Summary
Background/objective
Cosmetic factors are important when considering minimally invasive surgery. For cosmetic improvement, a complete thoracoscopic lobectomy is accomplished via the three-port two-instrument (TPTI) technique. The resected specimen is removed without extending the port wounds. Only three wounds at 1.2 cm are used to finish the procedure.
Methods
From June 2012 to December 2013, 60 patients with lung cancer were to undergo lobectomy and complete mediastinal lymph node dissection via the TPTI technique without an accessory wound. The initial 28 cases (learning curve group) and the latest 28 cases were compared to assess the learning curve.
Results
Excluding four cases of conversion, there were 28 cases in each group. There were no differences between these two groups with respect to age, sex, tumor size, location of the lobectomy, mean blood loss, mean postoperative drainage time, and mean hospitalization time (p > 0.05). The mean surgery time significantly decreased, the mean number of lymph nodes removed significantly increased, and the postoperative stage was significantly more advanced in the latest 28 cases (p < 0.05). The conversion rate was similar in both groups.
Conclusion
Three-port complete thoracoscopic lobectomy with the two-instrument technique is feasible for lung cancer treatment. The length of the learning curve consisted of 28 cases. This TPTI technique should be popularized.
Keywords
lung neoplasms;thoracic surgery;thoracoscopy;video-assisted
1. Introduction
Video-assisted thoracoscopic (VATS) lobectomy was first used in 1991.1 The standard VATS lobectomy approach involves two thoracoscopic ports and one accessory incision of 4 cm.2 Usually four instruments are required to perform the procedure. The ideal thoracoscopic lobectomy should maximize the cosmetic result, thereby minimizing the size of the incisions without compromising standard surgical oncologic principles. The three-port two-instrument (TPTI) thoracoscopic lobectomy technique is a complete thoracoscopic approach without an accessory wound. To maintain the advantage of less wound trauma of the TPTI technique, a distinct way to remove the resected specimen without extending the port-wound was designed. The initial cases were compared with the latest cases to detail the learning curve.
2. Methods
2.1. Patients
From June 2012 to December 2013, 60 patients with lung cancer underwent lobectomies and complete mediastinal lymph node dissections via the TPTI technique. All of them had the specimen removed without extending the thoracoscopic-port wounds. The TPTI technique was routinely performed to treat lung cancer starting in June 2012 at E-DA Hospital, Kaohsiung, Taiwan. The cases of bulky N2 disease were not included for surgery. All surgical procedures were performed by the author (Y.-J.C.). The TPTI is associated with a learning curve. These cases were analyzed and an attempt to define the length of the learning curve was made.
2.2. Surgical technique
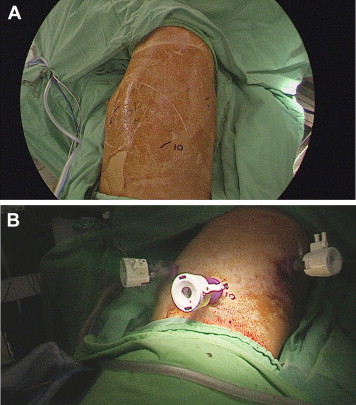
The TPTI technique is defined as a lobectomy performed during a complete thoracoscopic procedure. There are only three ports in three separate wounds of 1.2 cm without accessory thoracotomy wound (Fig. 1A and B). The patient is placed in the flexed lateral decubitus position tilted slightly backward for the upper/middle lobectomy, or forward for the lower lobectomy, for ease of the venous approach. The camera port is placed at the sixth interspace along the anterior axillary line for right-sided lesions and the seventh interspace along the posterior axillary line for left-sided lesions. The operator is always standing on the right side of the patient. The other two ports are created under the inspection of the camera. Usually they are placed at the 7th interspace along the posterior axillary line and 10th interspace along the scapular line in the right side. In the left side they are placed at the 6th interspace along the anterior axillary line and 10th interspace along the scapular line. Sometimes if the 10th interspace port is too low to be created, it will be replaced by the 9th interspace port. These two ports are for the two instruments, usually a Harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, Ohio, USA) and endo-forceps. The pulmonary vessels and the lobar bronchus are individually dissected. The small vessels are clamped with Hemolock (Hospiinz International, Coimbatore, Tamil Nadu, India), and larger vessels are applied with endo-staplers. During the procedure CO2 inflation is maintained at a pressure of 7–10 cm H2O to ease the manipulation. The resected lobe is cut into several strips using endo-staplers, with a cutting margin of at least 1 cm away from the tumor mass.1 The lung strips are then placed into a two-layer endo-bag, and are removed separately. The lung-strip with malignant tissue is the last to be removed. Following removal of all specimens, complete mediastinal lymph node dissection is carried out according to the European Society of Thoracic Surgeons guideline.3 All of the removed tissues is sent for detailed pathological examination.
