Summary
Objective
This is an initial review of the safety and efficacy of anterior preperitoneal modified Kugel (MK) mesh herniorrhaphy application without using optional onlay mesh.
Methods
We retrospectively reviewed patients who underwent herniorrhaphy by a single surgeon from July 1st, 2009 to December 31st, 2010. During these 18 months, a total of 72 patients underwent single-layer MK mesh herniorrhaphy. Anterior preperitoneal approach was used to place the mesh. If the patients inguinal hernia defect did not exceed the memory ring of MK mesh, the onlay mesh was omitted. Postoperative results (wound infection, recurrence, and chronic pain/discomfort) were recorded and analyzed.
Results
A total of 72 patients underwent anterior preperitoneal single layer MK mesh herniorrhaphy. One patient had recurrent hernia after 1 year and was treated with a laparoscopic transabdominal preperitoneal operation. The most common postoperative complaint was mild soreness which was self-resolving after 1 month. Mean total operative time (skin to skin) was 73 minutes. The average hospital stay was 2 days. Most of the postoperative complications including soreness (14%), pain for > 3 months (1.4%), and scrotal hematoma (1.4%) were self-resolving. One patient experienced wound infection, which was treated with oral antibiotics. One patient had recurrence 1 year after the operation.
Conclusion
The postoperative complication and recurrence rates of single-layer MK mesh herniorrhaphy was comparable with previously reported tension-free repair. Single-layer application is safe and feasible. A longer follow-up period and larger study group with a control group are needed to verify our method.
Keywords
herniorrhaphy;inguinal;Kugel;preperitoneal;transinguinal
1. Introduction
Inguinal hernia repair has a long history dating back to as early as the 19th century, the era of tension repair. As the technique and technologies evolved over time, the era of tension-free repair was ushered in with the discovery of synthetic polymers in 1935.1 In 1999, Dr Kugel introduced the posterior approach herniorrhaphy, which offers the benefits of both laparoscopic and open methods but at a cost of a higher learning curve compared with the traditional anterior approach.2; 3; 4; 5; 6 ; 7 To minimize the learning curve, BARD (BARD-Davol Inc., Cranston, RI, USA) introduced the modified Kugel (MK) mesh. The MK mesh is different from other commercial mesh due to the Posiflex ring, a hard spring-like material that prevents bending and folding, and therefore ensures flat placement of mesh.8 The anterior transinguinal preperitoneal (TIPP) approach in placement of MK mesh has been described in several studies with similar recurrence and complication rates as in the posterior approach.9 ; 10 The manufacturer recommends using the onlay mesh, when the MK mesh is unable to cover the entire groin area (direct, indirect, and femoral hernia).8 We feel this definition is vague and most surgeons at our institution rarely discard the onlay for fear of future recurrence. We wanted to narrow the definition and at the same time try to eliminate excess foreign material being inserted into patients, hence we started this retrospective study. Prosthetic mesh has been known to cause injuries to surrounding tissue.11; 12; 13; 14 ; 15 In theory, the inflammatory response will decrease with the amount of implanted prosthetic mesh. Chronic herniorrhaphy pain after Lichtenstein repair has been reported to be as high as 40% in some studies.3; 12; 16 ; 17 One explanation regarding the high postoperative discomfort of Lichtenstein repair is the involvement of the three nerves in the inguinal canal.17; 18 ; 19 This is a review of initial experience using single-layer MK mesh herniorrhaphy.
2. Methods
2.1. Patients
After obtaining approval from the institutions review board (IRB number: 130211), we searched patients who underwent herniorrhaphy from July 1st, 2009 to December 31st, 2010. A total of 73 consecutive patients underwent the TIPP approach single-layer MK mesh herniorrhaphy using a small, oval 8 cm × 12-cm MK mesh. An onlay patch was omitted if the MK mesh was able to cover the entire hernia defect.
2.2. Surgical method
A single surgeon (H.C.C.) performed the herniorrhaphy under spinal anesthesia. General anesthesia was performed in selected cases where spinal was contraindicated or patient refusal. A single dose of prophylactic antibiotics (cefazolin) was used 30 minutes before incision unless there was a previous allergic reaction. After the induction of anesthesia, the inguinal region was shaved, and then cleansed with Hibitane solution.
