–12), while moderately higher in the case of P. multocida indicating the effectiveness in controlling infection along with eradicating resistance.
Dosage regimen
Even though fluoroquinolones are concentration dependent, single administration of antibiotic achieving a higher Cmax is not enough for an effective antibacterial therapy. There is a necessity to maintain the concentration of antibacterial (for AUC) by multiple administrations. Since, Cmax and AUC are two important pharmacokinetic parameters used in PK-PD integration, it is necessary to achieve a higher value for maintaining antibacterial activity and against development of resistance irrespective of the post antibiotic effect for fluoroquinolones. So, the calculation of priming and maintenance dose helps in achieving both higher concentration as well as a higher AUC resulting in better antibacterial activity. In the case of mixed infections, we have to take into consideration the range of MICs or when bacterial load is very high (in case of acute infection) or in chronic cases there might be chances that some of microorganisms are less sensitive (mutant) to that particular antimicrobial. In such a situation, multiple administrations become necessary so as to maintain the plasma/serum concentration in the animal body for effective treatment. The priming and maintenance dose were also calculated by Ram et al. (2010) in buffalo calves; Javed et al. (2009) in ruminants; Dumka & Srivastava (2013) in cross bred calves for various fluoroquinolones.
After calculating the pharmacokinetic and pharmacodynamic parameters, the dosage regimen (D and D') was calculated and presented in Table 3. The calculated priming and maintenance doses of enrofloxacin for the treatment of infectious diseases caused by E. coli (MPC ≤ 0.2 μg mL−1) with an inter-dose interval of 24, 36 and 48 h after i.v. administration were found to be 1.95, 5.50, 15.54 and 1.70, 5.25 and 15.29 mg kg−1 body weight, respectively. Whereas, the priming and maintenance dose after i.m. administration were 2.39, 6.99, 20.52 and 2.11, 6.71 and 20.23 mg kg−1 body weight, respectively. For infections caused by Pasteurella spp. (MPC ≤ 1.2 μg mL−1), the priming and maintenance dose at an interdose interval of 24, 36 and 48 h were 11.72, 32.98, 93.24 and 10.22, 31.48, 91.74 mg kg−1 body weight after intravenous administration.. For the i.m. route priming and maintenance dose at 24, 36 and 48 h interval were 14.36, 41.94, 123.10 and 12.65, 40.24, 121.39 mg kg−1, respectively.
| Dosing interval (h) | |||||||
|---|---|---|---|---|---|---|---|
| 24 | 36 | 48 | |||||
| D | D1 | D | D1 | D | D1 | ||
| Escherichia coli (MPC – 0.2 μg mL−1) | i.v. | 1.95 ± 0.07 | 1.70 ± 0.08 | 5.50 ± 0.39 | 5.25 ± 0.41 | 15.54 ± 1.75 | 15.29 ± 1.76 |
| i.m. | 2.39 ± 0.35 | 2.11 ± 0.30 | 6.99 ± 0.90 | 6.71 ± 0.85 | 20.52 ± 2.50 | 20.23 ± 2.46 | |
| Pasteurella sp. (MPC – 1.2 μg mL−1) | i.v. | 11.72 ± 0.43 | 10.22 ± 0.46 | 32.98 ± 2.37 | 31.48 ± 2.43 | 93.24 ± 10.51 | 91.74 ± 10.58 |
| i.m. | 14.36 ± 2.07 | 12.65 ± 1.76 | 41.94 ± 5.37 | 40.24 ± 5.09 | 123.10 ± 14.98 | 121.39 ± 14.77 | |
| MPC, mutant prevention concentration. D and D1 is expressed in mg kg−1. All the values were expressed in mean ± SEM | |||||||
In the present day scenario of treating bacterial infections in animals together with an objective to prevent emergence of bacterial resistance, principled antimicrobial usage is important. With this objective in mind, the present study was planned to test the pharmacokinetics of a newer drug preparation in buffalo species which is an important part of future productive livestock but being ignored nutritionally as well as therapeutically making them susceptible to various diseases.
Conclusion
From the present study, it was concluded that the preparation of enrofloxacin used in the present study has a good pharmacokinetic property with longer elimination half-life as well as MRT when administered intramuscularly. Even though the drug was administered through different routes in buffalo calves, the PK-PD analysis has shown that the drug and/or its metabolite have persisted for longer periods in the plasma rendering multiple drug administration irrelevant when administered at a dose of 7.5 mg kg−1 body weight.