|
|
|
Figure 1. (A)The position of the port-wound in the left chest wall is marked. All three ports are located in the lower chest to upwardly manipulate the instruments and the thoracoscopy. (B) The three ports are shown. |
2.3. Statistical analysis
Quantitative data are presented as mean ± standard deviation. Two-sided Student t test is used to determine the significance between these two groups. The Pearson Chi-square test is used for comparison of category data between these two groups. A p value < 0.05 is considered statistically significant. The receiver operating characteristic (ROC) curve is graphically constructed by plotting sensitivity against the false-positive rate (1-specificity) for detecting the length of the learning curve based on the surgery time and the number of lymph nodes resected.
3. Results
Among the 60 intended surgical procedures there were four conversions. The remaining 56 surgical procedures were analyzed. There were 33 females and 23 males with mean age of 61.4 ± 9.9 years. The postoperative stages (pStages) were Stage Ia in 25 patients, Stage Ib in 14 patients, Stage IIa in 5 patients, Stage IIb in 2 patients, Stage IIIa in 9 patients, and Stage IV in 1 patient. The mean tumor size was 2.7 cm ± 1.0 cm. There were 11 right upper lobectomies (RUL), 6 right middle lobectomies (RML), 14 right lower lobectomies (RLL), 15 left upper lobectomies (LUL), and 10 left lower lobectomies (LLL). The mean number of lymph nodes removed was 25.5 ± 7.2. The mean surgical time was 300.4 ± 65.3 minutes, and the mean blood loss was 111.1 ± 125.6 mL. The mean time to chest tube removal was 5.8 ± 2.5 days, and the mean length of hospital stay was 9.5 ± 3.5 days (Table 1). There were no major complications. Minor complications that occurred in the initial cases consisted of a prolonged air leak of > 7 days in two patients, cerebral vascular accident in one patient, and pulmonary thrombosis on the surgical side in one patient aged 80 years, all of whom recovered well after nonsurgical treatment.
| Mean | Standard deviation | |
|---|---|---|
| Age (y) | 61.4 | 9.9 |
| Size (cm) | 2.7 | 1.0 |
| LN (no.) | 25.5 | 7.2 |
| Op time (min) | 300.4 | 65.3 |
| Blood (mL) | 111.1 | 125.6 |
| Hospitalization (d) | 9.5 | 3.5 |
| Drainage (d) | 5.8 | 2.5 |
| N | % | ||
|---|---|---|---|
| Sex | Male | 23 | 41.1 |
| Female | 33 | 58.9 | |
| Stage | Ia | 25 | 44.6 |
| Ib | 14 | 25.0 | |
| IIa | 5 | 8.9 | |
| IIb | 2 | 3.6 | |
| IIIa | 9 | 16.1 | |
| IV | 1 | 1.8 | |
| Lobe | RUL | 11 | 19.6 |
| RML | 6 | 10.7 | |
| RLL | 14 | 25.0 | |
| LUL | 15 | 26.8 | |
| LLL | 10 | 17.9 | |
| Type | Ade | 48 | 85.7 |
| SqCC | 5 | 8.9 | |
| SCC | 1 | 1.8 | |
| Ad-Sq | 1 | 1.8 | |
| Large | 1 | 1.8 |
ade = adenocarcinoma; Ad-Sq = adeno-squamous cell carcinoma; Blood = the blood lost during the operation; Drainage = the duration of the drainage tube intubation; large = large cell carcinoma; LLL = left lower lobectomies; LN = total number of the resected lymph nodes reported by the pathology; Lobe = resected lobe; LUL = left upper lobectomies; Op time = operation time from skin to skin; Stage = postoperative pathologic stage; RLL = right lower lobectomies; RUL = right upper lobectomies; SCC = small cell carcinoma; SqCC = squamous cell carcinoma; Type = tumor type.