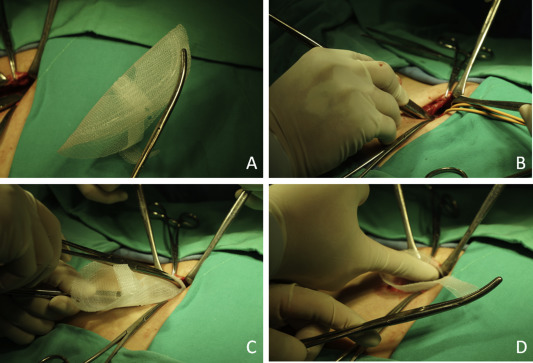
The incision skin was then disinfected with alcoholic povidone–iodine solution, and aqua–β-iodine was used for the scrotal region. The patient was then draped with sterile drapes. A 4–5-cm incision was made parallel to the inguinal ligament. After the hernia defect was identified and dissected, the preperitoneal space was then created using wet surgical gauze. We routinely use seven gauzes to create the preperitoneal space. Using the index finger to protect the major vessels, the gauzes were inserted in the direction of the pubic bone, internal ring, conjoint tendon, and inguinal ligament. An additional three gauzes were piled on top to dissect the preperitoneal space further and left in the space for 30 seconds allowing for compression. The MK mesh was then prepared for insertion into preperitoneal space with help of a Kelly clamp (Figure 1A). The wet gauzes were then removed and counted to insure no gauze was left in the preperitoneal space. After the dissected hernia sac was pushed back into the peritoneal space, a long forceps was used to depress the preperitoneal space and the MK mesh was pushed (Figure 1B and C) in the direction of the pubic bone. The operators index finger was inserted in the middle slit of the mesh to ensure flat placement (Figure 1D). The mesh was then secured onto the transversalis fascia and inguinal ligament with the help of prolene sutures. The inguinal canal and subcutaneous was then closed using absorbable sutures. The epidermis was approximated using 3M Steri-Strips (3M Health Care, St. Paul, MN, USA) and Tegaderm (3M Health Care, St. Paul, MN, USA), which were removed during subsequent outpatient department follow up. After the operation, we asked all of our patients to abstain from heavy work and exercise for a minimum of 4 weeks (1 month) and if possible up to 12 weeks (3 months).
|
|
|
Figure 1. (A) Preparation of modified Kugel (MK) mesh before insertion. The mesh is folded longitudinally like a taco. (B) Preparation of the preperitoneal space before insertion. The blunt end of the forceps is first inserted into the previously dissected space pushing the preperitoneal space downward. (C) Insertion of the MK mesh. The folded mesh is placed on top of the long forceps and slid down into the preperitoneal space. (D) Flattening of MK mesh. An index finger is inserted in the middle slit of the MK mesh. |
2.3. Patient evaluation
After obtaining review board approval (number: 130211), medical records were obtained and reviewed, including: preoperative and postoperative (7 days) physical examinations; postoperative pain evaluated with visual analog scale scores; and postoperative complications (wound infections, recurrences, and chronic pain/discomfort). A questionnaire with a series of questions (Table 1) was used during telephone interviews conducted 3 months, 6 months, and 12 months following the procedures. Any patients with suspicion of recurrence were asked to return to the OPD (Out-Patient Department) for further examination. The Clavien–Dindo system was used to record postoperative complications (Table 2). This system was described by Dindo et al20 in 2004 and is a widely used system to classify surgical complications.
| Question 1: | Since the last visit/interview, have you experience bulging appearance in the groin region during your daily activities? (if no, skip to question 4) |
| Question 2: | Does your job require heavy lifting? What other physical activity do you participate on a daily basis? |
| Question 3: | Did you notice the bulging mass or groin pain when you stand up, lift heavy objects, straining, or cough? |
| Question 4: | Have you seek another doctors advice or received surgical treatment for the bulging mass or groin pain? |
| Question 5: | Have you experience any pain in groin, scrotum or abdomen? (Assess pain with VAS score) |
| Question 6: | Is there anything that will make the pain worse or better? |
| Question 7: | If persistent pain, have you need to seek medical advice for the pain? Was pain medication prescribed? |
| Complications | N = 72 | (%) |
|---|---|---|
| Soreness | 10 | 14.0% |
| Recurrence | 1 | 1.4% |
| Pain < 3 months | 2 | 2.8% |
| Pain > 3 months | 1 | 1.4% |
| Wound infection | 1 | 1.4% |
| Scrotal hematoma | 1 | 1.4% |
3. Results
3.1. Patient characteristics
During the initial 1.5 years, H.C.C. performed anterior the TIPP approach single-layer MK mesh herniorrhaphy on 73 patients. One patient required an optional onlay patch; therefore, he was excluded from this retrospective study. At the time of the surgical interventions, the median age was 68 years and ranged from 30 years to 90 years. The median body mass index was 23.62 kg/m2, and the majority of the patients were male (Table 3). Most patients underwent spinal anesthesia (n = 63), and general anesthesia was done on patients who were deemed unfit by the anesthesiologist (n = 9). Seven patients had bilateral disease and three were recurrent cases. The median hernia repair time (from skin incision to mesh placement) was 25 minutes. The average total operative time including anesthesia and preoperative preparation was 73 minutes and hospital stay was 2 days ( Table 3).