Acknowledgements
The authors acknowledge the facilities provided by GADVASU. We are also thankful to Intas Pharmaceuticals Pvt. Ltd. for kindly providing Flobac®SA.
Source of funding
Council of Scientific and Industrial Research, New Delhi (India) in the form of research grant [Scheme No: 37 (1395)/10/EMR-II].
Contributions
Conceived and designed the experiments: PSD and VKD. Performed the experiments: PSD and BV. Estimation of drug in the collected samples: PSD and BV. Analyzed the data: PSD, BV, VKD, SKS. Wrote the paper: PSD and BV. Made the final revisions: VKD and SKS.
References
- Ahangar A.H. & Srivastava A.K. (2000) Pharmacokinetics of enrofloxacin in febrile cross-bred bovine calves. Indian Journal of Pharmacology32, 305–308.
- Aliabadi F.S., Landoni M.F. & Lees P. (2003) Pharmacokinetics (PK), pharmacodynamics (PD), and PK-PD integration of danofloxacin in sheep biological fluids. Antimicrobial Agents and Chemotherapy47, 626–635.
- Altreuther P. (1987) Data on chemistry and toxicology of Baytril. Veterinary Medical Review2, 87–89.
- Balaje R.M., Sidhu P.K., Kaur G. & Rampal S. (2013) Mutant prevention concentration and PK–PD relationships of enrofloxacin for Pasteurella multocida in buffalo calves. Research in Veterinary Science95, 1114–1124.
- Beri S., Sidhu P.K., Kaur G., Chandra M. & Rampal S. (2015) Comparative mutant prevention concentration and antibacterial activity of fluoroquinolones against Escherichia coli in diarrheic buffalo calves. Journal of Chemotherapy27(5), 312–316. doi:10.1179/1973947814Y.0000000173.
- Christensen J.M., Smith B.B., Murdane S.B. & Hollingshead N. (1996) The disposition of five therapeutically important antimicrobial agents in llamas. Journal of Veterinary Pharmacology and Therapeutics19, 431–438.
- Dumka V.K. & Srivastava A.K. (2013) Levofloxacin disposition and urinary excretion in febrile cross bred calves. Veterinary Archive84, 371–380.
- Elmas M., Tras B., Kaya S., Bas A.L., Yazar E. & Yarsan E. (2001) Pharmacokinetics of enrofloxacin after intravenous and intramuscular administration in Angora goats. Canadian Journal of Veterinary Research65, 64–67.
- Elsheikh H.A., Taha A.A.W., Khalafallah A.I. & Osman I.A.M. (2002) Disposition kinetics of enrofloxacin (Baytril 5%) in sheep and goats following intravenous and intramuscular injection using a microbiological assay. Research in Veterinary Science73, 125–129.
- Ferran A., Dupouy V., Toutain P.L. & Bousquet-Melou A. (2007) Influence of inoculum size on the selection of resistant mutants of Escherichia coli in relation to mutant prevention concentrations of marbofloxacin. Antimicrobial Agents and Chemotherapy51, 4163–4166.
- Forrest A., Nix D.E., Ballow C.H., Goss T.F., Birmingham M.C. & Schentag J.J. (1993) Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrobial Agents and Chemotherapy37, 1073–1081.
- Gavrielli R., Yagil R., Ziv G., Creveld C.V. & Glickman A. (1995) Effect of water deprivation on the disposition kinetics of enrofloxacin in camels. Journal of Veterinary Pharmacology and Therapeutics18, 333–339.
- Gibaldi M. & Perrier D. (eds.) (1982) Pharmacokinetics. Marcel and Dekker: New York. ISBN 0-8247-1042-8.
- Gips M. & Soback S. (1996) Norfloxacin nicotinate pharmacokinetics in unweaned and weaned calves. Journal of Veterinary Pharmacology and Therapeutics19, 130–134.
- Hooper D.C. (1999) Mode of action of fluoroquinolones. Drugs58 (S2), 6–10.
- Javed I., Iqbal Z., Zia-ur-Rahman N., Zargham Khan M., Muhammad F., Aslam B., et al. (2009) Disposition kinetics and optimal dosage of ciprofloxacin in healthy domestic ruminant species. Acta Veterinaria Brno78, 155–162.