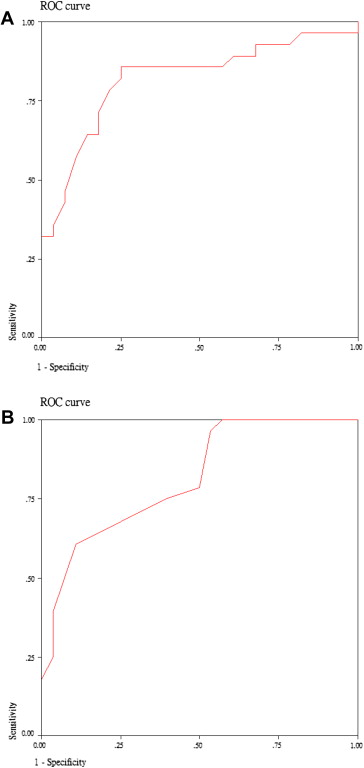
Based on the surgery time, the length of the learning curve was 28 cases with the area under the ROC curve being 0.820. If based on the number of lymph nodes resected, the length of the learning curve was also 28 cases with the area under the ROC curve being 0.814 (Fig. 2). Therefore, all cases were divided into two groups, the learning curve group (the initial 28 cases, Group I) and the latest 28 cases (Group L).
|
|
|
Figure 2. The receiver operating characteristic (ROC) curve is graphically constructed for detecting the length of the learning curve based on the surgery time (A) and the number of lymph node resected (B). |
There were no significant differences between these two groups with respect to sex (p = 0.786), age (p = 0.071), tumor size (p = 0.917), blood loss (p = 0.061), length of hospital stay (p = 0.135), time to chest tube removal (p = 0.057), location of the lobectomy (p = 0.132), and tumor cell type (p = 0.512). The number of lymph nodes removed was significantly higher (p = 0.000), and the surgery time was significantly less in Group L (p = 0.000). The pStage in Group L was more advanced (p = 0.013). The statistical data are listed in Table 2.
| Group | Mean | Standard deviation | p* | |
|---|---|---|---|---|
| Age (y) | I | 63.8 | 9.8 | |
| L | 59.0 | 9.6 | 0.071 | |
| Size (cm) | I | 2.7 | 1.0 | |
| L | 2.7 | 1.0 | 0.917 | |
| LN (n) | I | 22.1 | 4.6 | |
| L | 29.0 | 7.7 | 0.000 | |
| Op time (min) | I | 332.7 | 72.0 | |
| L | 268.2 | 36.7 | 0.000 | |
| Blood (mL) | I | 142.9 | 164.5 | |
| L | 79.3 | 54.6 | 0.061 | |
| Hospitalization (d) | I | 8.8 | 3.3 | |
| L | 10.2 | 3.6 | 0.135 | |
| Drainage (d) | I | 5.2 | 2.6 | |
| L | 6.4 | 2.3 | 0.057 | |
| Group | p** | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Male | Female | |||||
| I | 11 | 17 | |||||
| L | 12 | 16 | 0.786 | ||||
| Stage | Ia | Ib | IIa | IIb | IIIa | IV | |
| I | 13 | 10 | 4 | 1 | 0 | 0 | |
| L | 12 | 4 | 1 | 1 | 9 | 1 | 0.013 |
| Lobe | RUL | RML | RLL | LUL | LLL | ||
| I | 3 | 5 | 5 | 9 | 6 | ||
| L | 8 | 1 | 9 | 6 | 4 | 0.132 | |
| Type | Ade | SqCC | SCC | Ad-Sq | Large | ||
| I | 23 | 3 | 1 | 0 | 1 | ||
| L | 25 | 2 | 0 | 1 | 0 | 0.512 |
- There were no statistic differences in the distribution of the age, tumor size, operation blood loss, duration of hospitalization, and duration of drainage tube intubation in these two groups (Student t test, p = 0.071, p = 0.917, p = 0.061, p = 0.135, and p = 0.057, respectively). However, Group L had significantly less surgery time (p = 0.000) and higher number of lymph nodes removed (p = 0.000).
- There were no statistic differences in the distribution of the sex, resected lobe, and tumor type in these two groups (Pearson Chi-square test, p = 0.786, p = 0.132, and p = 0.052, respectively). The stage in Group L was significantly more advanced (p = 0.013).
ade = adenocarcinoma; Ad-Sq = adeno-squamous cell carcinoma; Blood = the blood lost during the operation; Drainage = the duration of the drainage tube intubation; large = large cell carcinoma; LLL = left lower lobectomies; LN = total number of the resected lymph nodes reported by the pathology; Lobe = resected lobe; LUL = left upper lobectomies; Op time = operation time from skin to skin; Stage = postoperative pathologic stage; RLL = right lower lobectomies; RUL = right upper lobectomies; SCC = small cell carcinoma; SqCC = squamous cell carcinoma; Type = tumor type.
4. Discussion
Pulmonary lobectomy has a curative intent for lung cancer. The role of less resection is still controversial.4 The prognostic influence of systemic lymph node dissection has been proved.5 Therefore, with the TPTI lobectomy for lung cancer, the systemic lymph node dissection is mandatory. In my previous study the mean number of lymph nodes removed was less in the whole TPTI group compared with that in the conventional VATS lobectomy with accessory wound (data not shown). There is still room for improvement. The number of retrieved lymph nodes is increased in the latest group. It is difficult to determine whether there are more lymph nodes existing in the latest group because there is not enough image information. However, with more experience it will be easier to attain resection of an increased number of lymph nodes.