| Basic Data | |
|---|---|
| Total number | 72 |
| Age (years) | 65.32 |
| BMI (kg/m2) | 23.62 |
| Male:Female | 70:2 |
| Right:Left:Bilateral | 33:32:7 |
| Anesthesia method | |
| Spinal | 63 |
| General | 9 |
| Mean hospital stay (day) | 2.30 ± 0.51 |
| Mean total OP time (min) | 73.32 ± 35.87 |
3.2. Complications
Self-resolving complications included 10 patients with soreness, two patients with pain for <3 months, and one scrotal hematoma (Clavien–Dindo Grade I; Table 4). One person experienced chronic postherniorrhaphy pain (>3 months) with a visual analog scale score of 3, which required pain medication (Clavien–Dindo Grade II). Wound infection occurred in one patient, which resolved with antibiotic use (Clavien–Dindo Grade II). One patient required laparoscopic preperitoneal repair for bilateral recurrence 1 year after the operation (Clavien–Dindo Grade IIIb).
| Grade | Definition |
|---|---|
| I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions |
| Allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgetics, diuretics, electrolytes, and physiotherapy. | |
| This grade also includes wound infections opened at the bedside | |
| II | Requiring pharmacological treatment with drugs other than such allowed for grade I complications. Blood transfusions and total parenteral nutrition are also included |
| III | Requiring surgical, endoscopic or radiological intervention |
| IIIa | Intervention not under general anesthesia |
| IIIb | Intervention under general anesthesia |
| IV | Life-threatening complication (including CNS complications)* requiring IC/ICU management |
| IVa | Single organ dysfunction (including dialysis) |
| IVb | Multiorgan dysfunction |
| V | Death of a patient |
4. Discussion
Since the introduction of Kugel posterior herniorrhaphy in 1999, the procedure offers minimal invasive preperitoneal mesh placement with low complication and recurrence rates.2; 4; 5; 6; 7 ; 21 However, the posterior approach is associated with a steep learning curve and high recurrence rate during the early learning period.6; 7; 10; 21 ; 22 By contrast, the anterior TIPP approach is advantageous for surgeons due to the familiar surgical approach.10 According to the manufacturers technical guide, the optional onlay mesh can be omitted if the MK mesh covers the entire groin area. Because most surgeons at our hospital insist on placing onlay mesh for fear of future recurrence, we feel that the definition of omitting onlay mesh needs to be more precise. In previous large patient population studies using MK mesh, the use of onlay mesh was not stated.9 ; 10 In this study, we have narrowed the definition of onlay omission (when MK mesh covers the posterior wall defect) and demonstrated the safety and efficacy. With any new surgical technique, a learning curve is to be expected.6; 21; 23 ; 24 In the series reported by Robert Kugel in 19992 and 2003,4 recurrence happened within the first 6 months of developing the new method with a total recurrence rate of 0.62%. Several studies have tried to reproduce the results without success.2; 4; 6; 7; 9 ; 10 The two studies with recurrence rates similar to Dr Kugels original study had >500 patients.25 ; 26 Van Nieuwenhove et al7 had a recurrence rate of 1.78% with a learning curve estimated to be around 25–35 cases. Schroder et al6 reported a recurrence rate of 7.25% with an estimated learning curve of 40 cases. Because most surgeons are acquainted with anterior approach herniorrhaphy, the anterior TIPP mesh placement will result in a lower learning curve compared with posterior or laparoscopic methods.6; 7; 22; 24; 27; 28; 29 ; 30 The unfamiliar anatomy, approach method, and inadequate mesh placement are culprits for recurrence when learning Kugels posterior approach.6 ; 10 The anterior TIPP approach, combined with adequate preperitoneal dissection, will result in a low learning curve and smooth mesh placement. We estimate the learning curve to be around 5–10 cases. Our recurrence rate of 1.4% (1 in 72) is similar to the