Summary
Undifferentiated embryonal sarcoma of the liver (UESL) is a rare primary liver tumor. Less than 100 adult cases were reported. It has female and right lobe preponderance. In pathological features, focal osteoid picture in UESL is never reported. We present a 63-year-old male patient with left lobe UESL with focal osteoid picture. He was admitted for a palpable solid mass, with left upper quadrant abdominal pain for 4 months. Abdominal computed tomography showed a huge well-circumscribed mass at left upper quadrant, 21.3 × 13 × 27.9 cm3 in size, with multiple septa in delayed phase. En bloc resection including lateral segmentectomy, splenectomy, and cholecystectomy were performed, but tumor rupture was noted. The pathologic diagnosis was ruptured UESL. The postoperative course was uneventful, and adjuvant radiotherapy without chemotherapy was performed. Peritoneal seeding with massive ascites was noted in the 9th month after operation. Even after receiving salvage chemotherapy, he died 1 year after operation. Early complete surgical resection with adjuvant chemotherapy may improve prognosis of UESL. But the overall survival of UESL did not improve until recently. We present this case along with a literature review of the clinical pictures, diagnosis, pathology presentation, pathologicogenesis of focal osteoid picture, treatment, and prognosis for UESL of another 23 new reported cases since 2007.
Keywords
focal osteoid picture;liver cystic tumor;malignant mesenchymoma;old age adult;undifferentiated embryonal sarcoma of liver
1. Introduction
Undifferentiated embryonal sarcoma of the liver (UESL) was first described in 1946 as a mesenchymoma, and was subsequently named “malignant mesenchymoma” by Stout. It was recognized as a unique clinicopathologic entity by Stocker and Ishak in 1978. It represents only approximately 0.2% of all primary liver tumor.1 The peak age of UESL is between 5 and 10 years. Less than 100 adult cases were reported. Adult cases over the age of 60 years are quite exceptional. Otherwise, it has a female preponderance, and the majority of tumor locations reported were in the right lobe. Only 22% UESL arise in the left lobe. To the best of our knowledge, there is no report about focal osteoid picture in UESL in pathology features. We present our experience of treating a 63-year-old male with left lobe UESL with focal osteoid picture. This report is presented along with a literature review of the clinical pictures, diagnosis, pathology presentation, treatment, and prognosis for UESL.
2. Case report
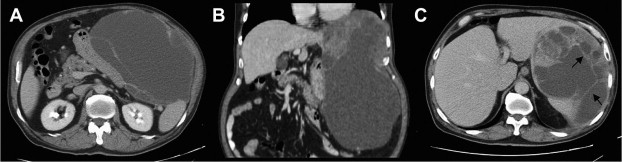
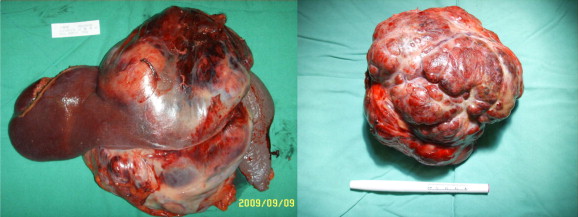
A 63-year-old male patient was admitted to our hospital due to a palpable solid mass with left upper quadrant abdominal pain for 4 months. He has no past history of Hepatitis B virus (HBV) or Hepatitis C virus (HCV) infection. Otherwise, he lost 10 kg of body weight within 8 months. Laboratory studies showed no elevation of liver function, alkaline phosphatase, and bilirubin. Abdominal ultrasonography showed a heterogeneous mass in the intra-abdomen, which was about 10 cm in diameter, with a peripheral cystic-like component near there about 13 cm in diameter. Abdominal computed tomography (CT) showed a huge well-circumscribed mass at left upper quadrant, up to 21.3 × 13 × 27.9 cm3 in size, seemed to have arisen from the gastric fundus (Fig. 1A and B), Much inner nonenhancing low-density collection, with enhancing soft parts, punctuate calcifications, and multiple septum in delayed phase were noted (Fig. 1C). It also exerts mass effect over the gastric body, liver, spleen, and pancreas. Surgical resection was recommended and accepted. During operation, a huge, pedunculated, and cystic liver tumor originating from inferior surface of left lateral segment was noted. The tumor was tightly adhesive to spleen, with multiple foci of rupture and some attached omental soft tissue. En bloc resection, including left lateral segmentectomy, splenectomy, and cholecystectomy, was performed ( Fig. 2).