- Kaartinen L., Salonen M., Alli L. & Pyorala S. (1995) Pharmacokinetics of enrofloxacin after single intravenous, intramuscular and subcutaneous injections in lactating cows. Journal of Veterinary Pharmacology and Therapeutics18, 357–362.
- Kaartinen L., Pyorala S., Moilanen M. & Raisanen S. (1997) Pharmacokinetics of enrofloxacin in newborn and one-week-old calves. Journal of Veterinary Pharmacology and Therapeutics20, 470–482.
- Kumar N. & Jayachandran C. (2013) Pharmacokinetic interactions and pharmacokinetic pharmacodynamic surrogate relationships of enrofloxacin and diclofenac in buffalo calves following intravenous administration. World Journal of Pharmaceutical Research3, 848–869.
- Kung K., Riond J.L. & Wanner M. (1993) Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin after intravenous and oral administration of enrofoxacin in dogs. Journal of Veterinary Pharmacology and Therapeutics16, 462–468.
- Malbe M., Salonen M., Fang W., Oopik T., Jalakas M., Klaassen M. & Sandholm M. (1996) Disposition of enrofloxacin (Baytril) into the udder after intravenous and intra-arterial injections into dairy cows. Journal of Veterinary Medicine A43, 377–386.
- McKellar Q.A. (1996) Clinical relevance of pharmacologic properties of fluoroquinolones. Compendium on Continuing Education for the Practicing Veterinarian18 (Suppl.), 14–21.
- McKellar Q.A., Gibson I., Monteiro A. & Bregante M. (1999) Pharmacokinetics of enrofloxacin and danofloxacin in plasma, inflammatory exudate and bronchial secretions of calves following subcutaneous administration. Antimicrobial Agents and Chemotherapy43, 1988–1992.
- McKellar Q.A., Sanchez-Bruni S.F. & Jones D.G. (2004) Pharmacokinetic/relationships of antimicrobial drugs used in veterinary medicine. Journal of Veterinary Pharmacology and Therapeutics27, 503–514.
- Nightingale C.H., Grant E.M. & Quintiliani R. (2000) Pharmacodynamics and pharmacokinetics of levofloxacin. Chemotherapy46, 6–14.
- Olofsson S.K., Marcusson L.L., Lindgren P.K., Hughes D. & Cars O. (2006) Selection of ciprofloxacin resistance in Escherichia coli in an in vitro kinetic model: relation between drug exposure and mutant prevention concentration. Journal of Antimicrobial Chemotherapy57, 1116–1121.
- Otero J.L., Mestorino N. & Errecalde J.O. (2009) Pharmacokinetics of enrofloxacin after single intravenous administration in sheep. Revue Scientifique et Technique (International Office of Epizootics)28, 1129–1142.
- Ram D., Dumka V.K., Sandhu H.S. & Raipuria M. (2010) Pharmacokinetics and dosage regimen of levofloxacin in buffalo calves after single subcutaneous administration. Veterinarski Arhiv80, 195–203.
- Rao G.S., Ramesh S., Ahmad A., Tripathi H.C., Sharma L.D. & Malik J.K. (2002) Disposition kinetics of enrofloxacin and ciprofloxacin following intravenous administration of enrofloxacin in goats. Small Ruminant Research44, 9–15.
- Saini S.P.S. & Srivastava A.K. (2001) The disposition kinetics, urinary excretion and dosage regimen of ciprofloxacin in buffalo calves (Bubalus bubalis). Veterinary Research Communications25, 641–649.
- Sanders C.C. (1988) Ciprofloxacin in vitro activity, mechanism of action and resistance. Reviews of Infectious Diseases10, 516–527.
- Sanjib K., Chandana C.B., Pritam M. & Mohan B. (2005) Pharmacokinetic studies of enrofloxacin in Yak after intramuscular administration. Iranian Journal of Pharmacology and Therapeutics4, 91–94.
- Sharma P.K., Ahmad A.H., Sharma L.D. & Varma R. (2003) Pharmacokinetics of enrofloxacin and the rate of formation of its metabolite ciprofloxacin following intravenous and intramuscular single dose administration to male buffalo calves. Veterinary Journal166, 101–104.
- Toutain P.L., Del Castillo J.R. & Bousquet-Melou A. (2002) The pharmacokinetic pharmacodynamic approach to a rational dosage regimen for antibiotics. Research in Veterinary Science73, 105–114.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?