The length of the learning curve has been suggested to consist of 50 VATS lobectomies.6 The learning curve is shorter in the TPTI technique, partly because the thinking process and the technique needed in the TPTI technique are similar to those in VATS lobectomies. It is supposed that the VATS lobectomy is the precursor to the TPTI technique. However, complete thoracoscopic surgery without the use of a utility thoracotomy requires surgeon skill. In the TPTI technique, lacking the third instrument for surgery, additional maneuvers must be used. The surgical table usually needs to be tilted to one side to open the lung tissue. Using CO2 inflation to increase the intrathoracic pressure up to 7–10 cm H2O can provide more space to manipulate the pulmonary structures. Encircling the lobe with a ribbon to space out the mediastinum is a way to ease the mediastinal lymph node dissection. The anesthetic one-lung ventilation with continuous endobronchial suction on the surgical side is the basis of a successful TPTI lobectomy.
It is just as it should be if the whole resected lobe is removed from the original wound without extension. If the wound has to be enlarged after the surgical procedure, it is not rational to promote the pure thoracoscopic lobectomy. By cutting the resected lung tissue into several strips with endo-staplers, the specimens can be easily removed through the small port-wound. My results show that tumors as large as 5.0 cm can be removed via the TPTI approach. It is important that the cutting edge should have a safe margin of at least 1 cm from the tumor to avoid tumor seeding in the thoracic cavity.7 The concept originates from the lobectomy done after pulmonary wedge resection for tissue proof of malignancy. To cut the cancerous lobe within the thoracic cavity is a controversial issue. Further follow-up is required for long-term prognostic evaluation. According to my short-term result, no increased risk is found.
The removal of the specimen with a double-layer protection bag provides greater security against breakage. It is better to use an elastic rubber glove as the inner layer that is covered by a retrieval bag (Endo Bag, Covidien, Mansfield, Massachusetts, USA).
The three-port pure thoracoscopic technique can decrease the length of the incisions. Some recent reports discuss uniportal thoracoscopic surgery for lung cancer lobectomy.8 ; 9 These authors advocate this technique because a small utility wound is necessary for surgical specimen retrieval. However, the uniportal wound is usually 3–4 cm in size, which is larger than the portal wounds in this TPTI method. For cosmetic consideration and pain score influence, the uniportal thoracoscopic lobectomy still needs further evaluation.
5. Conclusion
The results of this study demonstrate that TPTI thoracoscopic lobectomy without extending the port wound to remove the specimens is feasible. The length of the learning curve consisted of 28 cases. This TPTI technique should be popularized. Further follow-up is required for long-term prognostic evaluation.
References
- 1 G. Roviaro, F. Varoli, C. Rebuffat, et al.; Videothoracoscopic staging and treatment of lung cancer; Ann Thorac Surg, 59 (1995), pp. 971–974
- 2 R. Flores; Video-assisted thoracic surgery (VATS) lobectomy: focus on technique; World J Surg, 34 (2010), pp. 616–620
- 3 D. Lardinois, P. De Leyn, P. Van Schil, et al.; ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer; Eur J Cardiothorac Surg, 30 (2006), pp. 787–792
- 4 R.J. Ginsberg, L.V. Rubinstein; Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer; Ann Thorac Surg, 60 (1995), pp. 615–623
- 5 J. Zhang, T. Mao, Z. Gu, et al.; Comparison of complete and minimal mediastinal lymph node dissection for non-small cell lung cancer: results of a prospective randomized trial; Thorac Cancer, 4 (2013), pp. 416–421
- 6 R.J. McKenna Jr.; Complications and learning curves for video-assisted thoracic surgery lobectomy; Thorac Surg Clin, 18 (2008), pp. 275–280
- 7 M. Higashiyama, K. Kodama, K. Takami, et al.; Intraoperative lavage cytologic analysis of surgical margins as a predictor of local recurrence in pulmonary metastasectomy; Arch Surg, 137 (2002), pp. 469–474
- 8 D. Gonzalez-Rivas, E. Fieira, M. Delgado, M. de la Torre, L. Mendez, R. Fernandez; Uniportal video-assisted thoracoscopic lobectomy; J Thorac Dis, 5 (2013), pp. S234–245
- 9 C.Y. Liu, C.S. Lin, C.H. Shih, C.C. Liu; Single-port video-assisted thoracoscopic surgery for lung cancer; J Thorac Dis, 6 (2014), pp. 14–21
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?