|
|
|
Figure 1. (A and B) Abdominal computed tomography before surgery shows a huge well-circumscribed mass in the left upper quadrant which is attached to the spleen and pancreas firmly, with (C) multiple septa (arrows) in the delayed phase. |
|
|
|
Figure 2. Gross photograph of undifferentiated embryonal sarcoma of the liver. |
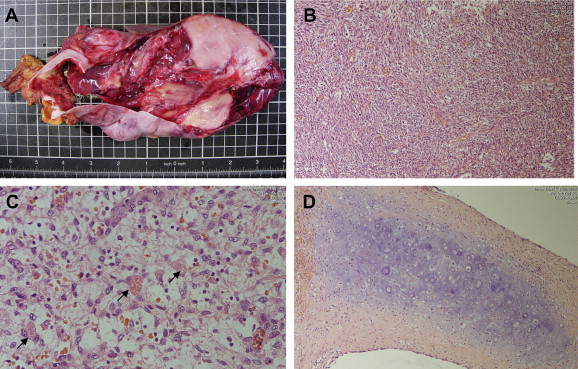
On microscopic examination, the tumor was found to be composed of pleomorphic tumor cells that are spindle and hyperchromatic, distributed in a fibrous or myxomatous stroma. Focally, tumor cells were highly bizarre, with atypical mitoses and mitotic and focal osteoid pictures (Fig. 3B and D). Prominent necrosis and hemorrhage were present. There were some eosinophilic granules in the mesenchymal part (Fig. 3C). Some dilated or small duct-like structures were found between the spindle cells in the peripheral area. The resection margin of liver was free of tumor. The immunohistochemistry stains showed positivity for actin-851, AiACT, and vimentin but no expression for cytokeratin. The pathologic diagnosis was ruptured UESL.
|
|
|
Figure 3. (A) Resected specimen under microscope, (B) area of embryonal sarcoma composed of pleomorphic spindle and hyperchromatic cells distributed in fibrous or myxomatous stroma, with (C) eosinophilic globules (arrows) and (D) some focal osteoid picture. |
The postoperative course was uneventful, and the patient was discharged 9 days after operation. One month after operation, he started to receive radiotherapy for 2 months without any adjuvant chemotherapy. Peritoneal seeding with massive ascites was noted on abdominal CT in the 9th month after operation. He received salvage chemotherapy as CYVADIC (cyclophosphamide + vincristine + adriamycin + dacarbazine). But disease still progressed and he died 1 year after operation.
3. Discussion
As we mentioned in the Introduction section, UESL is predominant in children and very rare in adult. Worldwide only 75 cases have been reported where patients are older than 15 years: 52 adult cases were reported from 1955 to 2007 and summarized by Pachera et al2 and another 23 from 2007 to 2011, including our case (Table 1)3; 4; 5; 6; 7; 8; 9; 10; 11; 12; 13; 14; 15; 16; 17 ; 18. In summary, the incidence of UESL is related to age. The incidence decreased with increasing age. In these 75 patients, 21 are between 15 and 20 years, 26 between 21 and 40 years, and 18 between 41 and 60 years of age, and only 10 patients are more than 60 years old. The relationship between age and survival is not clear now. Otherwise, female preponderance (female/male = 41/31) is noted. The incidence of right lobe tumor is higher (40/75, 53.3%) than left lobe (13/75, 17.3%) and bilateral lobe (10/75, 13.3%) tumors. Most tumor sizes exceed 10 cm (68/75, 90.6%). There is no static difference between the sizes of tumors at different sites (ANOVA test, p = 0.151).