previous reported series of the posterior and anterior approach (0.1–7%).6; 7; 9; 10; 25 ; 26 Our one recurrence happened within our first 20 cases; the patient underwent laparoscopic transabdominal preperitoneal operation. Upon reviewing this case, we suspect that inadequate dissection of the preperitoneal space (initially we used 3 wet gauzes for space dissection) resulted in an inadequate space for mesh placement. Along with the poor mesh placement, the patients work history, frequent exercise, and poor compliance (he resumed work and exercise within 3 weeks postoperatively) all contributed to his recurrence. Since then, we have standardized our preperitoneal dissection with seven wet gauzes. Since implementing the new dissection protocol, we noticed a smoother and flatter mesh placement. Previous reported series defined chronic pain as pain experienced for >3 months, which occurs in 1–20% of cases.11; 12; 31; 32 ; 33 In our current study, only one patient (1.4%) experienced chronic pain postoperatively, which is comparable with a series using Kugel mesh.2; 3; 4; 6; 7; 11 ; 22 Similar to other studies, we suspect that the low rate of chronic pain is due to decreased foreign body inflammatory response and uninvolved inguinal canal nerves.11; 17; 18; 19 ; 34
The limitations of our study include: (1) retrospective study without a control group; (2) the need for a longer follow-up period with office visits; and (3) telephone interview as the only follow up modality. Because of the limited resources and current medical environment in Taiwan, the second and third limitations would be very difficult to overcome even with a prospective randomized study. The questionnaires were set up to serve as history taking sessions. In cases with suspected recurrence, we would highly recommend an office visit for further physical examination.
5. Conclusion
In this study, we reported the safety and efficacy of the TIPP approach single-layer MK mesh herniorrhaphy. We have narrowed the definition for onlay mesh applications and described the method for adequate preperitoneal dissection. Our study showed that single-layer MK mesh is adequate without compromising recurrence or complication rates. A randomized prospective study using a larger study population and longer follow up would prove our theory.
References
- 1 R.C. Read; Herniology: past, present, and future; Hernia, 13 (2009), pp. 577–580
- 2 R.D. Kugel; Minimally invasive, nonlaparoscopic, preperitoneal, and sutureless, inguinal herniorrhaphy; Am J Surg, 178 (1999), pp. 298–302
- 3 B. Dasari, L. Grant, T. Irwin; Immediate and long-term outcomes of Lichtenstein and Kugel patch operations for inguinal hernia repair; Ulster Med J, 78 (2009), pp. 115–118
- 4 R.D. Kugel; The Kugel repair for groin hernias; Surg Clin North Am, 83 (2003), pp. 1119–1139
- 5 Y. Kurihara, T. Yamakawa, M. Yoshino, et al.; Experience with direct Kugel patch method for repair of adult inguinal hernia; J Nippon Med Sch, 75 (2008), pp. 28–31
- 6 D.M. Schroder, L.R. Lloyd, J.E. Boccaccio, C.A. Wesen; Inguinal hernia recurrence following preperitoneal Kugel patch repair; Am Surg, 70 (2004), pp. 132–136 discussion 6
- 7 Y. Van Nieuwenhove, F. Vansteenkiste, T. Vierendeels, K. Coenye; Open, preperitoneal hernia repair with the Kugel patch: a prospective, multicentre study of 450 repairs; Hernia, 11 (2007), pp. 9–13
- 8 BARD MK patch with Posiflex Memory Technology: Technique Guide (2008)
- 9 J. Li, Y. Zhang, H. Hu, W. Tang; Early experience of performing a modified Kugel hernia repair with local anesthesia; Surg Today, 38 (2008), pp. 603–608
- 10 X. Zhou; Comparison of the posterior approach and anterior approach for a Kugel repair of treatment of inguinal hernias; Surg Today, 43 (2013), pp. 403–407
- 11 R. Hompes, F. Vansteenkiste, H. Pottel, D. Devriendt, F. Van Rooy; Chronic pain after Kugel inguinal hernia repair; Hernia, 12 (2008), pp. 127–132
- 12 T. Inaba, K. Okinaga, R. Fukushima, et al.; Chronic pain and discomfort after inguinal hernia repair; Surg Today, 42 (2012), pp. 825–829