| Reference | Year | Age | Sex | Site | Size (cm) | Surgery | RT | CT | Recurrence | Treatment after recurrence | Followed up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al3 | 2007 | 61 | F | R | 17 | Only fine needle biopsy | No | VAIA | No | — | AWD 12 mo |
| McCarthy et al4 | 2007 | 21 | F | R | N/A | Resection | Yes | Ifosfamide + etoposide | Yes, 36 mo | Radio frequency ablation (RFA) for liver+ resection of lung lesion + palliative CT after pregnancy | AWD 60 mo |
| Kim et al5 | 2007 | 44 | F | R | 15 | Surgical excision | N/A | N/A | N/A | N/A | N/A |
| Gourgiotis et al6 | 2008 | 20 | M | B | 20 | Extensive left lobectomy | No | Adjuvant CT | Yes | N/A | DOD 9 mo |
| Ma et al7 | 2008 | 61 | F | R | 12 | Right lobectomy | No | N/A | N/A | N/A | DOD 8 mo |
| Fuertes et al8 | 2008 | 40 | M | R | 28 | Right trisegmentectomy (6 + 7 + 8 + 1) | No | No | Yes, 10 mo | Ifosfamide + adriamycin | ANED 36 mo |
| Yang et al9 | 2009 | 46 | M | R | 6.2 | Right lobectomy | N/A | N/A | N/A | N/A | N/A |
| Yang et al9 | 2009 | 54 | M | R | 13 | Right lobectomy | N/A | N/A | N/A | N/A | N/A |
| Yang et al9 | 2009 | 26 | F | R | 12.2 | N/A | N/A | N/A | N/A | N/A | N/A |
| Yang et al9 | 2009 | 20 | F | B | 18.2 | N/A | N/A | N/A | N/A | N/A | N/A |
| Kaur et al10 | 2009 | 23 | M | R | 15 | Partial hepatectomy | N/A | N/A | N/A | N/A | N/A |
| Li et al11 | 2010 | 63 | M | R | 20 | Right lobectomy | No | No | Yes, 9 mo | N/A | DOD 18 mo |
| Li et al11 | 2010 | 39 | M | L | 16 | Left lobectomy | No | TACE | Yes, 30 mo | N/A | DOD 32 mo |
| Li et al11 | 2010 | 56 | M | R | 12.5 | Right lobectomy | No | TACE | Yes, 14 mo | N/A | DOD 28 mo |
| Li et al11 | 2010 | 43 | F | R | 14 | Right lobectomy | No | No | Yes, 12 mo | N/A | DOD 24 mo |
| Xu et al12 | 2010 | 36 | F | L | 30 | Right lobectomy | N/A | N/A | N/A | N/A | N/A |
| Faraj et al13 | 2010 | 21 | M | R | 22 | Extensive right lobectomy | No | No | Yes, 3 wka | Ifosfamide + etoposide alternating with actinomycin-D, vincristine | ANED 5 mo |
| Massaniet al14 | 2010 | 47 | F | R | N/A | Resection | N/A | N/A | Yes, 24 mo | Liver resection + TACE | DOD 29 mo |
| Yoon et al 15 | 2010 | 53 | F | R | 13 | Resection | No | No | No | — | ANED 6 mo |
| Lee et al16 | 2010 | 72 | M | L | 3.4 | Only fine needle biopsy | N/A | N/A | N/A | N/A | N/A |
| Gasljevic et al17 | 2011 | 58 | F | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Kalra et al18 | 2011 | 24 | M | R | 14 | N/A | N/A | N/A | N/A | N/A | N/A |
| Chen et al (present case) | 2011 | 63 | M | L | 27.9 | Lateral segmentectomy + splenectomy + cholecystectomy | Yes | No | Yes, 9 mo | CYVADIC | DOD 12 mo |
ANED = alive with no evidence of disease; AWD = alive with disease; CT = computed tomography; CYVADIC = cyclophosphamide + vincristine + adriamycin + dacarbazine; DOD = died of disease; N/A = not available; TACE = transarterial chemoembolization; VAIA = vincristine + actinomycin + ifosfamide + doxorubicin.
a. Recurrence site: previous drainage insertion.
The most reported symptoms are all nonspecific, such as abdominal mass or hepatomegaly, with or without upper abdominal pain, but none of them are specific for UESL. Laboratory tests including liver test and tumor markers are usually within normal limit. Only 9.8% patients with elevation of AFP and 1.9% with elevation of CA-125 were noted. So, preoperative diagnosis should be aided by images. Under ultrasonography, UESL often presents as a solid mass. The CT image characters demonstrate it typically as a large mass, predominantly solid or cystic, with well-defined boarders. The solid part of the tumor has been seen at delayed images with internal septa.12 ; 14 This misleading cyst-like appearance on CT imaging compared with the ultrasonic findings, in both adults and children, is useful in preoperative diagnosis and in avoiding drainage to large cystic hepatic mass that is considered to be a benign lesion,2 ; 19 such as complicated hydatid cyst.18 The cytological features of fine needle aspiration of UESL along with radiological and other relevant clinical findings may help in the correct diagnosis of this uncommon primary hepatic tumor.10; 13 ; 18
In pathology, the microscopic characters of UESL in our case, such as fibrous pseudocapsule with compressed hepatic parenchyma, medium–large spindle or satellite cells in solid component, trapped hepatocytes and abnormal bile duct cells at peripheral area, and eosinophilic spherical globules, were similar to those described in previous journals. There is no specific histological staining pattern for UESL.2 ; 7 Unlike previously described pathological findings, in our case, focal osteoid picture was noted in the solid part of tumor. It is an unusual pathological presentation of UESL. To our knowledge, no other case presented until now has focal osteoid picture.
Focal osteoid presentation was ever mentioned in the teratoid hepatoblasma,20 malignant mixed epithelial and stromal tumor,21 and nested stromal epithelial tumor of the liver.22 ; 23 These tumors have arisen from the stem cell or mesenchymal tissue of liver. Although the histogenesis of UESL is debated, several journals mentioned that mesenchymal hamartoma of liver (MHL) may be a premalignancy of UESL.24; 25 ; 26 MHL is an uncommon hamartomatous growth of mesenchymal tissue or other parts of the liver. This can be indirectly proven that the stem cells in mesenchyma of liver may possibly induce focal osteoid picture, just like the teratoid hepatoblasma, malignant mixed epithelial and stromal tumor, and nested stromal epithelial tumor of the liver.
Until recently, radial excision of the tumor seemed to provide the only chance of cure. Most patients died due to tumor recurrence or metastases 1 year after operation. The risk of recurrence after surgery is relatively high for the first 2 years. The recurrence risk is associated with positive margin and spontaneous or iatrogenic rupture of UESL. Combined chemotherapy and/or radiotherapy has been used for the treatment of UES and has been found to achieve superior local control and disease-free survival, even long-term survival. Lenze et al1 analyzed 68 adult patients. The actuarial 1- and 2-year survival rates for all patients were 61% and 55%, respectively. Patients with complete tumor resection followed by adjuvant chemotherapy had significantly better survival than patients with surgery alone (p = 0.002). But, if complete resection cannot be achieved, just like in case of our patient, adjuvant chemotherapy had no benefit on overall and disease-free survivals (p = 0.07).
In conclusion, UESL is extremely rare in adults. Only in 11 reported cases, patients are older than 60 years. Right lobe and female preponderance are noted. Our case is extremely rare in all adult UESL cases because of the patients age (63 years old, i.e., more than 60 years old) and the tumors location (left lobe). Our patient is the first reported case of focal osteoid picture in UESL, which may develop from the stem cells in mesenchyma of liver. Once UESL is diagnosed, early definite surgical resection with adjuvant chemotherapy may improve survival rate, increase tumor-free survival, and even achieve long-term survival. In our case, because of ruptured tumor, incomplete resection was recognized and the prognosis of the patient was relatively poor.
References
- 1 F. Lenze, T. Birkfellner, P. Lenz, et al.; Undifferentiated embryonal sarcoma of the liver in adults; Cancer, 112 (2008), pp. 2274–2282
- 2 S. Pachera, H. Nishio, Y. Takahashi, et al.; Undifferentiated embryonal sarcoma of the liver: case report and literature survey; J Hepatobiliary Pancreat Surg, 15 (2008), pp. 536–544
- 3 K.T. Kim, S.Y. Han, E.H. Park, et al.; A case of the treatment in an adult with hepatic undifferentiated (embryonal) sarcoma; Korean J Hepatol, 13 (2007), pp. 96–102
- 4 F.P. McCarthy, M. Harris, L. Kornman; Management of undifferentiated embryonal sarcoma of the liver in pregnancy; Obstet Gynecol, 109 (2007), pp. 558–560
- 5 M. Kim, B. Tireno, P.J. Slanetz; Undifferentiated embryonal sarcoma of the liver; AJR Am J Roentgenol, 190 (2008), pp. W261–W262
- 6 S. Gourgiotis, P. Moustafellos, S. Germanos; Undifferentiated embryonal sarcoma of the liver in adult; Surgery, 143 (2008), pp. 568–569
- 7 L. Ma, Y.P. Liu, C.Z. Geng, Z.H. Tian, G.X. Wu, X.L. Wang; Undifferentiated embryonal sarcoma of liver in an old female: case report and review of the literature; World J Gastroenterol, 14 (2008), pp. 7267–7270
- 8 M. Jimenez Fuertes, R. Lopez Andujar, M. de Juan Burgueno, et al.; Hepatic undifferentiated (embryonal) sarcoma in an adult: a case report and literature review; Gastroenterol Hepatol, 31 (2008), pp. 12–17