- 13 I.L. Lichtenstein, A.G. Shulman, P.K. Amid, M.M. Montllor; Cause and prevention of postherniorrhaphy neuralgia: a proposed protocol for treatment; Am J Surg, 155 (1988), pp. 786–790
- 14 D. Shin, L.I. Lipshultz, M. Goldstein, et al.; Herniorrhaphy with polypropylene mesh causing inguinal vasal obstruction: a preventable cause of obstructive azoospermia; Ann Surg, 241 (2005), pp. 553–558
- 15 R.G. Uzzo, G.E. Lemack, K.P. Morrissey, M. Goldstein; The effects of mesh bioprosthesis on the spermatic cord structures: a preliminary report in a canine model; J Urol, 161 (1999), pp. 1344–1349
- 16 P.K. Amid; The Lichtenstein repair in 2002: an overview of causes of recurrence after Lichtenstein tension-free hernioplasty; Hernia, 7 (2003), pp. 13–16
- 17 G.G. Koning, F. Keus, L. Koeslag, et al.; Randomized clinical trial of chronic pain after the transinguinal preperitoneal technique compared with Lichtensteins method for inguinal hernia repair; Br J Surg, 99 (2012), pp. 1365–1373
- 18 G.G. Koning, H.J. de Schipper, H.J. Oostvogel, et al.; The Tilburg double blind randomised controlled trial comparing inguinal hernia repair according to Lichtenstein and the transinguinal preperitoneal technique; Trials, 10 (2009), p. 89 1–6
- 19 G.G. Koning, D. Koole, M.A. de Jongh, et al.; The transinguinal preperitoneal hernia correction vs Lichtensteins technique; is TIPP top?; Hernia, 15 (2011), pp. 19–22
- 20 D. Dindo, N. Demartines, P.A. Clavien; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey; Ann Surg, 240 (2004), pp. 205–213
- 21 W. Hoste, Y. Van Nieuwenhove, T. Vierendeels; Early Belgian experience with the Kugel patch inguinal hernia repair; Acta Chir Belg, 106 (2006), pp. 44–46
- 22 K.M. Reddy, W. Humphreys, A. Chew, J. Toouli; Inguinal hernia repair with the Kugel patch; ANZ J Surg, 75 (2005), pp. 43–47
- 23 J.D. Hernandez, S.D. Bann, Y. Munz, et al.; Qualitative and quantitative analysis of the learning curve of a simulated surgical task on the da Vinci system; Surg Endosc, 18 (2004), pp. 372–378
- 24 H. Lau, N.G. Patil, W.K. Yuen, F. Lee; Learning curve for unilateral endoscopic totally extraperitoneal (TEP) inguinal hernioplasty; Surg Endosc, 16 (2002), pp. 1724–1728
- 25 V. Ceriani, E. Faleschini, P. Bignami, et al.; Kugel hernia repair: open “mini-invasive” technique. Personal experience on 620 patients; Hernia, 9 (2005), pp. 344–347
- 26 M.E. Fenoglio, H.R. Bermas, W.E. Haun, J.T. Moore; Inguinal hernia repair: results using an open preperitoneal approach; Hernia, 9 (2005), pp. 160–161
- 27 B.M. Frost, J. Hilton, B.M. Stephenson, K.W. Millikan, A. Doolas; A long-term evaluation of the modified mesh-plug hernioplasty in over 2,000 patients; Hernia, 12 (2008), pp. 327–328
- 28 K.W. Millikan, B. Cummings, A. Doolas; The Millikan modified mesh-plug hernioplasty; Arch Surg, 138 (2003), pp. 525–529 discussion 9–30
- 29 S. Takayama, H. Imafuji, H. Funahashi, H. Takeyama; Laparoscopic Spigelian and inguinal hernia repair with the Kugel patch; Surg Laparosc Endosc Percutan Tech, 20 (2010), pp. e76–e78
- 30 S. Takayama, M. Sakamoto, H. Takeyama; Laparoscopic inguinal hernia repair with the Composix Kugel Patch; Int Surg, 95 (2010), pp. 54–56
- 31 J. Cunningham, W.J. Temple, P. Mitchell, J.A. Nixon, R.M. Preshaw, N.A. Hagen; Cooperative hernia study. Pain in the postrepair patient; Ann Surg, 224 (1996), pp. 598–602
- 32 S. Kumar, R.G. Wilson, S.J. Nixon, I.M. Macintyre; Chronic pain after laparoscopic and open mesh repair of groin hernia; Br J Surg, 89 (2002), pp. 1476–1479
- 33 J.E. Losanoff, B.W. Richman, J.W. Jones; Chronic pain after laparoscopic and open mesh repair of groin hernia; Br J Surg, 89 (2002), pp. 1476–1479
- 34 A.N. Singh, V.K. Bansal, M.C. Misra, et al.; Testicular functions, chronic groin pain, and quality of life after laparoscopic and open mesh repair of inguinal hernia: a prospective randomized controlled trial; Surg Endosc, 26 (2012), pp. 1304–1317
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?