- 9 L. Yang, L.B. Chen, J. Xiao, P. Han; Clinical features and spiral computed tomography analysis of undifferentiated embryonic liver sarcoma in adults; J Dig Dis, 10 (2009), pp. 305–309
- 10 J. Kaur, P. Dey, A. Das; Fine needle aspiration cytology of undifferentiated (embryonal) sarcoma of liver in an adult male; Diagn Cytopathol, 38 (2010), pp. 547–548
- 11 X.W. Li, S.J. Gong, W.H. Song, et al.; Undifferentiated liver embryonal sarcoma in adults: a report of four cases and literature review; World J Gastroenterol, 16 (2010), pp. 4725–4732
- 12 H.F. Xu, Y.L. Mao, S.D. Du, et al.; Adult primary undifferentiated embryonal sarcoma of the liver: a case report; Chin Med J (Engl), 123 (2010), pp. 250–252
- 13 W. Faraj, D. Mukherji, N. El Majzoub, A. Shamseddine, M. Khalife; Primary undifferentiated embryonal sarcoma of the liver mistaken for hydatid disease; World J Surg Oncol, 8 (2010), p. 58
- 14 M. Massani, E. Caratozzolo, M. Baldessin, L. Bonariol, N. Bassi; Hepatic cystic lesion in adult: a challenging diagnosis of undifferentiated primary embryonal sarcoma; G Chir, 31 (2010), pp. 225–228
- 15 J.Y. Yoon, J.M. Lee, Y. Kim do, et al.; A case of embryonal sarcoma of the liver mimicking a hydatid cyst in an adult; Gut Liver, 4 (2010), pp. 245–249
- 16 J.A. Lee, T.W. Kim, J.H. Min, et al.; A case of undifferentiated (embryonal) liver sarcoma mimicking klatskin tumor in an adult; Korean J Gastroenterol, 055 (2010), pp. 144–148
- 17 G. Gasljevic, J. Lamovec, J. Jancar; Undifferentiated (embryonal) liver sarcoma: synchronous and metachronous occurrence with neoplasms other than mesenchymal liver hamartoma; Ann Diagn Pathol, 15 (2011), pp. 250–256
- 18 N. Kalra, S. Vyas, P. Jyoti Das, R. Kochhar, R. Srinivasan, N. Khandelwal; Undifferentiated embryonal sarcoma of liver in an adult masquerading as complicated hydatid cyst; Ann Hepatol, 10 (2011), pp. 81–83
- 19 P.C. Buetow, J.L. Buck, L. Pantongrag-Brown, et al.; Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases; Radiology (1997), pp. 203779–203783
- 20 L. Kim, Y.N. Park, S.E. Kim, T.W. Noh, C. Park; Teratoid hepatoblastoma: multidirectional differentiation of stem cell of the liver; Yonsei Med J, 42 (2001), pp. 431–435
- 21 G. Heywood, L.J. Burgart, D.M. Nagorney; Ossifying malignant mixed epithelial and stromal tumor of the liver: a case report of a previously undescribed tumor; Cancer, 94 (2002), pp. 1018–1022
- 22 A. Heerema-McKenney, I. Leuschner, N. Smith, J. Sennesh, M.J. Finegold; Nested stromal epithelial tumor of the liver: six cases of a distinctive pediatric neoplasm with frequent calcifications and association with cushing syndrome; Am J Surg Pathol, 29 (2005), pp. 10–20
- 23 H.R. Makhlouf, H.M. Abdul-Al, G. Wang, Z.D. Goodman; Calcifying nested stromal-epithelial tumors of the liver: a clinicopathologic, immunohistochemical, and molecular genetic study of 9 cases with a long-term follow-up; Am J Surg Pathol, 33 (2009), pp. 976–983
- 24 G.Y. Lauwers, L.D. Grant, W.H. Donnelly, et al.; Hepatic undifferentiated (embryonal) sarcoma arising in a mesenchymal hamartoma; Am J Surg Pathol, 21 (1997), pp. 1248–1254
- 25 V. Rajaram, S. Knezevich, K.E. Bove, A. Perry, J.D. Pfeifer; DNA sequence of the translocation breakpoints in undifferentiated embryonal sarcoma arising in mesenchymal hamartoma of the liver harboring the t(11;19)(q11;q13.4) translocation; Genes Chromosomes Cancer, 46 (2007), pp. 508–513
- 26 S.M. Tucker, K. Cooper, S. Brownschidle, R. Wilcox; Embryonal (undifferentiated) sarcoma of the liver with peripheral angiosarcoma differentiation arising in a mesenchymal hamartoma in an adult patient; Int J Surg Pathol, 20 (2012), pp. 297–300
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?